Abstract
Purpose
The potential efficacy of metformin in breast cancer (BC) has been hotly discussed but never conclusive. This genetics-based study aimed to evaluate the relationships between metformin targets and BC risk.
Methods
Metformin targets from DrugBank and genome-wide association study (GWAS) data from IEU OpenGWAS and FinnGen were used to investigate the breast cancer (BC)-metformin causal link with various Mendelian Randomization (MR) methods (e.g., inverse-variance-weighting). The genetic association between type 2 diabetes (T2D) and the drug target of metformin was also analyzed as a positive control. Sensitivity and pleiotropic tests ensured reliability.
Results
The primary targets of metformin are PRKAB1, ETFDH and GPD1L. We found a causal association between PRKAB1 and T2D (odds ratio [OR] 0.959, P = 0.002), but no causal relationship was observed between metformin targets and overall BC risk (PRKAB1: OR 0.990, P = 0.530; ETFDH: OR 0.986, P = 0.592; GPD1L: OR 1.002, P = 0.806). A noteworthy causal relationship was observed between ETFDH and estrogen receptor (ER)-positive BC (OR 0.867, P = 0.018), and between GPD1L and human epidermal growth factor receptor 2 (HER2)-negative BC (OR 0.966, P = 0.040). Other group analyses did not yield positive results.
Conclusion
The star target of metformin, PRKAB1, does not exhibit a substantial causal association with the risk of BC. Conversely, metformin, acting as an inhibitor of ETFDH and GPD1L, may potentially elevate the likelihood of developing ER-positive BC and HER2-negative BC. Consequently, it is not advisable to employ metformin as a standard supplementary therapy for BC patients without T2D.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most prevalent malignant tumor among women, exhibiting an escalating incidence globally and serving as a leading cause of cancer-related mortality in women worldwide [1, 2]. Concurrently, Type 2 diabetes (T2D) has emerged as a significant public health issue on a global scale [3]. Research investigations have demonstrated a positive association between T2D and an elevated risk of BC [4], potentially attributable to the activation of insulin or insulin-like growth factor receptors within breast epithelial tissue, or the alteration of sex hormone levels due to insulin resistance and hyperinsulinemia [5]. In light of these mechanisms, metformin, a well-established therapeutic approach for T2D, is believed to have the potential to mitigate the risk of breast cancer and enhance BC outcomes [6]. Additionally, metformin exerts its effects on the AMPK and mTOR pathways, thereby potentially impeding the growth of BC [7, 8]. Although preclinical investigations offer evidence of metformin’s impact on all subtypes of BC, the translation of these findings to the clinical domain is not without challenges, primarily due to the utilization of supra-physiological concentrations of glucose, insulin, and metformin in in vitro and in vivo laboratory models employed in preclinical studies [9, 10].
However, numerous studies have cast doubt on the correlation between T2D and the risk of BC, concurrently underscoring the inadequacy of evidence supporting a definitive impact of metformin on BC patients [11,12,13]. Furthermore, inconsistencies prevail within the outcomes pertaining to distinct subtypes of BC [14, 15]. A recent investigation demonstrated that the inclusion of metformin, as compared to a placebo, alongside conventional BC treatment did not yield a statistically significant enhancement in invasive disease-free survival among individuals with high-risk operable BC and no preexisting diabetes [10].
Hence, the status of metformin as a potential standard adjuvant therapy for breast cancer remains inconclusive. Similar to the randomized controlled trial (RCT) methodology, the MR approach inherently allocates participants into groups through genetic predictions of drug target perturbation, effectively mitigating the influence of environmental factors due to the random assortment of genetic variants during conception [16]. Furthermore, this approach effectively reduces the potential for reverse causality, as the germline genotype remains unaltered by the onset and progression of disease [17, 18]. An extension of the MR paradigm, the drug-target approach, has been applied to clinical trials in order to predict the effectiveness and potential negative consequences of therapeutic interventions [19]. Using genetic variants to surrogate mechanistic impacts of drug targets, this technique enables characterization of protein function and perturbation of drug targets [20, 21]. In this study, we have employed the drug-target MR methodology to simulate prolonged exposure to metformin in European populations, aiming to evaluate its causal effects on BC and present novel genetic evidence in this regard.
