Abstract
Background
Increasing number of studies reported the positive effect of metformin on the prevention and treatment of cancers. However, the genetic causal effect of metformin utilization on the risk of common cancers was not completely demonstrated.
Methods
Two-sample Mendelian Randomization (two-sample MR) analysis was conducted to uncover the genetically predicted causal association between metformin use and 26 kinds of cancers. Besides, two-step Mendelian Randomization (two-step MR) assessment was applied to clarify the mediators which mediated the causal effect of metformin on certain cancer. We utilized five robust analytical methods, in which the inverse variance weighting (IVW) method served as the major one. Sensitivity, pleiotropy, and heterogeneity were assessed. The genetic statistics of exposure, outcomes, and mediators were downloaded from publicly available datasets, including the Open Genome-Wide Association Study (GWAS), FinnGen consortium (FinnGen), and UK Biobank (UKB).
Results
Among 26 kinds of common cancers, HER-positive breast cancer was presented with a significant causal relationship with metformin use [Beta: − 4.0982; OR: 0.0166 (95% CI: 0.0008, 0.3376); P value: 0.0077], which indicated metformin could prevent people from HER-positive breast cancer. Other cancers only showed modest associations with metformin use. Potential mediators were included in two-step MR, among which total testosterone levels (mediating effect: 24.52%) displayed significant mediating roles. Leave-one-out, MR-Egger, and MR-PRESSO analyses produced consistent outcomes.
Conclusion
Metformin use exhibited a genetically protective effect on HER-positive breast cancer, which was partially mediated by total testosterone levels.
Similar content being viewed by others
Introduction
The growing frequency and high mortality of malignant tumors imposed a significant burden on people all over the world [1]. Over the past few decades, healthcare professionals have tirelessly sought effective and safe strategies for cancer prevention, albeit with limited success. Metformin, a widely prescribed medication for managing type 2 diabetes, has garnered increasing interest for its potential anti-tumorigenic properties [2]. An increasing number of clinical studies attempted to reveal the efficacy of metformin on different types of cancer, but the controversial conclusions left the issue unsolved [3,4,5]. Biases induced by confounders which were hard to avoid in observational studies might be responsible for this.
Scientists were also interested in the biological pathways of metformin in cancer treatment. Apart from its well-documented benefits in improving glucose metabolism, recent years have witnessed extensive investigations into metformin's molecular mechanisms against various malignancies. These mechanisms include the reduction of leukocyte–endothelium interactions, modulation of oxidative stress, and the regulation of AMP-activated protein kinase (AMPK) [6, 7]. However, researches related to the genetic effect of metformin use on the risk of cancers were not complete yet.
Contrary to conventional observational research, Mendelian Randomization analysis (MR) provided a cost- and time-saving approach with high efficiency to investigate the genetically predicted causal relationships [8]. As Dr. Tobin stated, MR is also known as 'Mendelian deconfounding' since it attempts to present estimates of causal effects that are free of biases caused by confounding [9]. Robust-associated genetic variants were selected to explore the genetic association between exposures and outcomes. Genetic variations in the MR method are equal to lifetime changes caused by exposure and reflect the long-term implications of the alteration on certain illnesses [10]. In our study, two-sample MR was used to unveil the causal relationship between metformin use and 26 common types of cancer. Additionally, we supplemented our analysis with a two-step MR to identify potential mediators and assess their contribution to the genetic causal effect. Ultimately, we conducted a comprehensive review of previous clinical studies to enhance our understanding of the association between metformin use and cancer.
Materials and methods
Study design
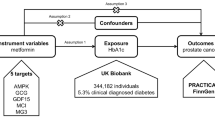
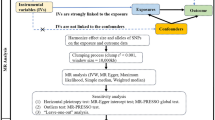
The overview of the study design is demonstrated in Fig. 1. As shown in Fig. 1a, the MR analysis requires three basic assumptions to be met: (1) instrumental variables (IVs) are strongly correlated to exposure; (2) IVs are independent of any potential confounders; and (3) IVs only affect the outcome through exposure. Two distinct genetic datasets should be integrated into a single MR study, which is the fundamental prerequisite of two-sample MR.
