Abstract
Aims
The frailty index (FI) is one way in which frailty can be quantified. While it is measured as a continuous variable, various cut-off points have been used to categorise older adults as frail or non-frail, and these have largely been validated in the acute care or community settings for older adults without cancer. This review aimed to explore which FI categories have been applied to older adults with cancer and to determine why these categories were selected by study authors.
Methods
This scoping review searched Medline, EMBASE, Cochrane, CINAHL, and Web of Science databases for studies which measured and categorised an FI in adults with cancer. Of the 1994 screened, 41 were eligible for inclusion. Data including oncological setting, FI categories, and the references or rationale for categorisation were extracted and analysed.
Results
The FI score used to categorise participants as frail ranged from 0.06 to 0.35, with 0.35 being the most frequently used, followed by 0.25 and 0.20. The rationale for FI categories was provided in most studies but was not always relevant. Three of the included studies using an FI > 0.35 to define frailty were frequently referenced as the rationale for subsequent studies, however, the original rationale for this categorisation was unclear. Few studies sought to determine or validate optimum FI categorises in this population.
Conclusion
There is significant variability in how studies have categorised the FI in older adults with cancer. An FI ≥ 0.35 to categorise frailty was used most frequently, however an FI in this range has often represented at least moderate to severe frailty in other highly-cited studies. These findings contrast with a scoping review of highly-cited studies categorising FI in older adults without cancer, where an FI ≥ 0.25 was most common. Maintaining the FI as a continuous variable is likely to be beneficial until further validation studies determine optimum FI categories in this population. Differences in how the FI has been categorised, and indeed how older adults have been labelled as ‘frail’, limits our ability to synthesise results and to understand the impact of frailty in cancer care.
Similar content being viewed by others
Introduction
Frailty is a dynamic state of diminished physiological reserve and increased vulnerability to adverse events. It has been recognised as a prevalent and important consideration in the individualised management of older adults with cancer [1, 2]. Routine screening for geriatric conditions has been recommended for all adults over 65 or 70 years of age with a new cancer diagnosis [2,3,4,5,6]. In contrast to older adults in the community-dwelling or acute care settings, those living with cancer face the additional acute stressors of cancer symptoms and potential treatment-related toxicities. Frailty has significant implications for not only understanding the underlying health status of a potentially-vulnerable individual with cancer, but also in influencing oncological treatment decisions and discourse, and tailoring non-oncological interventions or supports. Clinicians try to determine those who are too frail for treatment, those who require modified treatment or additional supports, and those who are deemed fit for standard therapy. However, there is currently no consensus regarding the optimum frailty screening or measurement tool in this population.
The frailty index (FI) is one way in which frailty can be quantified [7]. The FI conceptualises frailty as a multi-dimensional risk state which can be measured by the number, rather than the nature, of health problems. An FI is calculated as a proportion of deficits using a well-defined method [8] e.g., someone with 6 deficits out of 40 counted has an FI of 0.15. As a continuous variable, ranging from zero (most robust) to a theoretical maximum of one (most frail), the FI affords great precision in risk stratification by capturing frailty gradations. In a scoping review of FI in the community and acute care settings, an FI ≥ 0.25 was the most frequently used score to diagnose people as frail, however this was used in less than half of the identified studies [7]. This score was derived from work by Rockwood and colleagues, which demonstrated that FI = 0.25 had construct and predictive validity to categorise community-dwelling adults as frail or non-frail [9, 10]. It correlated with the crossing point between robust and frail groups according to Fried et al.’s phenotype model of frailty [10], another well validated yet conceptually distinct definition of frailty in older persons [11, 12], and was predictive of institutionalisation and death. It also presented the crossing point between Clinical Frailty Scale (CFS) ‘apparently vulnerable’ (mean FI = 0.22) and ‘mildly frail’ (mean FI = 0.27) [10].
However, little is known regarding the validity of FI categories in the context of cancer, and variation in who is deemed frail may be used to determine trial eligibility or treatment allocation [13], and referral for additional assessments or supports [14, 15]. It is therefore important to understand how the FI has been categorised in oncology literature, and to understand the rationale for these decisions [16].