Materials and methods
Study overview and data sources
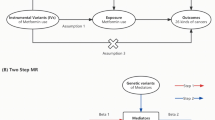
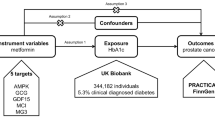
We employed a multi-group, two-sample MR design to investigate the potential efficacy of repurposing metformin for BC. Summary statistics of instrument exposure and instrument-outcome associations were derived from large-scale genome-wide association studies (GWAS) conducted in European populations (Fig. 1).
Initially, based on the Drugbank database, the target proteins of metformin were identified [22]. Subsequently, GWAS data corresponding to these genes were obtained from the IEU OpenGWAS project database, and independent variants within the gene locus were utilized as instrumental variables (IVs) to approximate the pharmacological modulation of the drug target protein [23].
The GWAS summary statistics for overall HER2-positive/negative BC and T2D were obtained from the FinnGen research project [24], while the GWAS summary statistics for ER-positive/negative BC were acquired from the Breast Cancer Association Consortium (BCAC) [25]. Due to the nature of the study, no specific ethical approval or written informed consent from participants was deemed necessary.
We subsequently assessed the causal link between genetic variations in metformin targets and the susceptibility to overall BC, and each subtype of BC, using a two-sample MR. Considering that the intended indication for metformin is T2DM, we concurrently investigated the causal relationship between metformin target variations and T2D as a positive control (ref: Guidelines for performing mendelian randomization investigations). Table 1 provides comprehensive details on all the GWAS included in our study.
Screening of IVs
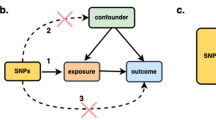
We implemented several quality control measures to identify suitable genetic instrumental tools [26]. Initially, we identified single nucleotide polymorphisms (SNPs) that achieved genome-wide significance (P < 5.0E − 08). Subsequently, a clumping process was applied, utilizing linkage disequilibrium (LD) estimates from Europeans in the 1000 Genomes project (R2 < 0.1, window size of 500 kb) to ensure the independence of genetic variables [27]. In cases where SNP pairs showed LD R2 values surpassing the threshold (0.1), we retained the SNP with the lower P value.
To detect potential weak instrumental variable bias, we calculated the F-statistic (F = R2(n - k − 1)/k (1 - R2)), where R2, n, and k denote the proportion of variance in exposure explained by selected genetic tools, the sample size of the exposure GWAS, and the number of chosen genetic tools, respectively. An average F-statistic exceeding 10 indicates suitable instrumental variables [28].
Adhering to the two fundamental IV analysis assumptions, we removed SNPs linked to potential confounding variables and adjusted for SNPs exhibiting horizontal pleiotropic effects through consultation with the PhenoScanner database [29].
MR analysis
In this study, we conducted two separate two-sample MR analyses. Initially, we aligned SNPs to standardize the exposure and outcome data. Subsequently, the inverse-variance weighting (IVW) method was used to assess heterogeneity between SNPs. A p-value greater than 0.05 for the Q-statistic indicated the absence of heterogeneity. Furthermore, for detecting horizontal pleiotropy, we performed the MR-Egger regression intercept test. Finally, based on the evaluation of between-SNP heterogeneity and horizontal pleiotropy, we selected the primary MR method. In order to ensure the robustness of the results, five distinct methods were employed in the present study. The fixed-effect IVW approach was utilized when neither heterogeneity nor pleiotropy were present, while the random-effect IVW method was employed in cases where heterogeneity was observed but pleiotropy was absent [30, 31]. In situations involving pleiotropy, with or without heterogeneity, the MR-Egger regression technique was applied [32, 33]. Additionally, the influence of SNPs was identified using leave-one-out analyses. All statistical analyses were performed using the R program (version 4.2.1), with MR analysis being implemented through the utilization of the TwoSampleMR packages [34]. Both sides of the test were considered statistically significant at 0.05.
Results
Genetic instruments for metformin
Three targets of metformin were retrieved from the DrugBank database: PRKAB1, ETFDH and GPD1L, with metformin acting as its inducer, inhibitor and inhibitor, respectively. All F-statistics fell within the range of 49.734 to 7601.343, suggesting that IVs were robust [28]. Further information regarding the SNPs can be found in Supplement Tables S1–16.