Overview of the study design. A We firstly applied two-sample MR analyses to figure out the genetic effect of metformin use on 26 prevalent cancers with five robust methods. B Two-step MR analysis and MVMR were further conducted to figure out the potential mediator who mediate the protective genetic effect of metformin on HER-positive breast cancer
To determine the mediating factors in the genetic causal relationship, two-step MR was performed as illustrated in Fig. 1b. In the first step, single-nucleotide polymorphisms (SNPs) to genetically predict metformin use were incorporated to evaluate the causal relationship of metformin use on 22 potential mediators (e.g. BMI, CRP, and testosterone levels) in the univariable MR method. And SNPs robustly related to mediators were used to calculate the causal association of mediators and cancer outcome(s) [11]. It should be noted that the genetic information utilized in this study is freely accessible to researchers around the world and is therefore not subject to additional ethical review or informed consent.
Selection of instrumental variants (IVs) of metformin use
Genetic variants of metformin use in European ancestry were obtained from the UK Biobank dataset (8392 cases/328,767 controls). The following inclusion criteria guided our selection of the IVs: (1) SNPs should have a genome-wide significance level (P < 5×10–8), which strongly indicates genetic association with exposure. (2) Genetic variants with linkage disequilibrium (LD) (r2 > 0.001) were excluded. The LD between SNPs was assessed to clump the independence of SNPs; (3) The F-statistics (beta2/se2) > 10. SNPs with F-statistics less than 10 may have inferior statistical power. Additional file 1: Table S1 summarizes the IVs of metformin use involved in this work.
Selection of cancer outcomes
The genetic information associated with the following types of malignant tumors was obtained from the FinnGen consortium: colorectal cancer (3022 cases/215,770 controls), stomach cancer (633 cases/218159 controls), pancreas cancer (605 cases/218187 controls), oral pharynx cancer (126 cases/218666 controls), oesophagus cancer (212 cases/218560 controls), bone and articular cartilage cancer (119 cases/218673 controls), kidney cancer (971 cases/217,821 controls), melanoma (98 cases/218694 controls), non-melanoma skin cancer (10,382 cases/208410 controls), thyroid gland cancer (989 cases/217803 controls), overall breast cancer (8401 cases/115178 controls), HER-negative breast cancer (3092 cases/99267 controls), HER-positive breast cancer (4263 cases/99267 controls), lung cancer (1681 cases, 217,111 controls), non-small cell lung cancer (NSCLC) (1627 cases/217165 controls) and small cell lung cancer (SCLC) (179 cases/218613 controls). The genetic information of other cancers was gotten from UKB: colon cancer (2226 cases/358968 controls), rectum cancer (1085 cases/461925 controls), liver cancer (168 cases/372016 controls), small intestine cancer (156 cases/337003 controls), bladder cancer (1554 cases/359640 controls), overall skin cancer (1436 cases/461497 controls). Only the European population was incorporated into this study, and no sample overlap in this MR study.
Statistical analyses
Two-sample Mendelian randomization
Two-sample MR studies were conducted using TwoSampleMR package (version 0.5.6) and R software (version 4.2.1) [12]. A total of five different approaches were used. The inverse variance weighting (IVW) method, which evaluates the causal influence of genetically predicted exposures on outcomes by weighted regression of SNP-specific Wald ratios, acted as the major approach. To examine the consistency and heterogeneity of our findings, four additional assessment techniques—weighted median, MR Egger, simple model, and weighted model—were performed [13,14,15]. When the variable in MR has an impact on illness independent of its impact on exposure, this is known as horizontal pleiotropy. To avoid the biases of horizontal pleiotropy, MR-PRESSO method was performed to identify the outliers with MRPRESSO package (version 1.0) [16]. Pleiotropy was tested by leave-one-out analysis and MR-Egger intercept method [17, 18]. Heterogeneity was evaluated by Cochran's Q-statistic, and any MR results with heterogeneity were excluded.