The objectives of this scoping review were: (i) to evaluate which FI categories (FI scores and labels) have been used in an oncology setting; and (ii) to identify why these categories were selected by the study authors.
Methods
Protocol and registration
The protocol for this scoping review protocol was prospectively registered with Open Science Framework (registration ID osf.io/gchq8) and developed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews criteria [17].
Eligibility criteria
Articles were considered eligible for inclusion if they utilised a frailty index (FI) that met the criteria described by Searle and colleagues [18], and if the FI was categorised. There was no limitation to study design or year of publication, however only studies conducted in human adults with solid organ malignancies were included. Articles were excluded if they were not an original study or were only available as an abstract or protocol.
Search strategy
A search of Medline, EMBASE, and Cochrane databases was conducted on 26 November 2021. Updated searches were performed to include CINAHL and Web of Science databases, as well as additional studies published before 22 July 2022 in all databases. Search results were imported into Covidence for screening, full text review, and data extraction. The full search strategy is available in the Supplementary appendix.
Study selection
Two reviewers (JF and BL) independently performed the screening and full text reviews. Disagreements were resolved by consensus with a third reviewer (NR).
Data extraction and analysis
Two reviewers (JF and BL) independently performed data extraction and disagreements were resolved by consensus with a third reviewer (NR). Extracted data included country, year of publication, study design, sample size, baseline demographics, and cancer-related details. FI data extracted included name, mean, categorised scores and labels, and justification for categorisation.
Results
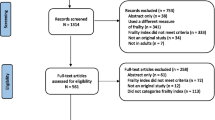
The primary search yielded 1994 articles (Fig. 1). After removal of duplicates, abstract and full text screening, 41 studies were ultimately included.
Study characteristics
All 41 studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] were published between 2014 and 2022, with the majority (n = 29, 71%) conducted in North America (Table 1). Thirty-six were of an observational study design, two studies reported secondary analyses of data from the same cluster randomised controlled trial, and three studies were non-randomised experimental trials. The median sample size was 541 (interquartile range [IQR] = 175–1136). The mean FI ranged from 0.05 to 0.31.
Fifteen studies were specifically conducted in the medical oncology setting [21, 22, 25, 26, 28,29,30,31, 34,35,36, 39, 44, 52, 55], ten studies in surgical oncology [42, 43, 46, 50, 53, 54, 56,57,58,59], and the remainder were either mixed or not specified [19, 20, 23, 24, 27, 32, 33, 37, 38, 40, 41, 45, 47,48,49, 51]. Fourteen studies included a range of cancers and the rest focused on individual cancer types [22,23,24,25,26,27, 32, 33, 37, 40, 41, 45, 47, 48, 51]. There were 11 studies of breast cancer [19,20,21, 28,29,30,31, 34, 38, 57, 59], of which six studies were different secondary analyses of the same prospective clinical trial [21, 28,29,30,31, 34]. Similarly, two [35, 52] of the five lung cancer studies utilised the same retrospective database [35, 36, 49, 50, 52], as did three [54,55,56] of five studies of gynaecological cancers [46, 54,55,56, 58]. Four studies evaluated gastrointestinal cancers [25, 39, 42, 43], one studied pancreatic cancer [32], and one specifically evaluated participants with meningiomas [53].
Frailty index categories
In 22 studies (54%) an FI ≥ 0.35 was used to categorise people as frail (Fig. 2) and an FI between 0.20 and 0.35 categorised people as prefrail [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Fourteen of the 22 studies referenced one of three oncology studies as rationale for their FI categorisation [22, 27, 34]. Sheppard et al., [34] published in 2014, referenced work which did not use similar FI categorisation. Sheppard et al. reported that prefrailty/frailty (FI ≥ 0.20) predicted treatment non-initiation in women with breast cancer. Cohen et al., [22] published 4 years later, referenced Sheppard et al. and reported that frailty predicted an increased likelihood of hospitalisation and treatment discontinuation in older adults commencing chemotherapy. Further, they determined the optimal FI cut-off for their individual outcomes was comparable to FI = 0.20. Guerard et al., [27] published in 2017, did not provide a rationale for their categorisation, however reported that frailty (defined as FI > 0.35) predicted an increased likelihood of all-cause and cancer-specific mortality in a heterogenous population of older adults with cancer, thereby establishing the predictive validity of their frailty categorisation.