Positive control analysis
Although no substantial causal relationship exists between long-term exposure to functional inhibition of ETFDH or GPD1L and T2D risk (ETFDH: OR[95%] = 1.022 [0.989 to 1.055], P = 0.189; GPD1L: OR[95%] = 0.993 [0.978 to 1.007], P = 0.324), the anticipated outcome was confirmed by the IVW method, which revealed that overexpression of PRKAB1 with AMP-activated protein kinase activity significantly reduced the risk of T2D (OR[95%] = 0.959 [0.935 to 0.984], P = 0.002). Consistency in results was also observed across MR Egger, simple model, weighted model, and MR-PRESSO analyses (Fig. 2).
The causal relationship between metformin targets and overall BC
No significant causal relationship found between inhibition or activation of any metformin target and risk of BC (PRKAB1: OR [95%] = 0.990 [0.961 to 1.020], P = 0.530; ETFDH: OR [95%] = 0.987 [0.939 to 1.036], P = 0.582; GPD1L: OR [95%] = 1.003 [0.981 to 1.025], P = 0.806). Consistency in results was also observed across MR Egger, simple model, weighted model, and MR-PRESSO analyses (Fig. 3)
The causal relationship between metformin targets and ER-positive/negative BC
Reduced risk of ER-positive BC was significantly associated with genetic variants in ETFDH (OR [95%] = 0.867 [0.770 to 0.976], P = 0.018), but not with GPD1L (OR [95%] = 0.987 [0.923 to 1.054], P = 0.689) and PRKAB1 (No valid genetic instruments were found) (Fig. 4A). For ER-negative BC, no significant causal association was found between any metformin target and BC risk (PRKAB1: No valid genetic instruments were found; ETFDH: OR [95%] = 0.954 [0.875 to 1.041], P = 0.294; GPD1L: OR [95%] = 1.011 [0.959 to 1.066], P = 0.680) (Fig. 4B). Consistency in results was also observed across MR Egger, simple model, weighted model, and MR-PRESSO analyses. (Fig. 4A, B)
The causal relationship between metformin targets and HER2-positive/negative BC
For HER-positive BC, no significant causal association was found between any metformin target and BC risk (PRKAB1: OR [95%] = 0.986 [0.949 to 1.023], P = 0.452; ETFDH: OR [95%] = 0.983 [0.929 to 1.039], P = 0.543; GPD1L: OR [95%] = 1.023 [0.996 to 1.050], P = 0.102) (Fig. 5A). Reduced risk of HER2-negative BC was significantly associated with genetic variants in GPD1L (OR [95%] = 0.966 [0.936 to 0.998], P = 0.040), but not with PRKAB1 (OR [95%] = 0.998 [0.961 to 1.036], P = 0.909); ETFDH: OR [95%] = 0.990 [0.928 to 1.057], P = 0.774 (Fig. 5B). Consistency in results was also observed across MR Egger, simple model, weighted model, and MR-PRESSO analyses (Fig. 5A, B).
Multiple causal path assessment
To address confounding issues among multiple causal factors, we attempted multivariable Mendelian randomization analysis to simultaneously evaluate the causal pathways between the three targets and BC [35, 36]. However, there was no enough overlap between effective IVs for the three targets and BC, making further analysis infeasible. This suggests that the likelihood of significant causal interactions among the three targets on breast cancer is low.
Sensitivity analysis
The level of heterogeneity and horizontal pleiotropy was assessed using Cochrane’s Q and MR Egger regression equation, and leave-one-out analysis was conducted to identify influential SNPs (Tables S17, Figure S1-6). Significant heterogeneity was observed when examining the causal relationship between PRKAB1 variation and overall BC, HER2-positive BC, and T2D. Importantly, the random-effect IVW method employed in this study effectively mitigates bias resulting from heterogeneity in the findings [30]. Additionally, no heterogeneity or pleiotropy was detected in other groups analyses.
