Abstract
Background
Breast cancer and frailty frequently co-occur in older women, and frailty status has been shown to predict negative health outcomes. However, the extent to which frailty assessments are utilized in observational research for the older breast cancer population is uncertain. Therefore, the aim of this review was to determine the frequency of use of frailty assessments in studies investigating survival or mortality, and characterize them, concentrating on literature from the past 5 years (2017–2022).
Methods
MEDLINE, EMBASE and Cochrane Library were systematically queried to identify observational studies (case-control, cohort, cross-sectional) published from 2017-2022 that focus on older females (≥ 65 years) diagnosed with breast cancer, and which evaluate survival or mortality outcomes. Independent reviewers assessed the studies for eligibility using Covidence software. Extracted data included characteristics of each study as well as information on study design, study population, frailty assessments, and related health status assessments. Risk of bias was evaluated using the appropriate JBI tool. Information was cleaned, classified, and tabulated into review level summaries.
Results
In total, 9823 studies were screened for inclusion. One-hundred and thirty studies were included in the final synthesis. Only 11 (8.5%) of these studies made use of a frailty assessment, of which 4 (3.1%) quantified frailty levels in their study population, at baseline. Characterization of frailty assessments demonstrated that there is a large variation in terms of frailty definitions and resulting patient classification (i.e., fit, pre-frail, frail). In the four studies that quantified frailty, the percentage of individuals classified as pre-frail and frail ranged from 18% to 29% and 0.7% to 21%, respectively. Identified frailty assessments included the Balducci score, the Geriatric 8 tool, the Adapted Searle Deficits Accumulation Frailty index, the Faurot Frailty index, and the Mian Deficits of Accumulation Frailty Index, among others. The Charlson Comorbidity Index was the most used alternative health status assessment, employed in 56.9% of all 130 studies. Surprisingly, 31.5% of all studies did not make use of any health status assessments.
Conclusion
Few observational studies examining mortality or survival outcomes in older women with breast cancer incorporate frailty assessments. Additionally, there is significant variation in definitions of frailty and classification of patients. While comorbidity assessments were more frequently included, the pivotal role of frailty for patient-centered decision-making in clinical practice, especially regarding treatment effectiveness and tolerance, necessitates more deliberate attention. Addressing this oversight more explicitly could enhance our ability to interpret observational research in older cancer patients.
Similar content being viewed by others
Introduction
Female breast cancer (BC) has surpassed lung cancer as the most commonly diagnosed cancer across the globe. At the same time, trends in the burden of breast cancer, measured by incidence and mortality, have continued to increase steadily [1]. GLOBOCAN estimates produced by the International Agency for Research on Cancer (IARC) revealed 2.3 million new cases of breast cancer worldwide, which accounted for 11.7% of all new cancer cases, and 685,000 deaths in 2020 [2]. Given that aging is the largest risk factor for breast cancer, older women develop BC at higher incidence rates compared to their younger counterparts [3]. Furthermore, as population life expectancy improves, the number of older women living with breast cancer is expected to rise.
Evidence supports the need for differential, tailored treatment between younger and older BC patients [4,5,6,7,8,9]. Clinical decision-making for the older cancer patient population (65+) is especially challenging because it is heterogeneous in nature and must take into account additional relevant factors such as frailty, multimorbidity, polypharmacy, limited life expectancy, and correspondingly death from competing causes besides the cancer of interest [10, 11]. However, these factors often lead to clinical study exclusions [12]. As a result, older women have been largely underrepresented in randomized clinical trials, therefore leading to a lack of evidence-based information on the best treatment within these age groups and a heavy reliance on observational research [13]. The prevalence of frailty increases with advancing age and more than 50% of older cancer patients are considered pre-frail or frail [14].
The notion of frailty has been historically difficult to capture considering its manifestation is highly complex and any underlying pathophysiological mechanisms are multifactorial [15]. Frailty is theoretically defined as an age-related syndrome of physiological decline and vulnerability, leading to an increased risk of adverse health outcomes [16,17,18,19]. Frailty has also been defined and quantified using several methods, two of which are particularly well-known and used in both clinical and research settings: the Frailty Phenotype [20] and the Frailty Index (FI ) [21]. The frailty phenotype by Fried and colleagues defines frailty as a condition meeting 3 of 5 phenotypic criteria, while the frailty index defines frailty through the proportion of accumulated deficits.
Many healthcare practitioners advocate for older adults to be evaluated via Comprehensive geriatric assessment (CGA), which is a multidimensional, multidisciplinary process which identifies their medical, social and functional needs, and supports the development of a care plan to address those needs [22]. In the field of geriatric oncology, CGA is used to detect disabilities, and conditions that potentially contribute to an older patient’s frailty status, which could predispose them to poor outcomes and treatment complications [23,24,25]. Furthermore, the insights gained from CGA can inform the coordination and planning of interventions designed to mitigate the impact of frailty on cancer treatment outcomes. CGA is often criticized for being time consuming, requiring the need for coordination of multidisciplinary specialties, and lacking consistency in collected data [26]. As a result, many cancer specialists seek a shorter screening tool that can separate fit older cancer patients, eligible for standard cancer treatment, from vulnerable patients who should subsequently receive a full assessment to guide tailoring of their treatment regimens. Additionally, although CGA can provide a comprehensive overview of a patient’s vulnerabilities, it alone does not provide a numerical measurement of frailty and must be operationalized on a scale or index for use in outcomes research [27].
Closely related concepts to frailty such as comorbidity and disability, as well as various geriatric parameters have been similarly utilized to characterize the health status of older breast cancer patients and have been shown to predict disease related survival, toxicity, patient reported outcomes (PROs), and mortality [28]. While comorbidity, disability, and other geriatric parameters can contribute to the development of frailty, it is crucial to recognize that frailty itself is a distinct and vital entity that holds paramount importance in the treatment of older cancer patients. Notably, frailty represents an aggregate expression of risk [29] that extends beyond the presence of individual conditions, and is considered preventable and partially reversable [30, 31].
Given the value of frailty assessments, it is crucial to understand their use in breast cancer research. To date, no reviews have yet quantified the use of frailty assessments in observational studies on breast cancer in older women. Therefore, the aim of this review was to determine the frequency of use of frailty assessments in such studies and characterize them, concentrating on literature from the past 5 years (2017-2022). The 5-year timeline was considered suitable since the intention was to capture current research practices.
Methods
This systematic review followed the PRISMA guidelines [32] (Preferred Reporting Items for Systematic reviews and Meta-Analyses). A protocol was developed a priori; however, it was not registered or published (see Appendix A.1). The specific objectives of this review were as follows:
Primary objectives:
-
1.
Quantify and characterize frailty assessments in included observational studies.
-
2.
Document which observational studies have been published in the last 5 years (2017–2022)
Secondary objectives:
-
1.