Distribution of frailty index cut-off points used to define frailty. The frailty index is a continuous measure between zero (least frail) and one (most frail). Here, the number of studies using different cut-off points to categorise adults with cancer as frail are highlighted. Significant variation in these frailty index cut-off points is evident
An FI ≥ 0.25 was the next most commonly used cut-off to categorise frailty in six (15%) studies [41,42,43,44,45,46]. Four of these studies either directly, or indirectly, referenced validation studies by Rockwood and colleagues [42, 44,45,46]. One of these reported predictive validity against comprehensive geriatric assessment [42], and another validated their FI with respect to Vulnerable Elderly Survey (VES-13) scores and treatment completion outcomes [44]. Six studies defined frailty as an FI ≥ 0.20 [47,48,49,50,51,52], one used FI > 0.21 [47], and one additional study arbitrarily defined an FI > 0.30 as ‘unfit’, commenting that using an FI > 0.20 would have categorised 99% of their population as ‘unfit’ and limited their statistical analysis [40]. Four of these studies defined categories of increasing frailty, most often with 0.1 increments in FI [48,49,50,51]. References for these studies varied, including work by Rockwood and colleagues [76, 80].
Two groups defined the optimum FI cut-off point for predicting adverse outcomes in women with ovarian cancer [54,55,56, 59]. The first [54,55,56] reported that an FI = 0.15 was the cut-off that yielded the highest Youden's index for their three binary outcomes (Accordion Grade 3 or 4 complication, 90-day mortality, receipt of chemotherapy within 42 days), while yielding the highest separation in outcome rates between frail and non-frail. Using similar methodology, another group [59] reported that FI > 0.26 showed the best discriminative ability for severe post-operative complications (≥ IIIb by Clavien-Dindo criteria) in a younger cohort of women with predominantly advanced ovarian cancer. An FI > 0.15 was determined to be the best cut-off for overall survival in this population.
The final study arbitrarily defined frailty as FI ≥ 0.06, and further divided this into mild frailty (FI = 0.06 – 0.11), moderate frailty (FI = 0.12 – 0.18) and severe frailty (FI ≥ 0.19) [57]. Internal validity was tested in a large cohort of women with breast cancer, and external validity in a second large representative cohort [57].
Discussion
This scoping review demonstrated significant variability in FI categorisation in older adults with cancer. An FI ≥ 0.35 was the most frequently used cut-off point to categorise frailty, followed by an FI ≥ 0.25. While most authors provided a rationale for their categorisation, many of the cited studies were not relevant, and the most frequently referenced oncological studies of FI in this context did not clearly justify or validate their cut-off points. Across treatment contexts, few studies sought to demonstrate construct validity or to establish optimal FI categories [22, 42, 51, 54, 57, 59].
This is the first review to evaluate FI categorisation in older adults with cancer. Significant variability in FI categorisation has also been reported in a recent review of the most highly-cited studies in older adults in the community, acute care, and residential care settings [7]. In contrast with this prior review, where an FI ≥ 0.25 was common, a significantly higher FI (≥ 0.35) was most frequently used to define frailty in oncology studies.
While the three most frequently cited oncology reference studies demonstrated associations between pre-frailty/frailty and adverse outcomes, the rationale for arriving at this FI categorisation was not clear [22, 27, 34]. Further, the nomenclature of ‘pre-frailty’ in this context would be more congruent with the measurement of frailty using the Fried Frailty Phenotype, which conceptualises frailty as a syndrome with three categories of fit, pre-frail, and frail. This is in contrast to the deficit accumulation model which considers frailty along a continuum, in which gradations of severity can be appreciated [18, 83]. It is also interesting to note that of these three studies, one collapsed ‘prefrailty’ (FI ≥ 0.20) and ‘frailty’ (FI ≥ 0.35) into a single category for their statistical analysis [34], and another identified an optimum FI cut-off point approximating their level of prefrailty (FI ≥ 0.20) [22]. FI cut-off points between 0.21 and 0.25 were used to define frailty, rather than ‘prefrailty’, in a further seven studies.