Discussion
This is the first MR study to investigate whether genetic variations in metformin targets are associated with the risk of overall BC, as well as ER-positive/negative BC and HER2-positive/negative BC. We retrieved the three key action targets of metformin from the Drugbank database: PRKAB1 [37], ETFDH [38, 39], and GPD1L [40, 41]. Among them, PRKAB1, also known as 5’AMP-activated protein kinase (AMPK) subunit beta-1 or AMPK, which as AMP-activated protein kinase activity, is the most critical target of metformin. AMPK is not solely the primary focus of glucose reduction; moreover, extant research indicates that metformin also depends on its anticancer properties [42]. The prevailing perspective posits that metformin triggers AMPK activation within cancerous cells, thereby instigating metabolic reprogramming and impeding the utilization of nutrient resources, ultimately impeding proliferation [43]. Regrettably, based on robust evidence, leveraging existing large-scale genetic association data on BC risk, our genetic investigation suggests that metformin might exert no protective effects on overall BC, ER-negative BC, and HER2-positive BC within European populations. The findings from this study indicate that there exists no discernible causal link between the functional activation of the star target AMPK (PRKAB1) and the mitigation of breast cancer risk, failing to manifest the anticipated anticancer efficacy. This suggests that despite certain preclinical investigations suggesting metformin’s potential in reducing cancer risk, its translation into the clinical context is not a straightforward process [9, 44].
Furthermore, our research suggests a significant causal association between elevated ETFDH expression as an exposure factor and a reduced probability of developing ER-positive BC. Similarly, a high expression level of GPD1L is correlated with a decreased likelihood of developing HER2-negative BC. These findings suggest that, overall among breast cancer patients, metformin not only fails to mitigate the risk of breast cancer in each subtype, but also possesses the potential to increase the risk of ER-positive BC and HER2-negative BC by inhibiting ETFDH and GPD1L.
This might appear disappointing at a first glance but not unexpected. While initial epidemiologic studies suggested a preventive role of metformin in BC [45, 46], subsequent research and meta-analyses presented conflicting conclusions [47, 48]. Most recently, the largest phase 3 randomized trial investigating metformin as adjuvant therapy for BC, comprising 3,649 women with a 5-year follow-up, revealed no discernible benefits in terms of disease-free survival or overall survival with metformin [10]. Oriana Hoi et al. highlighted in their review [49] that, despite experimental studies supporting metformin’s anticancer effects, many used suprapharmacological doses, reaching plasma levels 10 to 100 times higher than achievable in humans [50]. As such, findings from these studies may not translate to the same effects in humans [42, 50]. Numerous observational clinical studies have demonstrated susceptibility to time-related bias, leading to an overestimation of the drug’s advantages. Thus far, randomized trials investigating metformin as a therapeutic intervention for diverse cancer types have not yielded any evidence of diminished disease-free survival or overall survival rates [49]. Our study is the inaugural investigation into the causal link between metformin target variations and the risk of distinct breast cancer subtypes using drug-target MR stratification. The findings offer fresh evidence contradicting the notion of metformin as a standard adjunctive therapy for BC.
Furthermore, our findings imply that metformin, acting as an ETFDH inhibitor, might potentially exert a promoting effect on ER-positive BC. Previous studies have highlighted that low ETFDH expression correlates significantly with poorer survival in various tumors, including hepatocellular carcinoma and colorectal cancer [51, 52]. Some researchers suggest that although metformin may benefit ER-positive BC patients initially, the effect of long-term use may be reversed [15, 53]. Additionally, several investigations have reported metformin use was associated with an augmented incidence of ER-positive BC, aligning with our study results [54].
Our findings also indicate that metformin may enhance the development of HER 2-negative breast cancer by inhibiting GPD1L. Ye Du et al. have confirmed that the downregulation of GPD1L is linked to metabolic reprogramming in triple-negative breast cancer (TNBC) as a direct downstream target of aerobic glycolysis and oncogenic activity mediated by mir-210-3p [55]. A study indicates that metformin can induce metabolic adaptation in breast cancer cells, increasing resistance to metformin and promoting the accumulation of TNBC-derived BCSCs, which could eventually lead to cancer cell invasion, metastasis, and recurrence [56]. Matthew G. Costales et al. have identified a small molecule called Targapremir-210 in TNBC and observed a significant increase in GPD1L levels in TNBC tumor tissues in the Targapremir-210 treated group, disrupting the hypoxic adaptive response that promotes tumor growth [57]. While specific studies confirming the impact of GPD1L dysregulation on HER2-negative BC are lacking, the clear inhibitory effect of high GPD1L expression on TNBC may suggest evidence for the potential promoting effect of metformin on HER2-negative BC.