Assess the prevalence of frailty in older breast cancer patients
Search strategy and article selection
A systematic literature search was conducted to identify observational studies on older women with breast cancer reporting survival or mortality. Literature published from 2017-2022 was retrieved from 3 databases including: MEDLINE, EMBASE, and Cochrane Library. Additional articles were mined by searching on Google Scholar and inspecting reference lists of relevant systematic reviews. The search strategy can be accessed in Appendix A.1.
Studies were eligible for inclusion if they fulfilled the following criteria:
-
1.
Article was (or reported on) an observational study defined here as a case–control study, cross-sectional study, or cohort study.
-
2.
Article reported solely on older females ≥ 65 years of age with all stages of breast cancer who were patients receiving active oncological treatment at the time of enrollment.
-
3.
Article was written in English, German, Dutch, or Spanish.
-
4.
Article reported on survival or mortality before or after treatment.
-
5.
Article was published within the specified 5-year period (2017–2022)
Studies were excluded based the following criteria:
-
1.
Article was a letter, comment, conference abstract, partial text, or review.
-
2.
Article reported on a mixed population which includes individuals younger than 65 years of age, male patients, cancers besides breast cancer, and patients receiving best supportive care without active oncological treatment in the last stage of the disease.
-
3.
Article was about health technology assessment, (population) breast cancer screening, or a tool validation study.
-
4.
Article was primarily a molecular analysis (i.e., RNA, DNA, tumor structure, single cells, protein expression, biomarkers, genomic testing, gene expression etc.)
The list of excluded articles can be accessed in Appendix A.2.
Data retrieval, extraction, and synthesis
Collected references were managed using Covidence Software [33]. Duplicate articles were removed prior to the start of the review process. Eligibility of identified studies was determined by independently assessing titles and abstracts by two authors including DS, EB, MD, DM, FB, JP, or JV. Subsequently, the full texts of selected articles were independently assessed by DS and EB. Any disagreements on inclusion were resolved by consensus by DS and EB. A data extraction form was developed using Covidence, pilot tested on 10 randomly selected articles, and refined prior to use. Two unique sets of extracted data were independently collected (DS and EB) for each article and consolidated into a final version to ensure agreement and completeness. Extracted data included characteristics of each study such as title, DOI, country of publication, inclusion/exclusion criteria, aim, outcomes, study design, and funding sources. We also collected information on the population such as the number of patients used in the analysis, number of fit/pre-frail/frail patients, age, cancer stages and treatments, information on the use of frailty assessments, comorbidity assessments, or related health status assessments, as well as data source and setting. Variables were cleaned, classified, and tabulated into review level summaries for interpretation. Cancer stage, often described by TNM, or other stage descriptors were categorized to non-invasive non-metastatic, invasive non-metastatic, invasive metastatic, or unclear for simplicity. Descriptive statistics were performed using R (version 4.2.1, R Core Team, 2022) and Rstudio (version 2023.3.0.386, RStudio Team, 2023), while tables and figures were generated with the following attached packages: ggplot2 3.3.6, xtable 1.8-4, dplyr 1.0.9, and readr 2.1.2.
Quality assessment
Risk of bias was assessed separately by EB and DS using critical appraisal tools from the Joanna Briggs Institute (JBI) [34]. The appropriate checklist was selected per observational study type. Each checklist is composed of several questions answered as “yes”, “unclear”, “no”, or “not applicable”. Any disagreements were solved by consensus. Studies were labeled low, medium, or high risk of bias based on the applicable questions.
Results
Literature search and inclusion
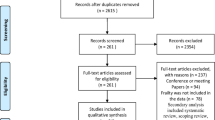
The search strategy yielded 14,036 records. After removing duplicate records, 9283 were screened on their titles and abstracts. Following this screening, 217 studies were deemed potentially eligible and were reviewed in full-text. Out of these, 130 studies met the inclusion criteria and were included in the systematic review. The PRISMA Flow Diagram (Fig. 1) shows an overview of the study selection and reasons for exclusion.
Study characteristics
From the 130 included studies, 71 used data from North America, 39 from Europe, 13 from Asia, 5 from Europe and Asia, 1 from North America and Asia, and 1 from Europe and North America. One-hundred twenty-eight were cohort studies (114 retrospective studies, 14 prospective studies), 1 was a retrospective case-control study, and 1 was a cross-sectional study. Fifty-six studies had a minimum age under 70 years and 73 had a minimum age above 70 years. Ninety-nine studies examined patients with invasive non- metastatic cancer, 8 with invasive metastatic cancer, 1 with non-invasive non-metastatic cancer, 15 examined a combination of invasive metastatic, invasive non-metastatic, and non-invasive non-metastatic cancers, and the remaining 7 were unclear. Patient data stemmed from various sources; however, the majority were from single cancer registries or institutional databases. The complete overview of study characteristics is detailed in Table 1.
Due to the nature of the review, all studies were included in the synthesis. Risk of bias was assessed for 130 studies using the appropriate JBI Critical Appraisal tool. The quality of the studies was mixed; however, all were determined to have low or medium risk of bias overall. Full details of the risk of bias assessment are displayed in Appendix A.3.
Frailty assessments
Eleven studies [42, 73, 86, 93, 98, 105, 120, 121, 144, 154, 155] (8.5% of 130 included studies) assessed frailty in their patient population, however only 4 studies [42, 98, 121, 155] classified patients into fit, pre-frail, or frail categories. Frailty was only assessed at baseline and there were no studies which assessed frailty post-treatment. Patients in each study included those treated with surgery, radiotherapy, hormonal therapy, chemotherapy, or targeted therapy. The assessments included the Balducci Score, the Geriatric 8 tool, the CGA, the Adapted Searle Deficits of Accumulations Index, Activities of Daily Living/Instrumental Activities of Daily Living, the Faurot Frailty Index, the Mian Deficits of Accumulations Index, and various combinations of geriatric tests. The identified frailty assessments were highly heterogeneous in terms of their operationalization, definitions, and patient classification. In total, there were ten unique definitions of frailty from eleven studies. Surprisingly, 4 studies [42, 121, 144, 154] identified frailty using a novel definition based off select geriatric assessments. One study [105] used Activities of Daily Living and Instrumental Activities of Daily Living to define and assess frailty.
The most common approaches to operationalizing frailty included the use of scores, binary scales, or indices. However, it was observed that the results of these frailty measurements were frequently either not reported or not utilized in subsequent analyses or interpretations within the studies. Furthermore, all identified frailty assessments incorporated at least one of two key components in their definition of frailty: comorbidity and functional status, with the latter most always encompassing disability. In addition to these core elements, many frailty assessments also included other geriatric parameters, such as cognitive function, nutritional status, polypharmacy, as well as various others.