The predictive validity of these cut-off points (FI > 0.21, > 0.25, > 0.35) have been tested in community-dwelling adults by Hoover et al. [84], who reported four frailty categories for hospital-related outcomes (non-frail FI < 0.1, pre-frail 0.1 < FI ≤ 0.21, frail FI > 0.21, and most frail FI ≥ 0.45). These cut-offs also correspond well with the mean FI for increasing levels of the Clinical Frailty Scale (CFS): very fit (CFS 1, mean FI = 0.09), apparently vulnerable (CFS 4, mean FI = 0.22), and severely frail (CFS 7, mean FI = 0.43) [71].
Variability in the categorisation of frailty contributes to inconsistency in understanding the true impact of frailty on outcomes in older adults with cancer [2, 7]. These disparities should be taken into account when interpreting the data, as one patient may be categorised as robust in one study, and frail in another. Much like chemotherapy toxicity calculators [85], the FI is intended to inform intrinsic vulnerability and risk, rather than to discriminate between treatment options. Maintaining the FI as a continuous variable can be advantageous to mitigate this and to understand the associations between frailty and outcomes, however the importance of validated categorisations must also be acknowledged. Given the previously discussed findings, it could be suggested that an FI greater than 0.20 or 0.25 may be most appropriate to identify those who are at increased risk of adverse events and to categorise this group as frail. It is likely that studies defining frailty with an FI ≥ 0.35 captured a significantly more frail, and therefore more vulnerable population than studies using other validated cut-offs [71]. Graded frailty severity (e.g., mild, moderate, severe), reported in only five of the included oncology studies, may be a more useful method to assist researchers and clinicians. This would parallel chemotherapy toxicity calculators [85, 86], however more research is required to determine optimum FI cut-offs to discriminate between outcomes, and this may vary across tumour streams [42, 54].
Despite a number of studies including some patients with lymphomas, a limitation to this review was the exclusion of studies evaluating solely haematological malignancies. While the aim of this review was to determine FI categories and their rationale, the lack of validated FI categories in the present review means that these findings cannot likely be extrapolated to other haematological populations.
Conclusion
This scoping review demonstrated variability in how oncological studies categorise frailty in older adults with cancer. While some studies sought to determine optimal cut-off points to define frailty in specific populations, FI categories were otherwise not well validated in the general oncology setting. Further work is therefore required to validate frailty categories in this context, and at the present time, the FI may be best reported as a continuous variable to understand an older adult’s level of frailty.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67(5):362–77. https://doi.org/10.3322/caac.21406.
Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–101. https://doi.org/10.1093/annonc/mdu540.
Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326–47. https://doi.org/10.1200/jco.2018.78.8687.
Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–603. https://doi.org/10.1200/jco.2013.54.8347.
Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015;26(2):288–300. https://doi.org/10.1093/annonc/mdu210.
Garcia MV, Agar MR, Soo WK, To T, Phillips JL. Screening Tools for Identifying Older Adults With Cancer Who May Benefit From a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021;7(4):616–27. https://doi.org/10.1001/jamaoncol.2020.6736.
Gordon EH, Reid N, Khetani IS, Hubbard RE. How frail is frail? A systematic scoping review and synthesis of high impact studies. BMC Geriatrics. 2021;21(1)https://doi.org/10.1186/s12877-021-02671-3.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. https://doi.org/10.1186/1471-2318-8-24.
Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. Cancer J Clin. 2017;67(5):362–377. https://doi.org/10.3322/caac.21406.
Rockwood K, Andrew M, Mitnitski A. A Comparison of Two Approaches to Measuring Frailty in Elderly People. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. https://doi.org/10.1093/gerona/62.7.738.
Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57. https://doi.org/10.1093/gerona/56.3.m146.
Cesari M, Gambassi G, Abellan Van Kan G, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. https://doi.org/10.1093/ageing/aft160.