Certainly, we need to consider whether metformin interacts with other major BC treatments such as surgery, radiotherapy, chemotherapy, endocrine therapy, and targeted therapy. Currently, no evidence links metformin’s efficacy to surgical intervention. Its radiosensitizing effects have been preliminarily explored in mice, suggesting that while metformin with oxygen microbubbles may enhance short-term radiosensitivity, it does not reverse treatment resistance and may promote metastasis [58]. Although some studies suggest metformin can reverse chemotherapy resistance in vitro, this does not translate effectively in vivo or clinically [59, 60]. Additionally, although some studies have explored combining metformin with endocrine and targeted therapies, evidence shows that metformin promotes the survival of dormant estrogen receptor-positive breast cancer cells by activating AMPK, cautioning against widespread use of AMPK activators [61]. Long-term metformin treatment may also lead to dual resistance to tamoxifen and metformin through the Akt/Snail1/E-cadherin signaling axis [62]. Existing clinical data supporting the inclusion of metformin in BC treatment primarily come from survival analyses of BC patients with diabetic patients [63,64,65]. This may be because diabetes itself is a high-risk factor for BC [66, 67], and metformin might help combat diabetes-related BC risk while treating diabetes. However, this does not justify expanding its use to non-diabetic populations with normal blood glucose levels.
Not only that, the compliance of metformin cannot be ignored. Metformin is recognized for inducing gastrointestinal side effects, including nausea, diarrhea, and abdominal discomfort, affecting 20–30% of the general population, notably during initial dose adjustment [68, 69]. In a substantial double-blind trial for ER-positive early BC, non-adherence was more prevalent among metformin-treated patients [70]. Consequently, metformin does not appear to be a viable standard complementary treatment for breast cancer due to concerns about both anticancer efficacy and patient compliance.
Our study exhibits notable strengths. Firstly, it pioneers a unique MR investigation comparing metformin and breast cancer (both overall and subtypes) in European populations, filling a gap in the current literature. The two-sample design ensures a causal inference devoid of confounding bias and reverse causation. Moreover, the large F statistic underscores minimal risk of a weak instrument bias. Lastly, we meticulously selected SNPs showing significant associations with the exposure variable but lacking direct associations with the outcome variable. It is worth mentioning that our study, compared to previous epidemiological studies, has a much larger sample size. We not only concluded that there is no significant causal relationship between metformin use and reduced overall breast cancer risk but also suggested that metformin might actually increase the risk of ER-positive and HER2-negative BC by inhibiting ETFDH and GPD1L. Although there is not yet sufficient clinical data to support this finding, much laboratory evidence supports our conclusion.
Despite the aforementioned strengths, it is crucial to acknowledge several inescapable limitations in our study. Firstly, our results should primarily be interpreted as a test of causal association and cannot replace clinical trials in the real-world setting. Secondly, our analyses did not consider the combined efficacy of drug interactions in clinical scenarios. Thirdly, the drug target MR effect estimates mainly correspond to continuous, long-term modulation of drug targets and may not reflect the impact of short-term drug use on breast cancer. Lastly, our MR analysis was limited to the European population due to inadequate GWAS data resources, raising uncertainty about the generalizability of our findings to different ethnic populations. Additionally, due to the limited information in the databases, further stratified analysis may not have been conducted for patients with different clinical-pathological characteristics. Therefore, future research should conduct subgroup analyses on different racial populations, expand the study samples for each breast cancer subtype, and supplement the design with considerations for short-term and long-term medication. A specific and thorough tracking investigation should be implemented to assess short-term and long-term benefits, aiming to derive more comprehensive conclusions.