Median analysis
The genetic information of potential mediators was downloaded from publicly accessible GWAS consortia, and relevant GWAS identifiers or available references were listed in Table 2. Two-step MR analysis was applied to figure out if the potential mediator attributed any mediating effect between exposure and outcome [11]. Of note, the mediator has to meet the premise of a continuous variable [19]. In the first step, genetic variants of exposure (metformin use) were obtained to determine the causal effect of exposure on potential mediators. After that, genetic variants of mediators were also acquired to assess the causal role of mediators on outcomes (cancers) in the second step. Beta 1 and beta 2 were calculated in step one and step two respectively (Fig. 1b). Potential mediator which presented supporting evidence in two-step MR would be included in the median analysis. Multivariable MR (MVMR) analysis was performed on metformin use-TT level-HER(+) breast cancer. The mediation effect was obtained by multiplying beta1 by beta 2.
Comparison with clinical studies
To further confirm our findings, we reviewed the prevention and treatment effects of metformin on breast cancer in previous clinical studies. Phrase II, Phrase III randomized clinical trials (RCT), prospective studies and retrospective studies that published on Pubmed, Medline and Embase were included.
Results
Selected genetic instrumental variants (IVs)
We meticulously followed the aforementioned criteria when selecting the IVs. As a consequence, 26 independent SNPs were selected out of the total amount of 10,894,596 SNPs, acting as the IVs of metformin use. Detailed information could be found in Additional file 1: Table S1. F-statistics, which were also presented in the supplementary document, showed no evidence of weak instrumental bias.
Assessment of the genetic causal effect of metformin on cancers
Two-sample MR results
The brief results of two-sample MR analyses of metformin use on 26 prevalent cancers were listed in Table 1. IVW results presented the genetically predicted protective effect of metformin use on HER-positive breast cancer (Beta: − 4.0982; OR: 0.0166 (95% CI: 0.0008, 0.3376); P value: 0.0077). The scatter plots and funnel plots were illustrated in Additional file 1: Fig. S1. The leave-one-out analysis showed no pleiotropy in the MR result (Additional file 1: Fig. S2). And no significant genetic relationship existed between metformin use and other types of cancers.
Median analysis results
The following 20 probable mediators were investigated to figure out whether MR is shown to be causally related to both the effect of metformin use on them (step one) and the mediators' effects on HER-positive breast cancer (step two): inflammation-related factors (white blood cell counts and C-reactive protein), body shape-related index (BMI, weight, waist circumference, body fat percentage, visceral adipose tissue volume, and abdominal subcutaneous adipose tissue volume), metabolism-related biomarkers (HbA1c, fasting insulin, and fasting glucose) and sex hormone-related biomarkers (SHBG, estradiol, total testosterone levels, and bioavailable testosterone levels). As shown in Table 2, we determined that metformin treatment had a causal influence on HDL cholesterol, LDL cholesterol, SHBG, total testosterone, bioavailable testosterone, estradiol, and fasting glucose levels. MR analyses were further conducted to evaluate the causal effect of the seven mediators above on HER-positive breast cancer (Table 3). Significant causal associations was exhibited in total testosterone levels (Beta: 0.4058, 95% CI: 0.0562 to 0.7556, P value: 0.0229). Hence, total testosterone (TT) levels was selected for mediation effect calculation (Additional file 1: Figs. S3–S10).
In the MVMR of metformin-TT-HER(+) breast cancer, the direct effect of metformin on HER(+) breast cancer was OR 0.0992 (95% CI: 0.0038 to 2.5986, P value: 0.1655) after being adjusted by TT levels, and the direct effect of TT on HER(+) breast cancer was OR 1.5964 (95% CI: 1.1334 to 2.2486, P value: 0.0074) after being adjusted by metformin use (Additional file 1: Table S2). The mediation effect of TT levels was 24.52%.