Corre R, Greillier L, Caër HL, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non–Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. 2016;34(13):1476–83. https://doi.org/10.1200/jco.2015.63.5839.
Mohile SG, Mohamed MR, Culakova E, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J Clin Oncol. 2020;38(15_suppl):12009–12009. https://doi.org/10.1200/JCO.2020.38.15_suppl.12009.
Ørum M, Eriksen SV, Gregersen M, et al. The impact of a tailored follow-up intervention on comprehensive geriatric assessment in older patients with cancer - a randomised controlled trial. J Geriatr Oncol. 2021;12(1):41–8. https://doi.org/10.1016/j.jgo.2020.07.011.
Mudge AM, Hubbard RE. Frailty: mind the gap. Age Ageing. 2018;47(4):508–11. https://doi.org/10.1093/ageing/afx193.
PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Internal Med. 2018;169(7):467–473. https://doi.org/10.7326/m18-0850 %m30178033.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. https://doi.org/10.1186/1471-2318-8-24.
Ahles TA, Schofield E, Li Y, et al. Relationship between cognitive functioning and frailty in older breast cancer survivors. J Geriatr Oncol. 2021. https://doi.org/10.1016/j.jgo.2021.07.011.
Ahles TA, Schofield E, Li Y, et al. Relationship between cognitive functioning and frailty in older breast cancer survivors. J Geriatr Oncol. 2022;13(1):27–32. https://doi.org/10.1016/j.jgo.2021.07.011.
Bluethmann SM, Alfano CM, Clapp JD, et al. Cognitive function and discontinuation of adjuvant hormonal therapy in older breast cancer survivors: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;165(3):677–86. https://doi.org/10.1007/s10549-017-4353-y.
Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–72. https://doi.org/10.1002/cncr.30269.
Gilmore N, Kehoe L, Bauer J, et al. The Relationship Between Frailty and Emotional Health in Older Patients with Advanced Cancer. Oncologist. 2021;26(12):e2181–91. https://doi.org/10.1002/onco.13975.
Gilmore N, Xu H, Kehoe L, et al. Evaluating the association of frailty with communication about aging-related concerns between older patients with advanced cancer and their oncologists. Cancer. 2022;128(5):1101–9. https://doi.org/10.1002/cncr.34010.
Giri S, Al-Obaidi M, Weaver A, et al. Association between chronologic age and geriatric assessment-identified impairments: Findings from the care registry. J Natl Comprehensive Cancer Network. 2021;19(8):922–927. https://doi.org/10.6004/jnccn.2020.7679.
Giri S, Mir N, Al-Obaidi M, et al. Use of Single-Item Self-Rated Health Measure to Identify Frailty and Geriatric Assessment-Identified Impairments Among Older Adults with Cancer. Oncologist. 2022;27(1):e45–52. https://doi.org/10.1093/oncolo/oyab020.
Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Comprehensive Cancer Network. 2017;15(7):894–902. https://doi.org/10.6004/jnccn.2017.0122.
Mandelblatt JS, Clapp JD, Luta G, et al. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer. 2016;122(22):3555–63. https://doi.org/10.1002/cncr.30208.
Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treatment. 2017;164(1):107–17. https://doi.org/10.1007/s10549-017-4222-8.
Mandelblatt JS, Small BJ, Luta G, et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol. 2018;36(32):3211–22. https://doi.org/10.1200/jco.18.00140.
Mandelblatt JS, Zhou X, Small BJ, et al. Deficit Accumulation Frailty Trajectories of Older Breast Cancer Survivors and Non-Cancer Controls: The Thinking and Living with Cancer Study***MD, MPH. J Natl Cancer Institute. 2021;113(8):1053–1064. https://doi.org/10.1093/jnci/djab003.
Negrete-Najar JP, Sehovic M, Rodriquenz MG, Garcia-Martinez J, Extermann M. Development of a health data derived frailty index as a predictor of adverse outcomes in older patients with pancreatic cancer. J Geriatric Oncol. 2022;13(3):308–14. https://doi.org/10.1016/j.jgo.2021.10.009.