Conclusions
To sum up, the main target of metformin, PRKAB1, does not demonstrate a substantial causal association with the risk of BC. Conversely, metformin, acting as an inhibitor of ETFDH and GPD1L, may elevate the risk of developing ER-positive BC and HER2-negative BC. This research presents innovative genetic evidence indicating that metformin may not serve as a promising standard adjunctive therapy for BC patients without T2D.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Abbreviations
- BC:
-
Breast cancer
- T2D:
-
Type 2 diabetes
- MR:
-
Mendelian Randomization
- OR:
-
Odds ratio
- ER:
-
Estrogen receptor
- RCT:
-
Randomized controlled trial
- GWAS:
-
Genome-wide association studies
- BCAC:
-
Breast Cancer Association Consortium
- SNPs:
-
Single nucleotide polymorphisms
- LD:
-
Linkage disequilibrium
- IVs:
-
Instrumental variables
- IVW:
-
Inverse-variance weighting
- AMPK:
-
5’AMP-activated protein kinase
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians 2021, 71(1).
Naganathan G, Bilgen I, Cleland J, Reel E, Cil T. #COVID19 and #Breastcancer: a qualitative analysis of tweets. Curr Oncol. 2022;29(11):8483–500.
Casana E, Jimenez V, Jambrina C, Sacristan V, Muñoz S, Rodo J, Grass I, Garcia M, Mallol C, León X. AAV-mediated BMP7 gene therapy counteracts insulin resistance and obesity. Mol Therapy-Methods Clin Dev. 2022;25:190–204.
Boyle P, Boniol M, Koechlin A, Robertson C, Autier P. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608–17.
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Ztschrift Für Ernhrungswissenschaftjournal Nutritional Encessupplementa. 2005;14(2):Suppl.
Scordamaglia D, Cirillo F, Talia M, Santolla MF, Rigiracciolo DC, Muglia L, Zicarelli A, De Rosis S, Giordano F, Miglietta AM. Metformin counteracts stimulatory effects induced by insulin in primary breast cancer cells. J Transl Med. 2022;20(1):1–19.
Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC. Metformin activates AMPK through the Lysosomal Pathway. Cell Metabol. 2016;24(4):521–2.
Hampsch RA, Wells JD, Traphagen NA, Mccleery CF, Miller TW. AMPK activation by Metformin promotes survival of dormant ER + breast Cancer cells. Clin Cancer Res. 2020;26(14):clincanres02692020.
Saini N, Yang X. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin. 2017;50(2):1–11.
Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, Ligibel JA, Hershman DL, Mayer IA, Hobday TJ. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in patients with breast Cancer: the MA32 Randomized Clinical Trial. JAMA. 2022;327(20):1963–73.
Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D, Xie J, Tao M, Mao Y. Metformin Use is Associated with Better survival of breast Cancer patients with diabetes: a Meta-analysis. Oncologist 2015:1236.
Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013;36(10):3018–26.
Hosio M, Urpilainen E, Hautakoski A, Marttila M, Arffman M, Sund R, Ahtikoski A, Puistola U, Karihtala P, Jukkola A. Survival after breast cancer in women with type 2 diabetes using antidiabetic medication and statins: a retrospective cohort study. Acta Oncol. 2020;59(9):1110–7.
Sonnenblick A, Agbor-Tarh D, Bradbury I, Cosimo SD, Azambuja ED. Impact of diabetes, insulin, and Metformin Use on the outcome of patients with human epidermal growth factor receptor 2-Positive primary breast Cancer: analysis from the ALTTO Phase III Randomized Trial. J Clin Oncol. 2017;35(13):1421–9.
Park Y-M, Bookwalter D, O’Brien K, Jackson C, Weinberg C, Sandler D. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann Oncol. 2021;32(3):351–9.
Yuan S, Wang L, Zhang H, Xu F, Zhou X, Yu L, Sun J, Chen J, Ying H, Xu X. Mendelian randomization and clinical trial evidence supports TYK2 inhibition as a therapeutic target for autoimmune diseases. EBioMedicine 2023, 89.
Rosoff DB, Bell AS, Jung J, Wagner J, Mavromatis LA, Lohoff FW. Mendelian randomization study of PCSK9 and HMG-CoA reductase inhibition and cognitive function. J Am Coll Cardiol. 2022;80(7):653–62.
Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for mendelian randomization. Stat Methods Med Res. 2017;26(5):2333–55.
Carss KJ, Deaton AM, Del Rio-Espinola A, Diogo D, Fielden M, Kulkarni DA, Moggs J, Newham P, Nelson MR, Sistare FD. Using human genetics to improve safety assessment of therapeutics. Nat Rev Drug Discovery. 2023;22(2):145–62.
Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, Tyl B, Chopade S, Faraway R, Zwierzyna M. Genetic drug target validation using mendelian randomisation. Nat Commun. 2020;11(1):3255.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet 2016.
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Oxford University Press; 2006. (90001).
Elsworth BL, Lyon MS, Alexander T, Liu Y, Hemani G. The MRC IEU OpenGWAS data infrastructure. Cold Spring Harbor Lab 2020.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18.
Michailidou K, Lindström S, Dennis J, Beesley J, Hui S, Kar S, Lemaçon A, Soucy P, Glubb D, Rostamianfar A. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92–4.
Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18(1):1–19.
Sun L, Guo D, Jia Y, Shi M, Yang P, Wang Y, Liu F, Chen G-C, Zhang Y, Zhu Z. Association between Human Blood Metabolome and the risk of Alzheimer’s Disease. Ann Neurol. 2022;92(5):756–67.
Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, Butterworth AS, Staley JR. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, Kazmi N, Robinson TM, Albanes D, Aleksandrova K, et al. Physical activity and risks of breast and colorectal cancer: a mendelian randomisation analysis. Nat Commun. 2020;11(1):597.
Yuan S, Kim JH, Xu P, Wang Z. Causal association between celiac disease and inflammatory bowel disease: a two-sample bidirectional mendelian randomization study. Front Immunol. 2022;13:1057253.
Jiang H, Hu D, Wang J, Zhang B, He C, Ning J. Adiponectin and the risk of gastrointestinal cancers in East asians: mendelian randomization analysis. Cancer Med. 2022;11(12):2397–404.
Wang X, Wang X, Wang H, Yang M, Dong W, Shao D. Association between psoriasis and lung cancer: two-sample mendelian randomization analyses. BMC Pulm Med 2023.
Gibran H, Jie Z, Benjamin E, Wade KH, Valeriia H, Denis B, Charles L, Stephen B, Jack B, Ryan L. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–27.
Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med 2021, 11(2).
Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Reviews Endocrinol. 2019;15(10):569–89.
Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;122(6):253–70.
Fontaine E. Metformin-Induced mitochondrial complex I inhibition: facts, uncertainties, and consequences. Front Endocrinol (Lausanne) 2018, 9.
Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6.
Baur JA, Birnbaum MJ. Control of gluconeogenesis by metformin: does redox trump energy charge? Cell Metabol. 2014;20(2):197–9.
Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, MacDonald R, Hollenberg MD, Hill MA. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133:155223.
Mahvash Z, Ryan D, Fantus GI. Nahum, Sonenberg, Michael, Pollak: Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006.
Dowling RJO, Niraula S, Stambolic V. P., J.: Metformin in cancer: translational challenges. J Mol Endocrinol 2012.
Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135(3):639–46.
Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, Ipp E, Kaklamani VG, Vitolins M, Wallace R. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30(23):2844.
Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schünemann HJ, Muti P. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: a GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018;27(6):627–35.
Ting Y, Yuan Y, Shengchun L. Association between metformin therapy and breast Cancer incidence and mortality: evidence from a Meta-analysis. J Breast Cancer. 2015;18(3):264–70.
Yu OHY, Suissa S. Metformin and Cancer: Solutions to a Real-World Evidence Failure. Diabetes care 2023.
He L, Wondisford FE. Metformin action: concentrations matter. Cell Metabol. 2015;21(2):159–62.
Yaxun X, Zhang R, Shen. Jieyu, Huang, Xiaoyun, Guihua, Zheng: expression and significance of ETFDH in hepatocellular carcinoma. Pathol Res Pract. 2019;215(12):152702–152702.
Maurya N, Kushwaha S, Chawade A, Mani A. Transcriptome profiling by combined machine learning and statistical R analysis identifies TMEM236 as a potential novel diagnostic biomarker for colorectal cancer. Sci Rep 2021, 11.
Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: where are we now? Int J Mol Sci. 2022;23(5):2705.
Aksoy S, Sendur MAN, Altundag K. Demographic and clinico-pathological characteristics in patients with invasive breast cancer receiving metformin. Med Oncol. 2013;30(2):590.
Du Y, Wei N, Ma R, Jiang S, Song D. A mir-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death Dis. 2020;11(9):731.