Among the four studies which quantified frailty, the percentage of pre-frail individuals ranged from 18 to 29 percent, while the percentage of frail individuals ranged from 0.7 to 21 percent (percentage of frail patients was not reported by 7 studies). Two of these studies [42, 121] operationally defined frailty using a novel index based on seven geriatric assessments (Charlson Comorbidity Index, Activities of Daily Living, Instrumental Activities of Daily Living, Eastern Cooperative Oncology Group (ECOG) Performance Status, Mini Mental State Examination, and Abridged Patient-Generated Subjective Global Assessment), and the remaining two used established indices, namely the Adapted Searle Deficits Accumulation Frailty Index [98], and the Mian Deficits of Accumulation Frailty Index [155]. A summary of characteristics including details on the domains and geriatric parameters which define each assessment is indicated in Table 2. Author provided frailty definitions can be found in Appendix A.4.
Compared to frailty assessments, the use of comorbidity assessments was more frequent, with 56.9% of all studies employing them. The distribution of studies by combination of assessments used is displayed in Fig. 2. Nearly 75% (55/74) of studies that included comorbidity assessments utilized either the Charlson Comorbidity Index or a modified version. A list of other health status assessments categorized by CGA domain is available in Appendix A.5.
Additional comorbidity assessments included the Elixhauser Comorbidity Score (n = 1), comorbidity counts (n = 13), lists (n = 2), and binary scales (n = 2). The full distribution of comorbidity assessments is shown below (Fig. 3).
Discussion
This systematic review summarizes the current use of frailty assessments in observational studies investigating survival or mortality outcomes for older breast cancer patients. The findings show that less than 10 percent of these observational studies utilize frailty assessments. Additionally, there is significant variation in how frailty is defined and how patients are subsequently classified based on these definitions. It also illustrates that the majority of researchers tend to rely on less comprehensive health indicators such as comorbidity, which are often used as a substitute for frailty. The majority of frailty assessments identified in our systematic review have been previously validated [164], however, a small subset of assessments were novel, generated from combinations of individual geriatric parameters [42, 121, 144, 154], or single assessments [105]. The proportion of baseline pre-frail or frail patients captured by studies included in our review ranged from 0.07-21.07% for frail and 18.26-29.41% for pre-frail patients. While there was substantial heterogeneity in the estimates, it is clear that a high proportion of older breast cancer patients are frail. Currently there is no specific assessment recommended for use in observational studies centered on older breast cancer patients.
Frail older patients need personalized care strategies to optimize treatment outcomes and post-treatment recovery. In the clinical setting, frailty assessments are primarily useful because they enable clinicians to determine the most suitable cancer treatment for their patients while minimizing excess harm. In observational research, the primary motivations for utilizing frailty information include improving predictive and causal analyses, which can be used to inform the design of future RCTs. Interpreting the findings of observational studies becomes challenging in the absence of frailty information, as frailty has a significant impact on various health outcomes for older cancer patients. Incomplete measurements and adjustments for frailty in relevant analyses can therefore lead to confounding bias and diminish our ability to make accurate predictions or causal estimations.
A systematic review published by Wang et al [165] published in 2022, estimated that the prevalence of pre-frailty and frailty in breast cancer patients were 32% and 30%, respectively and confirmed that age is positively associated with higher levels of frailty. Another review which looked at population levels of frailty, found that frailty was higher for women compared to men [166]. Considering this information, and the possibility of ascertainment bias due to the likelihood of missing data for frail older patients, we believe the proportion of frail individuals are likely underestimated in the studies we identified. It is known that classification of patients, i.e., who is considered fit, pre-frail or frail, depends heavily on the assessment used [166], and that frailty prevalence rates exhibit less variation when arranged by definition [167]. In our review, two [42, 121] out of four [42, 98, 121, 155], studies used similar definitions for their frailty assessments and had close estimates. Estimates derived from studies which used different definitions, and different cohorts, showed much greater variability. However, the similarity could also be attributed to use of the same cohort.
Limited use of frailty assessments in observational research may stem from the overall lack of knowledge on special considerations for older adults. First, it is crucial for health care specialists in clinical practice to routinely collect this data for all older adults and to make it accessible for use in research. Second, researchers should distinguish between the health status assessments that describe vulnerabilities commonly found in older adults, as the distinctions between these may not always be clear. In particular, it’s essential to understand that frailty represents a unique dimension of aging, which sets it apart from comorbidity and disability [29, 168]. Another reason for their limited use is that much of the data in observational studies comes from healthcare databases that have been long established, and they are not required to collect data on frailty. Ideally, the assessment of frailty for older adults should be consistently and systematically conducted within clinical settings, with their integration into healthcare databases mandated as standard practice. Addressing this oversight in data repositories is essential for a comprehensive understanding of health outcomes. However, until this becomes feasible, one possible solution is to generate a frailty measure from information present in healthcare databases, which can be done with or without a reference standard [169]. For example, frailty assessments derived from electronic health records have been shown to exhibit similar performance to in-person evaluations, retain their predictive ability, and demonstrate convergent validity between research standard frailty assessments [170,171,172].
Many of the studies we identified, which utilized a frailty assessment, failed to classify patients and/or report levels of frailty for their study population. This was also the case for the single study [93] which assessed frailty with CGA. One difficulty with using CGA is that the information must be operationalized as an index or scale to distinguish between levels of frailty. Additionally, although CGA is meant to determine vulnerabilities comprehensively, there is debate on the best assessments to use for each CGA domain. This means there is likely variation between CGAs conducted in clinical settings. The frailty assessments we identified, including indices and scales, reflect this reality. In our review, each frailty assessment had a unique definition for frailty, and used differing sets of geriatric parameters (tests). The CGA domains captured by the parameters, however, were frequently overlapping between frailty assessments. As the classification of frailty hinges on each assessment’s definition, this makes comparing frailty across populations inherently complex. Furthermore, results on the prevalence of frailty are limited by small number of studies [42, 98, 121, 155] that used these assessments, with two studies [42, 121] using the same patient cohort.
Additionally, a group of researchers attempting to compare frailty assessments in different clinical and social settings determined that there is limited consensus among tools across both areas, implying they might assess distinct dimensions of frailty [173]. Thus, there is a compelling case for exploring frailty assessments that are specifically aligned with health outcomes which impact older breast cancer patients, aiming for a standardized approach. Adopting this perspective would acknowledge the diverse impact of frailty on different diseases, highlighting that certain tools may offer insights on specific aspects of frailty which are more relevant to this population. This would promote field specific, contextualized, and interpretable findings in future research.
Surprisingly, a majority of the studies we identified in our review use comorbidity in their analyses, but many do not consider any dimension of health status in their older population. In the absence of exhaustive data to define a frailty assessment, it is ethically and methodologically justifiable to employ alternative health assessments as surrogate indicators. However, relying on a singular, or less comprehensive health metric risks overlooking the multidimensional nature inherent to older adult health.