Nishijima TF, Deal AM, Williams GR, Guerard EJ, Nyrop KA, Muss HB. Frailty and inflammatory markers in older adults with cancer. Aging. 2017;9(3):650–64. https://doi.org/10.18632/aging.101162.
Sheppard VB, Faul LA, Luta G, et al. Frailty and Adherence to Adjuvant Hormonal Therapy in Older Women With Breast Cancer: CALGB Protocol 369901. J Clin Oncol. 2014;32(22):2318–27. https://doi.org/10.1200/jco.2013.51.7367.
Wang Y, Zhang R, Shen Y, Su L, Dong B, Hao Q. Prediction of chemotherapy adverse reactions and mortality in older patients with primary lung cancer through frailty index based on routine laboratory data. Clin Interv Aging. 2019;14:1187–97. https://doi.org/10.2147/CIA.S201873.
Weiss JM, Pennell N, Deal AM, et al. Nab-paclitaxel in older patients with non–small cell lung cancer who have developed disease progression after platinum-based doublet chemotherapy. Cancer. 2020;126(5):1060–7. https://doi.org/10.1002/cncr.32573.
Williams GR, Deal AM, Muss HB, et al. Frailty and skeletal muscle in older adults with cancer. J Geriatr Oncol. 2018;9(1):68–73. https://doi.org/10.1016/j.jgo.2017.08.002.
Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer. 2019;27(7):2693–8. https://doi.org/10.1007/s00520-018-4558-6.
Williams GR, Al-Obaidi M, Harmon C, et al. Racial disparities in frailty and geriatric assessment impairments in older adults with cancer in the Deep South: Results from the CARE Registry. Cancer. 2022;128(12):2313–9. https://doi.org/10.1002/cncr.34178.
Martinez-Tapia C, Laurent M, Paillaud E, et al. Predicting Frailty and Geriatric Interventions in Older Cancer Patients: Performance of Two Screening Tools for Seven Frailty Definitions—ELCAPA Cohort. Cancers. 2022;14(1). https://doi.org/10.3390/cancers14010244.
Geessink N, Schoon Y, Van Goor H, Olde Rikkert M, Melis R. Frailty and quality of life among older people with and without a cancer diagnosis: Findings from TOPICS-MDS. PLOS ONE. 2017;12(12):e0189648. https://doi.org/10.1371/journal.pone.0189648.
Giannotti C, Sambuceti S, Signori A, et al. Frailty assessment in elective gastrointestinal oncogeriatric surgery: Predictors of one-year mortality and functional status. J Geriatr Oncol. 2019;10(5):716–23. https://doi.org/10.1016/j.jgo.2019.04.017.
Giannotti C, Massobrio A, Carmisciano L, et al. Effect of Geriatric Comanagement in Older Patients Undergoing Surgery for Gastrointestinal Cancer: A Retrospective, Before-and-After Study. J Am Med Directors Assoc. 2022; https://doi.org/10.1016/j.jamda.2022.03.020.
McCarthy AL, Peel NM, Gillespie KM, et al. Validation of a frailty index in older cancer patients with solid tumours. BMC Cancer. 2018;18(1):892. https://doi.org/10.1186/s12885-018-4807-6.
Pérez-Zepeda MU, Cárdenas-Cárdenas E, Cesari M, Navarrete-Reyes AP, Gutiérrez-Robledo LM. Cancer and frailty in older adults: a nested case-control study of the Mexican Health and Aging Study. J Cancer Surviv. 2016;10(4):736–42. https://doi.org/10.1007/s11764-016-0519-6.
Reiser E, Pötsch N, Seebacher V, et al. Impact of frailty on the management of patients with gynecological cancer aged 80 years and older. Arch Gynecol Obstetr. 2021;303(2):557–63. https://doi.org/10.1007/s00404-020-05807-9.
Zhang D, Mobley EM, Manini TM, et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: Evidence from the National Health and Nutrition Examination Survey, 1999–2014. Cancer. 2022;128(15):2978–87. https://doi.org/10.1002/cncr.34258.
Bensken WP, Schiltz NK, Warner DF, et al. Comparing the association between multiple chronic conditions, multimorbidity, frailty, and survival among older cancer patients. J Geriatr Oncol. 2022; https://doi.org/10.1016/j.jgo.2022.06.011.