Banerjee A, Birts CN, Darley M, Parker R, Mirnezami AH, West J, Cutress RI, Beers SA, Rose-Zerilli MJJ, Blaydes JP. Stem cell-like breast cancer cells with acquired resistance to metformin are sensitive to inhibitors of NADH-dependent CtBP dimerization. Carcinogenesis. 2019;40(7):871–82.
Costales MG, Haga CL, Velagapudi SP, Childs-Disney JL, Phinney DG, Disney MD. Small molecule inhibition of microRNA-210 reprograms an Oncogenic Hypoxic Circuit. J Am Chem Soc 2017.
Drzał A, Dziurman G, Hoła P, Lechowski J, Delalande A, Swakoń J, Pichon C, Elas M. Murine breast Cancer Radiosensitization using Oxygen Microbubbles and Metformin: vessels are the Key. Int J Mol Sci 2023, 24(15).
Samuel SM, Varghese E, Koklesová L, Líšková A, Kubatka P, Büsselberg D. Counteracting chemoresistance with metformin in breast cancers: Targeting Cancer Stem cells. Cancers (Basel) 2020, 12(9).
Morio K, Kurata Y, Kawaguchi-Sakita N, Shiroshita A, Kataoka Y. Efficacy of metformin in patients with breast Cancer receiving chemotherapy or endocrine therapy: systematic review and Meta-analysis. Ann Pharmacother. 2022;56(3):245–55.
Hampsch RA, Wells JD, Traphagen NA, McCleery CF, Fields JL, Shee K, Dillon LM, Pooler DB, Lewis LD, Demidenko E, et al. AMPK activation by Metformin promotes survival of dormant ER + breast Cancer cells. Clin Cancer Res. 2020;26(14):3707–19.
Scherbakov AM, Sorokin DV, Tatarskiy VV, Prokhorov NS, Semina SE, Berstein LM, Krasil’nikov MA. The phenomenon of acquired resistance to metformin in breast cancer cells: the interaction of growth pathways and estrogen receptor signaling. IUBMB Life. 2016;68(4):281–92.
Kim HJ, Kwon H, Lee JW, Kim HJ, Lee SB, Park HS, Sohn G, Lee Y, Koh BS, Yu JH, et al. Metformin increases survival in hormone receptor-positive, HER2-positive breast cancer patients with diabetes. Breast Cancer Res. 2015;17(1):64.
Rao M, Gao C, Guo M, Law BYK, Xu Y. Effects of metformin treatment on radiotherapy efficacy in patients with cancer and diabetes: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:4881–90.
Peairs KS, Barone BB, Snyder CF, Yeh H-C, Stein KB, Derr RL, Brancati FL, Wolff AC. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncology: Official J Am Soc Clin Oncol. 2011;29(1):40–6.
Martin SD, McGee SL. Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J Endocrinol. 2018;237(2):R35–46.
Samuel SM, Varghese E, Varghese S, Büsselberg D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat Rev 2018, 70.
McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–35.
Flory JH, Justin KS, David S, Mushlin AI. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. Bmj Open. 2018;8(7):e021505.
Hershman DLL, Chen BEE, Sathe C, Parulekar WRR, Lemieux J, Ligibel JAA, Gelmon KAA, Whelan TJJ, Goodwin PJJ. Metformin, placebo, and endocrine therapy discontinuation among participants in a randomized double-blind trial of metformin vs placebo in hormone receptor-positive early-stage breast cancer (CCTG MA32). Breast cancer research and treatment 2023.
Acknowledgements
Not applicable.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Jing-Xuan Xu: Conceptualization, Formal analysis, Methodology, Writing-original draft; Qi-Long Zhu: Validation, Visualization, Writing-original draft; Yu-Miao Bi: Resources, Writing-review & editing; Yu-Chong Peng: Investigation, Validation, Resources, Writing-review & editing.
Corresponding authors
Ethics declarations
Ethics approval and informed consent
Ethics approval and informed consent were not required for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, JX., Zhu, QL., Bi, YM. et al. New evidence: Metformin unsuitable as routine adjuvant for breast cancer: a drug-target mendelian randomization analysis. BMC Cancer 24, 691 (2024). https://doi.org/10.1186/s12885-024-12453-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12453-w