Review of previous clinical studies
With the help of the three databases mentioned above, we listed the literature reviews of clinical studies concerning metformin use on breast cancer in Table 4, both therapeutic and preventive effect were reviewed here.
Discussion
In addition to its well-established role in reducing persistently high plasma glucose and insulin levels, metformin stands out as a promising candidate for the prevention and treatment of malignant tumors. Recent years have witnessed the promising efficacy of metformin in the management of several types of cancer. However, clinical outcomes have been inconsistent [5, 20, 21]. To optimize the anti-tumor effect of metformin, researchers have focused on the underlying mechanisms for decades. As previously mentioned, numerous pathways and mediating molecules connecting metformin to its effects on cancer have come to light. These include the activation of AMPK-related pathways [22, 23], the promotion of apoptotic cell death in cancer cells [24, 25], and the inhibition of mitochondrial metabolism [26, 27]. These mechanistic studies have shed light on the pivotal role of metformin in cancer therapy and the regulatory pathways involved. Their findings hold significant promise for advancing future clinical management and pharmaceutical development in the field of cancer treatment.
There is a growing focus on investigating the genetic aspects of metformin's role in cancer treatment. In this study, we employed MR analysis to uncover the genetically predicted connections between metformin usage and the risk of common cancers. Unlike traditional clinical studies, MR analysis offers several advantages. It helps eliminate the influence of irrelevant confounding factors and environmental exposures, mitigates the impact of reverse causality, and enhances the strength of evidence for causal inference [28]. As a result, MR analysis stands as a relatively reliable and cost-effective method, leveraging global genome databases to advance our understanding of the relationship between metformin and cancer risk.
Several MR studies have demonstrated the genetic influence of metformin on a variety of diseases. For instance, Zhou et al.'s MR analysis examined the relationship between metformin use and lung cancer risk, finding no genetic causality between the two, a result consistent with our own findings [29]. Modest genetic associations were also reported in the context of breast cancer and prostate cancer [30]. Notably, the MR study on breast cancer encompassed overall, estrogen receptor (ER)-positive, and ER-negative subtypes, yielding results congruent with our research. Beyond cancers, the causal role of metformin on other diseases has been assessed as well. Zhang et al. reported the protective causal relationship between metformin targets and osteoarthritis, highlighting AMPK and GDF-15 as promising targets for osteoarthritis treatment [31]. However, GDF-15 as a therapeutic target of metformin might increase the risk of gallstone disorders [32]. Given metformin's multiple drug targets, which cannot be simplified into one or two specific targets, the accuracy of drug target MR analysis for explaining its therapeutic effects may be limited.
The relationship between sex hormone levels and breast cancer is indeed intricate and has been the focus of extensive research. A comprehensive review study, encompassing 44 breast cancer research studies, revealed that the risk of breast cancer increased with the use of oral contraceptives [33]. Furthermore, this risk was positively correlated with the duration of oral contraceptive use, shedding light on the association between estrogen and progestogen and the prevalence of breast cancer [34]. Testosterone, another sex hormone, also plays a significant role in the development of breast cancer. Evidence from a case–control analysis within the European Prospective Investigation into Cancer and Nutrition cohort established a link between elevated blood testosterone concentrations and an increased incidence of breast cancer (OR: 1.73, 95% CI: 1.16 to 2.57; P value: 0.01) among premenopausal individuals [35]. Similar findings have been reported by researchers from various countries [36,37,38]. Moreover, two-sample MR studies conducted by UK scientists underscored the potential impact of sex steroid hormones on breast cancer risk, These studies pointed out that testosterone and bioavailable testosterone could elevate the risk of both overall and estrogen receptor-positive (ER-positive) breast cancer [39, 40]. These findings align closely with our own research, solidifying the notion of a robust association between testosterone levels and breast cancer risk.