Cheng D, Dumontier C, Sheikh AR, et al. Prognostic value of the veterans affairs frailty index in older patients with non-small cell lung cancer. Cancer Med. 2022. https://doi.org/10.1002/cam4.4658.
Cooper L, Gong Y, Dezube AR, et al. Thoracic surgery with geriatric assessment and collaboration can prepare frail older adults for lung cancer surgery. J Surg Oncol. 2022;126(2):372–82. https://doi.org/10.1002/jso.26866.
Hembree T, Theou O, Thirlwell S, et al. A simple test-based frailty index to predict survival among cancer patients with an unplanned hospitalization: An observational cohort study. Cancer Med. 2021;10(17):5765–74. https://doi.org/10.1002/cam4.4107.
Shen Y, Wang Y, Shi Q, et al. The electronic frailty index is associated with increased infection and all-cause mortality among older patients with primary lung cancer: A cohort study. Clin Interv Aging. 2021;16:1825–33. https://doi.org/10.2147/CIA.S335172.
Tariciotti L, Fiore G, Carapella S, et al. A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas. Cancers (Basel). 2022;14(13). https://doi.org/10.3390/cancers14133065.
Kumar A, Langstraat CL, DeJong SR, et al. Functional not chronologic age: Frailty index predicts outcomes in advanced ovarian cancer. Gynecol Oncol. 2017;147(1):104–9. https://doi.org/10.1016/j.ygyno.2017.07.126.
Narasimhulu DM, McGree ME, Weaver AL, et al. Frailty is a determinant of suboptimal chemotherapy in women with advanced ovarian cancer. Gynecol Oncol. 2020;158(3):646–52. https://doi.org/10.1016/j.ygyno.2020.05.046.
Yao T, DeJong SR, McGree ME, Weaver AL, Cliby WA, Kumar A. Frailty in ovarian cancer identified the need for increased postoperative care requirements following cytoreductive surgery. Gynecologic Oncol. 2019;153(1):68–73. https://doi.org/10.1016/j.ygyno.2018.12.025.
Jauhari Y, Gannon MR, Dodwell D, et al. Construction of the secondary care administrative records frailty (SCARF) index and validation on older women with operable invasive breast cancer in England and Wales: A cohort study. BMJ Open. 2020;10(5). https://doi.org/10.1136/bmjopen-2019-035395.
Zhou J, Cueto J, Ko NY, et al. Population-based recurrence rates among older women with HR-positive, HER2-negative early breast cancer: Clinical risk factors, frailty status, and differences by race. Breast. 2021;59:367–75. https://doi.org/10.1016/j.breast.2021.08.005.
Inci MG, Anders L, Woopen H, et al. Frailty Index for prediction of surgical outcome in ovarian cancer: Results of a prospective study. Gynecol Oncol. 2021;161(2):396–401. https://doi.org/10.1016/j.ygyno.2021.02.012.
Guerard EJ, Deal AM, Williams GR, Jolly TA, Wood WA, Muss HB. Construction of a frailty index for older adults with cancer using a geriatric assessment. Conference Abstract. J Clin Oncol. 2015;33(15).
Song X, Mitnitski A, Rockwood K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J Am Geriatr Soc. 2010;58(4):681–7. https://doi.org/10.1111/j.1532-5415.2010.02764.x.
Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of Frailty Using Eight Commonly Used Scales and Comparison of Their Ability to Predict All-Cause Mortality. J Am Geriatr Soc. 2013;61(9):1537–51. https://doi.org/10.1111/jgs.12420.
Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Medicine. 2018;16(1). https://doi.org/10.1186/s12916-018-1223-3.
Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-Term Risks of Death and Institutionalization of Elderly People in Relation to Deficit Accumulation at Age 70. J Am Geriatr Soc. 2006;54(6):975–9. https://doi.org/10.1111/j.1532-5415.2006.00738.x.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. https://doi.org/10.1016/s0140-6736(12)62167-9.