Four recent randomized controlled trials have assessed the effectiveness of CGA in improving post- treatment outcomes for older cancer patients [23, 25, 174, 175]. The results demonstrated that treatment decisions based on CGA reduce the incidence of toxic effects from chemotherapy and may improve rates of treatment continuation/completion and unplanned hospital admissions; however, there was no evidence for differences in overall survival or progression-free survival between patients receiving CGA based intervention and standard care. In all trials, evaluating frailty status helped physicians choose the best care strategies for their patients. Regardless, of the direct effect on survival, frail patients are more susceptible to mortality from other causes [149]. This increased susceptibility can in turn influence the extent to which patients can benefit from treatment, including the duration of survival time. In light of this information, it is important to explore the role of frailty assessments in observational studies focusing on additional metrics such as patient reported outcomes, time without symptoms, or time to treatment failure, as these may be more meaningful for older breast cancer patients [176]. Given our findings, however, it is likely that frailty assessments are also overlooked for other research outcomes as well. All things considered, we recommend frailty assessment use in clinical decision-making and along care and recovery pathways.
A strength of this review is the comprehensive search strategy used to identify target studies and a thorough evaluation of evidence through rigorous critical appraisal. To our knowledge, this is the first review to synthesize evidence to quantify and characterize the use of frailty assessments in observational studies for the older breast cancer population. Our review was limited by the narrow examination of outcomes (survival, mortality) in a short time frame. We also report recent use of frailty assessments and are therefore unable to capture time trends. Lastly, due to a lack of translation resources, we considered studies only in English, German, Spanish, and Dutch. This restriction may have potentially reduced the pool of eligible studies screened.
Conclusion and recommendations
Frailty is an important determinant of health outcomes in older breast cancer patients. However, the majority of observational studies focusing on survival and mortality outcomes do not include frailty assessments. Missing frailty data in these studies may lead to incomplete or biased conclusions about appropriate cancer treatment. To increase their use, it is crucial to prioritize routine and standardized data collection in the clinical setting for use in health databases, and to improve education on health status assessments for researchers. To understand the use of frailty assessments more comprehensively, future research should examine the application of these assessments in studies with endpoints besides survival and mortality. By restructuring frailty measures into observational data, we can gain a better understanding of its impact and inform evidence-based guidelines to optimize patient-centered treatment in this vulnerable group of patients.
Availability of data and materials
The following can be acquired from the corresponding author upon reasonable request: raw and manipulated data and code used to generate material in the publication.
References
Sondik EJ. Breast cancer trends Incidence, mortality, and survival. Cancer. 1994;74(S3):995–9.
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Breast cancer statistics. Cancer Research UK. 2015;(https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer). (Accessed April 29, 2024)
Biganzoli L, Battisti NML, Wildiers H, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327–40.
Schonberg MA, Marcantonio ER, Li D, et al. Breast Cancer Among the Oldest Old: Tumor Characteristics, Treatment Choices, and Survival. JCO. 2010;28(12):2038–45.
Ring A, Harder H, Langridge C, et al. Adjuvant chemotherapy in elderly women with breast cancer (AChEW): an observational study identifying MDT perceptions and barriers to decision making. Ann Oncol. 2013;24(5):1211–9.
Husain LS, Collins K, Reed M, et al. Choices in cancer treatment: a qualitative study of the older women’s (>70 years) perspective. Psychooncology. 2008;17(4):410–6.
Wyld L, Reed MWR, Collins K, et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg. 2021;108(5):499–510.
Battisti NML, Reed MWR, Herbert E, et al. Bridging the Age Gap in breast cancer: Impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer. 2021;144:269–80.
Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–7.
Jørgensen TL, Hallas J, Land LH, et al. Comorbidity and polypharmacy in elderly cancer patients: The significance on treatment outcome and tolerance. Journal of geriatric oncology. 2010;1(2):87–102.
Bernard MA, Clayton JA, Lauer MS. Inclusion Across the Lifespan: NIH Policy for Clinical Research. JAMA. 2018;320(15):1535.
Marum RJ. Underrepresentation of the elderly in clinical trials, time for action. Br J Clin Pharmacol. 2020;86(10):2014–6.
Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–101.
Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433.
Bortz WM II. The physics of frailty. J Am Geriatr Soc. 1993;41(9):1004–8.
Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26(4):315–8.
Lipsitz LA, Goldberger AL. Loss of’complexity’and aging: potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–9.
Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39(1):46–52.
Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57
Rockwood K, Mitnitski A. Frailty in Relation to the Accumulation of Deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7.
Parker SG, McCue P, Phelps K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review Age and Ageing. 2018;47(1):149–55.
Nadaraja S, Matzen L-E, Jørgensen TL, et al. The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: A randomized parallel-group clinical trial. J Geriatr Oncol. 2020;11(3):488–95.
Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241–52.
Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894–904.
Maas HAAM, Janssen-Heijnen MLG, Rikkert MGMO, et al. Comprehensive Geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43(15):2161–9.
Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–33.
De Boer AZ, Bastiaannet E, Putter H, et al. Prediction of other-cause mortality in older patients with breast cancer using comorbidity. Cancers [electronic article]. 2021;13(7):1627. https://doi.org/10.3390/cancers13071627
Fried LP, Ferrucci L, Darer J, et al. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J Gerontol A Biol Sci Med Sci. 2004;59(3):M255–63.
Morley JE, Vellas B, Abellan Van Kan G, et al. Frailt y Consensus: A Call to Action. Journal of the American Medical Directors Association. 2013;14(6):392–7.
Lee JS, Auyeung T-W, Leung J, et al. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15(4):281–6.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906.
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. 2024. Available at www.covidence.org.
JBI Critical Appraisal Tools | JBI. (https://jbi.global/critical-appraisal-tools). (Accessed 18 Oct 2023).
Agborbesong O, Helmer SD, Reyes J, et al. Breast cancer treatment in the elderly: Do treatment plans that do not conform to NCCN recommendations lead to worse outcomes? Am J Surg. 2020;220(2):381–4.
Akushevich I, Yashkin AP, Greenup RA, et al. A medicare-based comparative mortality analysis of active surveillance in older women with DCIS. npj Breast Cancer [electronic article]. 2020;6(1):57. https://doi.org/10.1038/s41523-020-00199-0.
Alatawi Y, Hansen RA, Chou C, et al. The impact of cognitive impairment on survival and medication adherence among older women with breast cancer. Breast Cancer. 2021;28(2):277–88.
Ali AA, Tawk R, Xiao H, et al. Comparative effectiveness of radiotherapy for early-stage hormone receptor-positive breast cancer in elderly women using real-world data. Cancer Med. 2019;8(1):117–27.
Al-Rashdan A, Xu Y, Quan ML, et al. Higher-risk breast cancer in women aged 80 and older: Exploring the effect of treatment on survival. Breast. 2021;59:203–10.
Aly A, Shah R, Hill K, et al. Overall survival, costs and healthcare resource use by number of regimens received in elderly patients with newly diagnosed metastatic triple-negative breast cancer. Future Oncol. 2019;15(9):1007–20.