Remarkably, our current study unveiled a novel finding, demonstrating that metformin has the potential to reduce the risk of HER-positive (HER+) breast cancer, and this reduction is partially mediated through its impact on total testosterone levels. The testosterone reduction effect of metformin has been observed in previous reports [41, 42]. Some clinical trials have administrated metformin on non-diabetic breast cancer women, ending with a significant reduction of both insulin and testosterone levels [43, 44]. Furthermore, metformin primarily lowered estradiol levels by diminishing testosterone levels, and these hormonal alterations may hold relevance in certain clinical contexts. This underscores the multifaceted effects of metformin on hormonal regulation and its potential implications in breast cancer prevention and treatment.
Our study boasts several notable strengths. To our knowledge, this is the first study figuring out the genetic effect of metformin use on multiple prevalent cancer risks. And the mediators on the genetic pathway were clarified, and their mediating effects were calculated. Moreover, the genetic information incorporated in this study is giant, which increases the credibility of the conclusion.
While our study presents valuable insights, it's important to acknowledge its limitations. First, to ascertain the consistency of genetic background, this MR analysis only concluded European populations, which could not be extended to other ethnicities. Second, MR analysis of tumors with a small number of instances was less accurate (fewer than 1000). For the validation analysis, more genetic data from large samples need to be added. The association between metformin use and other malignancies cannot be determined at this time; however, this will be clarified in follow-up research.
Conclusion
The current MR study revealed that metformin use could genetically shield individuals from HER-positive breast cancer, which was mediated by total testosterone levels. Further investigation is required to determine whether metformin-induced changes in total testosterone levels could potentially serve as a predictor or biomarker in HER-positive breast cancer development and progression.
Availability of data and materials
All of the genetic data used in this work was publicly available. The relevant data can be found here: Open GWAS summary dataset (https://gwas.mrcieu.ac.uk/); UK Biobank (https://www.ukbiobank.ac.uk/); FinnGen database (https://www.finngen.fi/).
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–49.
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–19.
Lai SW, et al. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13(2):143–8.
Bragagnoli AC, et al. Metformin plus lrinotecan in patients with refractory colorectal cancer: a phase 2 clinical trial. Br J Cancer. 2021;124(6):1072–8.
Barakat HE, et al. The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: an open-labelled randomized controlled trial. Sci Rep. 2022;12(1):7656.
Vancura A, et al. Metformin as an anticancer agent. Trends Pharmacol Sci. 2018;39(10):867–78.
Apostolova N, et al. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte–endothelium interactions. Redox Biol. 2020;34:101517.
Sekula P, et al. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–65.
Tobin MD, et al. Commentary: development of Mendelian randomization: from hypothesis test to ‘Mendelian deconfounding.’ Int J Epidemiol. 2004;33(1):26–9.
Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330(7499):1076–9.
Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41(1):161–76.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Bowden J, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Jones HJ, et al. Associations between plasma fatty acid concentrations and schizophrenia: a two-sample Mendelian randomisation study. Lancet Psychiatry. 2021;8(12):1062–70.
Verbanck M, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–208.
Bowden J, et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–802.
Carter AR, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78.
Yang BY, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG. 2020;127(7):848–57.
Skuli SJ, et al. Metformin and Cancer, an Ambiguanidous Relationship. Pharmaceuticals (Basel). 2022;15(5):1.
Zheng Z, et al. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089–104.
Chen YH, et al. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol Med Rep. 2021;23(1):1.
Haugrud AB, et al. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res Treat. 2014;147(3):539–50.
Klose K, et al. Metformin and sodium dichloroacetate effects on proliferation, apoptosis, and metabolic activity tested alone and in combination in a canine prostate and a bladder cancer cell line. PLoS ONE. 2021;16(9):e0257403.
Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32(3):341–52.
Wheaton WW, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242.
Zoccali C, et al. Mendelian randomization: a new approach to studying epidemiology in ESRD. Am J Kidney Dis. 2006;47(2):332–41.