Peña FG, Theou O, Wallace L, et al. Comparison of alternate scoring of variables on the performance of the frailty index. BMC Geriatr. 2014;14(1):25. https://doi.org/10.1186/1471-2318-14-25.
Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K. Identifying Common Characteristics of Frailty Across Seven Scales. J Am Geriatr Soc. 2014;62(5):901–6. https://doi.org/10.1111/jgs.12773.
Negrete-Najar JP, Sehovic M, Rodriquenz MG, Garcia-Martinez J, Extermann M. Development of a health data derived frailty index as a predictor of adverse outcomes in older patients with pancreatic cancer. J Geriatr Oncol. 2021. https://doi.org/10.1016/j.jgo.2021.10.009.
Qian CL, Knight HP, Ferrone CR, et al. Randomized trial of a perioperative geriatric intervention for older adults with cancer. J Clin Oncol. 2020;38(15_suppl):12012–12012. https://doi.org/10.1200/JCO.2020.38.15_suppl.12012.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. https://doi.org/10.1093/gerona/62.7.722.
Rockwood K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95. https://doi.org/10.1503/cmaj.050051.
Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–470. https://doi.org/10.1016/j.archger.2015.01.016.
Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate–vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80(2):187–191. https://doi.org/10.1016/j.maturitas.2014.11.010.
Chen C, Winterstein AG, Fillingim RB, Wei Y-J. Body weight, frailty, and chronic pain in older adults: a cross-sectional study. BMC Geriatrics. 2019;19(1). https://doi.org/10.1186/s12877-019-1149-4.
Miller AJ, Theou O, McMillan M, Howlett SE, Tennankore KK, Rockwood K. Dysnatremia in Relation to Frailty and Age in Community-dwelling Adults in the National Health and Nutrition Examination Survey. J Gerontol Series A: Biol Sci Med Sci. 2016:glw114. https://doi.org/10.1093/gerona/glw114.
Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol Ser A. 2018;73(7):980–7. https://doi.org/10.1093/gerona/glx229.
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;183(8):E487–94. https://doi.org/10.1503/cmaj.101271.
Franco I, Chen Y-H, Chipidza F, et al. Use of frailty to predict survival in elderly patients with early stage non-small-cell lung cancer treated with stereotactic body radiation therapy. J Geriatr Oncol. 2018;9(2):130–7. https://doi.org/10.1016/j.jgo.2017.09.002.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of Deficits as a Proxy Measure of Aging. Sci World J. 2001;1:323–36. https://doi.org/10.1100/tsw.2001.58.
Jayanama K, Theou O, Blodgett JM, Cahill L, Rockwood K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Medicine. 2018;16(1). https://doi.org/10.1186/s12916-018-1176-6.
Armstrong JJ, Andrew MK, Mitnitski A, Launer LJ, White LR, Rockwood K. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing. 2015;44(4):709–12. https://doi.org/10.1093/ageing/afv016.
Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71(5):649–55. https://doi.org/10.1093/gerona/glv228.
Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative Deficits Better Characterize Susceptibility to Death in Elderly People than Phenotypic Frailty: Lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. https://doi.org/10.1111/j.1532-5415.2008.01656.x.
Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24(9):10–7.
Hurria A, Togawa K, Mohile SG, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. J Clin Oncol. 2011;29(25):3457–65. https://doi.org/10.1200/jco.2011.34.7625.
Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–86. https://doi.org/10.1002/cncr.26646.
Acknowledgements
Not applicable.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
JF was responsible for conceptualization, methodology, primary review, analysis, and writing of the original manuscript. BL was second reviewer and involved in analysis and editing/reviewing the manuscript. NR was third reviewer, and involved in conceptualisation, methodology, and manuscript review and editing. EG assisted with methodology, analysis, and reviewing the manuscript. RL was involved with review conceptualization, methodology, analysis, and reviewing and editing of the manuscript. REH was senior author, involved from review conceptualization to final manuscript. All authors reviewed and approved the final manuscript submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fletcher, J.A., Logan, B., Reid, N. et al. How frail is frail in oncology studies? A scoping review. BMC Cancer 23, 498 (2023). https://doi.org/10.1186/s12885-023-10933-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10933-z