Aytekin A, Karatas F, Sahin S, et al. Clinicopathological features of patients with breast cancer aged 70 years or over. J BUON. 2017;22(1):200–7.
Battisti NML, Hatton MQ, Reed MWR, et al. Observational cohort study in older women with early breast cancer: Use of radiation therapy and impact on health-related quality of life and mortality. Radiother Oncol. 2021;161:166–76.
Bertolo A, Rosso C, Voutsadakis IA. Breast cancer in patients 80 years-old and older. Meme Sagligi Dergisi / Journal of Breast Health. 2020;16(3):208–12.
Blanchette PS, Lam M, Richard L, et al. Factors associated with endocrine therapy adherence among post-menopausal women treated for early-stage breast cancer in Ontario. Canada Breast Cancer Research & Treatment. 2020;179(1):217–27.
Blay Aulina L, Louro Aldamiz-Echevarría J, Ribes Cajas P, et al. Breast cancer treatment in octogenarian patients. Clinica e Investigacion en Ginecologia y Obstetricia [electronic article]. 2022;49(2):100722. https://doi.org/10.1016/j.gine.2021.100722.
Buszek SM, Lin HY, Bedrosian I, et al. Lumpectomy Plus Hormone or Radiation Therapy Alone for Women Aged 70 Years or Older With Hormone Receptor-Positive Early Stage Breast Cancer in the Modern Era: An Analysis of the National Cancer Database. Int J Radiat Oncol Biol Phys. 2019;105(4):795–802.
Cao KI, Salviat F, Laki F, et al. Outcomes of postoperative radiation therapy for breast cancer in older women according to age and comorbidity status: An observational retrospective study in 752 patients. Journal of Geriatric Oncology. 2018;9(6):600–5.
Chadha M, Shao T, Cate S, et al. Patterns of relapse in older women diagnosed with estrogen receptor (ER)–positive early-stage breast cancer (BC) treated with lumpectomy without adjuvant endocrine therapy (ET). J Radiat Oncol. 2019;8(3):349–53.
Chagpar AB, Hatzis C, Pusztai L, et al. Association of LN Evaluation with Survival in Women Aged 70 Years or Older With Clinically Node-Negative Hormone Receptor Positive Breast Cancer. Ann Surg Oncol. 2017;24(10):3073–81.
Chen J, Wu X, Christos P, et al. Adjuvant Radiation Therapy for T3N0 Breast Cancer Patients Older Than 75 Years After Mastectomy: A SEER Analysis. Clin Breast Cancer. 2018;18(5):e967–73.
Chen YQ, Xu JW, Xu XF, et al. Predicting the survival benefit of local surgery in patients aged 70 years or older with stage IV breast cancer: A population-based analysis. Breast. 2021;59:124–34.
Chu QD, Zhou M, Peddi P, et al. Outcomes in real-world practice are different than cooperative trial for elderly patients with early breast cancer treated with adjuvant radiation therapy. Surgery (United States). 2018;163(6):1213–9.
Cil I, Kucukarda A, Atcı MM, et al. Efficacy and safety of trastuzumab emtansine in older patients with HER2-positive advanced breast cancer: a real-world study. Tumori. 2022;108(1):19–25.
Corso G, Magnoni F, Montagna G, et al. Long-term outcome and axillary recurrence in elderly women (≥70 years) with breast cancer: 10-years follow-up from a matched cohort study. Eur J Surg Oncol. 2021;47(7):1593–600.
Crozier JA, Pezzi TA, Hodge C, et al. Addition of chemotherapy to local therapy in women aged 70 years or older with triple-negative breast cancer: a propensity-matched analysis. Lancet Oncol. 2020;21(12):1611–9.
Dahn H, Wilke D, Walsh G, et al. Radiation and/or endocrine therapy? Recurrence and survival outcomes in women over 70 with early breast cancer after breast-conserving surgery. Breast Cancer Res Treat. 2020;182(2):411–20.
de Boer AZ, van de Water W, Bastiaannet E, et al. Early stage breast cancer treatment and outcome of older patients treated in an oncogeriatric care and a standard care setting: an international comparison. Breast Cancer Res Treatment. 2020;184(2):519–26.
De Luca R, Alù M, Genova G, et al. Use of Eribulin mesylate as second-line therapy in elderly patients with HER/2 negative metastatic breast cancer (MBC): Efficacy, tolerability and Quality of Life. Eur Rev Med Pharmacol Sci. 2021;24(24):12727–34.
Derks MGM, Bastiaannet E, Kiderlen M, et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer. 2018;119(1):121–9.
De Santis MC, Bonfantini F, Di Salvo F, et al. Hypofractionated Whole-Breast Irradiation With or Without Boost in Elderly Patients: Clinical Evaluation of an Italian Experience. Clin Breast Cancer. 2018;18(5):e1059–66.
Downs-Canner S, Zabor EC, Wind T, et al. Radiation Therapy After Breast-Conserving Surgery in Women 70 Years of Age and Older: How Wisely Do We Choose? Ann Surg Oncol. 2019;26(4):969–75.
Du XL, Song L. Age and Racial Disparities in the Utilization of Anticancer, Antihypertension, and Anti-diabetes Therapies, and in Mortality in a Large Population-Based Cohort of Older Women with Breast Cancer. J Racial Ethn Health Disparities [electronic article]. 2023;10(1):446–61. https://doi.org/10.1007/s40615-022-01235-4.
Dumontier C, Clough-Gorr KM, Silliman RA, et al. Motivation and mortality in older women with early stage breast cancer: A longitudinal study with ten years of follow-up. J Geriatr Oncol. 2017;8(2):133–9.
El Badri S, Tahir B, Balachandran K, et al. Palbociclib in combination with aromatase inhibitors in patients ≥ 75 years with oestrogen receptor-positive, human epidermal growth factor receptor 2 negative advanced breast cancer: A real-world multicentre UK study. Breast. 2021;60:199–205.
Enomoto K, Fukumoto S, Mori S, et al. Survival With Surgery Is Superior to Survival Without Surgery in Breast Cancer Patients Aged 85 years or Older: A Retrospective Study. Am Surg. 2021;87(11):1746–51.
Escott CE, Zaenger D, Switchencko JM, et al. The Influence of Histologic Grade on Outcomes of Elderly Women With Early Stage Breast Cancer Treated With Breast Conserving Surgery With or Without Radiotherapy. Clin Breast Cancer. 2020;20(6):e701–10.
Faiz AS, Guo S, Kaveney A, et al. Venous thrombosis and breast cancer in older women: Racial differences in risk factors and mortality. Thromb Res. 2018;171:130–5.
Fattoruso SIS, De Luca R, Grassadonia A, et al. Non-pegylated liposomal doxorubicin plus cyclophosphamide as first-line therapy in elderly women with HER2 negative metastatic breast cancer. Clin Ter. 2022;173(2):121–7.
Frebault J, Bergom C, Cortina CS, et al. Invasive Breast Cancer Treatment Patterns in Women Age 80 and Over: A Report from the National Cancer Database. Clin Breast Cancer. 2022;22(1):49–59.