Zhou H, et al. Mendelian randomization study showed no causality between metformin use and lung cancer risk. Int J Epidemiol. 2020;49(4):1406–7.
Au Yeung SL, Schooling CM. Impact of glycemic traits, type 2 diabetes and metformin use on breast and prostate cancer risk: a Mendelian randomization study. BMJ Open Diabetes Res Care. 2019;7(1):00872.
Zhang Y, et al. Evaluating the impact of metformin targets on the risk of osteoarthritis: a Mendelian randomization study. Osteoarthr Cartil. 2022;30(11):1506–14.
Yu L, et al. GDF-15 as a therapeutic target of diabetic complications increases the risk of gallstone disease: Mendelian randomization and polygenic risk score analysis. Front Genet. 2022;13:814457.
Gierisch JM, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1931–43.
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159–68.
Kaaks R, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2005;97(10):755–65.
Micheli A, et al. Plasma testosterone and prognosis of postmenopausal breast cancer patients. J Clin Oncol. 2007;25(19):2685–90.
Arthur RS, Dannenberg AJ, Rohan TE. The association of prediagnostic circulating levels of cardiometabolic markers, testosterone and sex hormone-binding globulin with risk of breast cancer among normal weight postmenopausal women in the UK Biobank. Int J Cancer. 2021;149(1):42–57.
Watts EL, et al. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. Int J Cancer. 2021;149(3):573–84.
Nounu A, et al. Sex steroid hormones and risk of breast cancer: a two-sample Mendelian randomization study. Breast Cancer Res. 2022;24(1):66.
Tang SN, Zuber V, Tsilidis KK. Identifying and ranking causal biochemical biomarkers for breast cancer: a Mendelian randomisation study. BMC Med. 2022;20(1):457.
Cai T, et al. Effect of metformin on testosterone levels in male patients with type 2 diabetes mellitus treated with insulin. Front Endocrinol (Lausanne). 2021;12:813067.
Andræ F, et al. Sustained maternal hyperandrogenism during PCOS pregnancy reduced by metformin in non-obese women carrying a male fetus. J Clin Endocrinol Metab. 2020;105(12):3762–70.
Campagnoli C, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer. 2012;12(3):175–82.
Campagnoli C, et al. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin Breast Cancer. 2013;13(6):433–8.
Wood AR, et al. Variants in the FTO and CDKAL1 loci have recessive effects on risk of obesity and type 2 diabetes, respectively. Diabetologia. 2016;59(6):1214–21.
Shungin D, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96.
Liu Y, et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife. 2021;2021:10.
Astle WJ, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167(5):1415-1429.e19.
Richardson TG, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. 2020;17(3):e1003062.
Willer CJ, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83.
Ruth KS, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–8.
Chen J, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021;53(6):840–60.
Manning AK, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–69.
Soranzo N, et al. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–39.
Goodwin PJ, et al. Effect of metformin vs. placebo on invasive disease-free survival in patients with breast cancer: The MA.32 randomized clinical trial. JAMA. 2022;327(20):1963–73.
Huang J, et al. Neoadjuvant docetaxel, epirubicin, and cyclophosphamide with or without metformin in breast cancer patients with metabolic abnormality: results from the randomized Phase II NeoMET trial. Breast Cancer Res Treat. 2023;197(3):525–33.
Pimentel I, et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast. 2019;48:17–23.
Nanni O, et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res Treat. 2019;174(2):433–42.
Yam C, et al. Efficacy and safety of the combination of metformin, everolimus and exemestane in overweight and obese postmenopausal patients with metastatic, hormone receptor-positive, HER2-negative breast cancer: a phase II study. Invest New Drugs. 2019;37(2):345–51.
Essa NM, et al. Efficacy of metformin as adjuvant therapy in metastatic breast cancer treatment. J Clin Med. 2022;11(19):1.
Sonnenblick A, et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: analysis from the ALTTO phase III randomized trial. J Clin Oncol. 2017;35(13):1421–9.
Feng JL, Qin X. Metformin and cancer-specific survival among breast, colorectal, or endometrial cancer patients: a nationwide data linkage study. Diabetes Res Clin Pract. 2021;175:108755.
Kim BH, Cho MJ, Kwon J. Potential intrinsic subtype dependence on the association between metformin use and survival in surgically resected breast cancer: a Korean national population-based study. Int J Clin Oncol. 2021;26(11):2004–16.
Hui T, et al. Metformin improves the outcomes in Chinese invasive breast cancer patients with type 2 diabetes mellitus. Sci Rep. 2021;11(1):10034.
Hosio M, et al. Survival after breast cancer in women with type 2 diabetes using antidiabetic medication and statins: a retrospective cohort study. Acta Oncol. 2020;59(9):1110–7.
El-Benhawy SA, El-Sheredy HG. Metformin and survival in diabetic patients with breast cancer. J Egypt Public Health Assoc. 2014;89(3):148–53.
Kim HJ, et al. Metformin increases survival in hormone receptor-positive, HER2-positive breast cancer patients with diabetes. Breast Cancer Res. 2015;17(1):64.
Park YM, et al. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann Oncol. 2021;32(3):351–9.
Chlebowski RT, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30(23):2844–52.
Acknowledgements
We are grateful to the public open accessible databases mentioned above.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82100542 to B. Xie), Zhejiang Provincial Natural Science Foundation of China (No. LQ21H160027 to B. Xie), the China Postdoctoral Science Foundation (No. 2020M681893 to B. Xie).
Author information
Authors and Affiliations
Contributions
YC, SY and XZ: conceptualization and writing of the manuscript. BB, XG and KY: making and correcting the tables and figures. HP and BX: reviewing, editing, and providing critical discussion.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All participating studies involved in the GWAS obtained informed consent from the study populations. As we utilized publicly available datasets to conduct MR, no additional ethics approval was required. A certification of ethics approval waiver was consented to by the ethics committee of Zhejiang University Affiliated Sir Run Run Shaw Hospital.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1
: SNPs associated with metformin use, which performed as instrumental variants (IVs) in two-sample MR analysis. Table S2: The genetic effect obtained from MVMR analysis. TT: total testosterone levels. Fig. S1: Scatter plots and funnel plots of metformin use on HER-positive breast cancer. A Scatter plots of the genetic association between metformin use and HER-positive breast cancer. The genetic predicted metformin use is associated with a lower risk of HER-positive breast cancer. The slope of each line shows the estimated causal effect of metformin use on HER-positive breast cancer for each approach. B Funnel plots showing the statistical association between metformin use and the risk of HER-positive breast cancer. Fig. S2: Leave-one-out analysis and Forest plots results. A Leave-one-out analysis of sensitivity test. After one by one eliminating the IVs, calculate the MR outcomes for the remaining IVs. B Forest plot of the causal effects of metformin use associated SNPs on HER-positive breast cancer. B Showed the Mendelian randomization estimated effects sizes for metformin use on HER-positive breast cancer. Fig. S3: Leave-one-out analysis result of metformin use on total testosterone levels. Fig. S4: Leave-one-out analysis result of total testosterone levels on HER-positive breast cancer. Fig. S5: Scatter plot of metformin use on total testosterone levels. Fig. S6: Scatter plot of total testosterone levels on HER-positive breast cancer. Fig. S7: Forest plot of metformin use on total testosterone levels. Fig. S8: Forest plot of total testosterone levels on HER-positive breast cancer. Fig. S9: Funnel plot of metformin use on total testosterone levels. Fig. S10: Funnel plot of total testosterone levels on HER-positive breast cancer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Bai, B., Ye, S. et al. Genetic effect of metformin use on risk of cancers: evidence from Mendelian randomization analysis. Diabetol Metab Syndr 15, 252 (2023). https://doi.org/10.1186/s13098-023-01218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01218-3