Gal O, Ishai Y, Sulkes A, et al. Early Breast Cancer in the Elderly: Characteristics, Therapy, and Long-Term Outcome. Oncology (Switzerland). 2018;94(1):31–8.
Goldberg M, Sutradhar R, Paszat L, et al. Patterns of adjuvant care and outcomes of elderly women with stage I breast cancer after breast-conserving surgery: a population-based analysis. Breast Cancer Res Treat. 2019;176(3):657–67.
Goyal RK, Carter GC, Nagar SP, et al. Treatment patterns, survival and economic outcomes in Medicare-enrolled, older patients with HR+/HER2- metastatic breast cancer. Curr Med Res Opin. 2019;35(10):1699–710.
Hannoun-Lévi JM, Montagne L, Sumodhee S, et al. APBI Versus Ultra-APBI in the Elderly With Low-Risk Breast Cancer: A Comparative Analysis of Oncological Outcome and Late Toxicity. Int J Radiat Oncol Biol Phys. 2021;111(1):56–67.
Haque W, Kee Yuan DM, Verma V, et al. Radiation therapy utilization and outcomes for older women with breast cancer: Impact of molecular subtype and tumor grade. Breast. 2017;35:34–41.
Haque W, Verma V, Butler EB, et al. Omission of radiotherapy in elderly women with early stage metaplastic breast cancer. Breast. 2018;38:154–9.
Haque W, Verma V, Hsiao KY, et al. Omission of radiation therapy following breast conservation in older (≥70 years) women with T1–2N0 triple-negative breast cancer. Breast Journal. 2019;25(6):1126–33.
Herskovic AC, Wu X, Christos PJ, et al. Omission of Adjuvant Radiotherapy in the Elderly Breast Cancer Patient: Missed Opportunity? Clin Breast Cancer. 2018;18(5):418–31.
Hornova J, Bortlicek Z, Majkova P, et al. Locally advanced breast cancer in elderly patients. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2017;161(2):217–22.
Huang K, Zhang J, Yu Y, et al. The impact of chemotherapy and survival prediction by machine learning in early Elderly Triple Negative Breast Cancer (eTNBC): a population based study from the SEER database. BMC Geriatr. 2022;22(1):268.
Iglay K, Santorelli ML, Hirshfield KM, et al. Impact of preexisting mental illness on all-cause and breast cancer-specific mortality in elderly patients with breast cancer. J Clin Oncol. 2017;35(36):4012–8.
Janeva S, Zhang C, Kovács A, et al. Adjuvant chemotherapy and survival in women aged 70 years and older with triple-negative breast cancer: a Swedish population-based propensity score-matched analysis. The Lancet Healthy Longev. 2020;1(3):e117–24.
Jhawar SR, Alpert N, Taioli E, et al. Adjuvant radiation therapy alone is associated with improved overall survival compared to hormonal therapy alone in older women with estrogen receptor positive early stage breast cancer. Cancer Med. 2020;9(22):8345–54.
Jobsen JJ, Middelburg JG, van der Palen J, et al. Breast-conserving therapy in older patients with breast cancer over three decades: progress or stagnation. Journal of Geriatric Oncology. 2019;10(2):330–6.
Jobsen JJ, van der Palen J, Siemerink E, et al. Limited Impact of Breast Cancer and Non-breast Malignancies on Survival in Older Patients with Early-Stage Breast Cancer: Results of a Large, Single-Centre, Population-Based Study. Clin Oncol [electronic article]. 2022;34(6):355–62. https://doi.org/10.1016/j.clon.2021.11.005.
Karanlik H, Klllç B, Ylldlrlm I, et al. Breast-Conserving Surgery under Local Anesthesia in Elderly Patients with Severe Cardiorespiratory Comorbidities: A Hospital-Based Case-Control Study. Breast Care. 2017;12(1):29–33.
Kędzierawski P, Mężyk R. Breast cancer in women aged 75 years and older – tumour characteristics and treatment options. Przeglad Menopauzalny. 2021;20(1):14–20.
Kinj R, Chand ME, Gal J, et al. Single fraction of accelerated partial breast irradiation in the elderly: Early clinical outcome. Radiat Oncol [electronic article]. 2018;13(1):174. https://doi.org/10.1186/s13014-018-1119-6.
Kinj R, Chand ME, Gal J, et al. Five-year oncological outcome after a single fraction of accelerated partial breast irradiation in the elderly. Radiat Oncol [electronic article]. 2019;14(1):234. https://doi.org/10.1186/s13014-019-1448-0.
Klint L, Kovács A, Rönnerman E, et al. Real world data on adjuvant treatment of older HER2-positive breast cancer patients – A single institution experience through 8 years. Cancer Treat Res Commun [electronic article]. 2021;28:100430. https://doi.org/10.1016/j.ctarc.2021.100430.
Kong AL, Nattinger AB, McGinley E, et al. The relationship between patient and tumor characteristics, patterns of breast cancer care, and 5-year survival among elderly women with incident breast cancer. Breast Cancer Res Treat. 2018;171(2):477–88.
La Rocca E, Meneghini E, Dispinzieri M, et al. Hypofractionated irradiation in 794 elderly breast cancer patients: An observational study. Breast Journal. 2020;26(2):188–96.
La Rocca E, Meneghini E, Lozza L, et al. Older age and comorbidity in breast cancer: is RT alone the new therapeutic frontier? J Cancer Res Clin Oncol. 2020;146(7):1791–800.
Leo S, Arnoldi E, Repetto L, et al. Eribulin Mesylate as Third or Subsequent Line Chemotherapy for Elderly Patients with Locally Recurrent or Metastatic Breast Cancer: A Multicentric Observational Study of GIOGer (Italian Group of Geriatric Oncology)-ERIBE. Oncologist. 2019;24(6):e232–40.
Lin KH, Hsu HM, Hsu KF, et al. Survival outcomes in elderly Taiwanese women according to breast cancer subtype and lymph node status: A single-center retrospective study. PLoS One [electronic article]. 2021;16(12):e0261258. https://doi.org/10.1371/journal.pone.0261258.
Liu X, Zheng D, Wu Y, et al. Treatment patterns and outcomes in older women with early breast cancer: a population-based cohort study in China. BMC Cancer [electronic article]. 2021;21(1):226. https://doi.org/10.1186/s12885-021-07947-w.
Luo SP, Zhang J, Wu QS, et al. Association of Axillary Lymph Node Evaluation With Survival in Women Aged 70 Years or Older With Breast Cancer. Front Oncol [electronic article]. 2021;28(10):596545. https://doi.org/10.3389/fonc.2020.596545.
Luo C, Zhong X, Luo T, et al. Postmastectomy radiation therapy and survival outcome in older patients with T1–2N1 breast cancer. Breast (Edinburgh, Scotland). 2021;59:308–13.
Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164(1):107–17.
Marks CE, Ren Y, Rosenberger LH, et al. Surgical Management of the Axilla in Elderly Women With Node-positive Breast Cancer. J Surg Res. 2020;254:275–85.
Martin C, Shrestha A, Morgan J, et al. Treatment choices for older women with primary operable breast cancer and cognitive impairment: Results from a prospective, multicentre cohort study. J GERIATR ONCOL. 2021;12(5):705–13.
McKevitt E, Cheifetz R, DeVries K, et al. Sentinel Node Biopsy Should Not be Routine in Older Patients with ER-Positive HER2-Negative Breast Cancer Who Are Willing and Able to Take Hormone Therapy. Ann Surg Oncol. 2021;28(11):5950–7.
Mermut Ö, İnanç B. Prognostic factors and survival of elderly women with breast cancer aged ≥70 years. Turk Geriatri Dergisi. 2019;22(4):426–33.
Merrill AY, Brown DR, Klepin HD, et al. Outcomes after mastectomy and lumpectomy in octogenarians and nonagenarians with early-stage breast cancer. Am Surg. 2017;83(8):887–94.
Mogal HD, Clark C, Dodson R, et al. Outcomes After Mastectomy and Lumpectomy in Elderly Patients with Early-Stage Breast Cancer. Ann Surg Oncol. 2017;24(1):100–7.
Morgan JL, George J, Holmes G, et al. Breast cancer surgery in older women: outcomes of the Bridging Age Gap in Breast Cancer study. Br J Surg. 2020;107(11):1468–79.
Morita M, Shimomura A, Tokuda E, et al. Is adjuvant chemotherapy necessary in older patients with breast cancer? Breast Cancer [electronic article]. 2022;29(3):498–506. https://doi.org/10.1007/s12282-021-01329-7.
Nayyar A, Strassle PD, Iles K, et al. Survival Outcomes of Early-Stage Hormone Receptor-Positive Breast Cancer in Elderly Women. Ann Surg Oncol. 2020;27(12):4853–60.
Nichol AM, Chan EK, Lucas S, et al. The Use of Hormone Therapy Alone Versus Hormone Therapy and Radiation Therapy for Breast Cancer in Elderly Women: A Population-Based Study. Int J Radiat Oncol Biol Phys. 2017;98(4):829–39.
Ogawa Y, Ikeda K, Watanabe C, et al. Super-elderly patient-specific perioperative complications in breast cancer surgery. Surg Today. 2019;49(10):843–9.
Ojala K, Meretoja TJ, Mattson J, et al. Surgical treatment and prognosis of breast cancer in elderly - A population-based study. Eur J Surg Oncol. 2019;45(6):956–62.
Oktay E, Keskin Ö, Degirmencioglu S. What are the clinicopathological features of elderly early-stage breast cancer patients and is there any difference in patients over 70 years of age? J Geriatr Oncol. 2019;5(2):49–53.
Onega T, Zhu W, Weiss JE, et al. Preoperative breast MRI and mortality in older women with breast cancer. Breast Cancer Res Treat. 2018;170(1):149–57.
Park JH, Choi IS, Kim KH, et al. Treatment patterns and outcomes in elderly patients with metastatic breast cancer: A multicenter retrospective study. J Breast Cancer. 2017;20(4):368–77.
Park C, Park SK, Woo A, et al. Health-related quality of life among elderly breast cancer patients treated with adjuvant endocrine therapy: a U.S Medicare population-based study. Qual Life Res [electronic article]. 2022;20(11):1320–9. https://doi.org/10.1007/s11136-021-03059-x.
Peng Y, Hu T, Cheng L, et al. Evaluating and Balancing the Risk of Breast Cancer-Specific Death and Other Cause-Specific Death in Elderly Breast Cancer Patients. Front. Oncol. [electronic article]. 2021;11((Peng Y.; Hu T.; Cheng L.; Tong F.; Cao Y.; Liu P.; Zhou B.; Liu M.; Liu H.; Guo J.; Xie F.; Yang H.; Wang S.; Wang C.; Wang S., shuwang@pkuph.edu.cn) Breast Center, Peking University People’s Hospital, Beijing, China). (https://www.embase.com/search/results?subaction=viewrecord&id=L634597974&from=export).
Pinsky PF, Miller EA, Heckman-Stoddard BM, et al. Breast Cancer Characteristics and Survival among Users versus Nonusers of Raloxifene. Cancer Prev Res. 2020;13(1):83–90.
Poodt IGM, Schipper RJ, Vugts G, et al. The rationale for and long-term outcome of incomplete axillary staging in elderly women with primary breast cancer. Eur J Surg Oncol. 2018;44(11):1714–9.
Rais F, Tsui JMG, Daianska A, et al. Extreme weekly locoregional hypofractionated radiation in elderly women with non-metastatic breast cancer: Extreme locoregional hypofractionated breast RT. Radiother Oncol. 2021;162((Rais F., fadoua.rais.med@ssss.gouv.qc.ca; Tsui J.M.G., james.tsui@mail.mcgill.ca; Daianska A.; Faye M.D.; Lambert C.; David M.; Panet-Raymond V.; Azoulay M.; Saidi A.; Hijal T.) McGill University Health Cancer Centre, Canada):156–161.
Reeder-Hayes KE, Meyer AM, Hinton SP, et al. Comparative toxicity and effectiveness of trastuzumab-based chemotherapy regimens in older women with early-stage breast cancer. J Clin Oncol. 2017;35(29):3298–305.
Reeder-Hayes KE, Wheeler SB, Meyer AM, et al. Adoption and effectiveness of de-escalated radiation and endocrine therapy strategies for older women with low-risk breast cancer. Journal of Geriatric Oncology. 2021;12(5):731–40.
Ring A, Battisti NML, Reed MWR, et al. Bridging The Age Gap: observational cohort study of effects of chemotherapy and trastuzumab on recurrence, survival and quality of life in older women with early breast cancer. Br J Cancer. 2021;125(2):209–19.
Schuil H, Derks M, Liefers GJ, et al. Treatment strategies and survival outcomes in older women with breast cancer: A comparative study between the FOCUS cohort and Nottingham cohort. Journal of Geriatric Oncology. 2018;9(6):635–41.
Schwartz KL, Simon MS, Bylsma LC, et al. Clinical and economic burden associated with stage III to IV triple-negative breast cancer: A SEER-Medicare historical cohort study in elderly women in the United States. Cancer. 2018;124(10):2104–14.
Showalter SL, Meneveau MO, Keim-Malpass J, et al. Effects of Adjuvant Endocrine Therapy Adherence and Radiation on Recurrence and Survival Among Older Women with Early-Stage Breast Cancer. Ann Surg Oncol. 2021;28(12):7395–403.
Sieluk J, Haiderali A, Huang M, et al. Early triple-negative breast cancer in women aged ≥65: Retrospective study of outcomes, resource use and costs, 2010–2016. Future Oncol. 2021;17(9):1039–54.
Smith-Graziani D, Lei X, Giordano SH, et al. Delayed initiation of adjuvant chemotherapy in older women with breast cancer. Cancer Med. 2020;9(19):6961–71.
Stueber TN, Diessner J, Bartmann C, et al. Effect of adjuvant radiotherapy in elderly patients with breast cancer. PLoS One [electronic article]. 2022;15(5):e0229518. https://www.embase.com/search/results?subaction=viewrecord&id=L2005979447&from=export.
Suarez-Almazor ME, Herrera R, Lei X, et al. Survival in older women with early stage breast cancer receiving low-dose bisphosphonates or denosumab. Cancer. 2020;126(17):3929–38.
Suen TKD, Luk WP, Fung LH, et al. Matched case-control survival analysis of older chinese breast cancer patients treated with surgery or primary endocrine therapy. Cancer Treat Res Commun [electronic article]. 2020;25:100227. https://doi.org/10.1016/j.ctarc.2020.100227.
Sumodhee S, Levy J, Chamorey E, et al. Accelerated partial breast irradiation for elderly women with early breast cancer: A compromise between whole breast irradiation and omission of radiotherapy. Brachytherapy. 2017;16(5):929–34.
Sun Z-H, Chen C, Kuang X-W, et al. Breast surgery for young women with early-stage breast cancer: Mastectomy or breast-conserving therapy? Medicine (Baltimore). 2021;100(18): e25880.
Takada K, Asano Y, Goto W, et al. Prognostic value of quality of life in endocrine therapy for elderly patients with breast cancer: A retrospective study. Anticancer Res. 2019;39(6):2941–50.
Tamirisa N, Thomas SM, Fayanju OM, et al. Axillary Nodal Evaluation in Elderly Breast Cancer Patients: Potential Effects on Treatment Decisions and Survival. Ann Surg Oncol. 2018;25(10):2890–8.
Tamirisa N, Lin H, Shen Y, et al. Association of Chemotherapy with Survival in Elderly Patients with Multiple Comorbidities and Estrogen Receptor-Positive, Node-Positive Breast Cancer JAMA Oncology. 2020;6(10):1548–54.
Tamirisa N, Lin H, Shen Y, et al. Impact of adjuvant endocrine therapy in older patients with comorbidities and estrogen receptor-positive, node-negative breast cancer—A National Cancer Database analysis. Cancer. 2021;127(13):2196–203.
Tang V, Zhao S, Boscardin J, et al. Functional Status and Survival after Breast Cancer Surgery in Nursing Home Residents. JAMA Surg. 2018;153(12):1090–6.
Tang L, Ma Z, et al. Effect of radiotherapy after breast-conserving surgery in elderly patients with early breast cancer according to the AJCC 8th Edition Breast Cancer Staging System in Japan. Breast Cancer. 2021;28(2):465–70.
Tang Z, Ji Y, Min Y, et al. Prognostic Factors and Models for Elderly (≥70 Years Old) Primary Operable Triple-Negative Breast Cancer: Analysis From the National Cancer Database. Front Endocrinol [electronic article]. 2022;17(13):856268. https://doi.org/10.3389/fendo.2022.856268.
Tannenbaum S, Soulos PR, Herrin J, et al. Regional Medicare Expenditures and Survival among Older Women with Localized Breast Cancer. Med Care. 2017;55(12):1030–8.
Thompson MR, Niu J, Lei X, et al. Association of endocrine therapy and dementia in women with breast cancer. Breast Cancer. 2021;13((Thompson M.R.) Department of Epidemiology and Environmental Health, University at Buffalo School of Public Health and Health Professions, Buffalo, NY, United States):219–224.
Tringale KR, Berger ER, Sevilimedu V, et al. Breast conservation among older patients with early-stage breast cancer: Locoregional recurrence following adjuvant radiation or hormonal therapy. Cancer. 2021;127(11):1749–57.
Valachis A, Nyström P, Fredriksson I, et al. Treatment patterns, risk for hospitalization and mortality in older patients with triple negative breast cancer. Journal of Geriatric Oncology. 2021;12(2):212–8.
Valli M, Cima S, Fanti P, et al. The role of radiotherapy in elderly women with early-stage breast cancer treated with breast conserving surgery. Tumori. 2018;104(6):429–33.
van der Plas-Krijgsman WG, Morgan JL, de Glas NA, et al. Differences in treatment and survival of older patients with operable breast cancer between the United Kingdom and the Netherlands – A comparison of two national prospective longitudinal multi-centre cohort studies. Eur J Cancer. 2022;163:189–99.
Vyas A, Mantaian T, Kamat S, et al. Association of guideline-concordant initial systemic treatment with clinical and economic outcomes among older women with metastatic breast cancer in the United States. J Geriatr Oncol. 2021;12(7):1092–9.
Wang Z, Zhou Z, Li W, et al. Treatment strategies and predicting prognoses in elderly patients with breast cancer. Cancer Manag Res. 2018;10:3207–18.
Ward SE, Richards PD, Morgan JL, et al. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Br J Surg. 2018;105(11):1454–63.
Ward SE, Holmes GR, Ring A, et al. Adjuvant Chemotherapy for Breast Cancer in Older Women: An Analysis of Retrospective English Cancer Registration Data. Clin Oncol. 2019;31(7):444–52.
Wasif N, Neville M, Gray R, et al. Competing Risk of Death in Elderly Patients with Newly Diagnosed Stage I Breast Cancer. J Am Coll Surg. 2019;229(1):30–36.e31.
Wickberg Å, Liljegren G, Killander F, et al. Omitting radiotherapy in women ≥ 65 years with low-risk early breast cancer after breast-conserving surgery and adjuvant endocrine therapy is safe. Eur J Surg Oncol. 2018;44(7):951–6.
Wittayanukorn S, Qian J, Westrick SC, et al. Prevention of Trastuzumab and Anthracycline-induced Cardiotoxicity Using Angiotensin-converting Enzyme Inhibitors or β-blockers in Older Adults with Breast Cancer. Am J Clin Oncol: Cancer Clinical Trials. 2018;41(9):909–18.
Wu SG, Zhang WW, Wang J, et al. 21-gene recurrence score assay and outcomes of adjuvant radiotherapy in elderly women with early-stage breast cancer after breast-conserving surgery. Front Oncol [electronic article]. 2019;15(14):1629–39. https://doi.org/10.3389/fonc.2019.00001.
Wu SG, Li FY, Wang J, et al. Omission of adjuvant radiotherapy following breast-conserving surgery for elderly women with early-stage pure mucinous breast carcinoma. Radiat Oncol [electronic article]. 2019;14(1):190. https://doi.org/10.1186/s13014-019-1394-x.
Wyld L, Reed MWR, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48–62.
Yan CH, Coleman C, Nabulsi NA, et al. Associations between frailty and cancer-specific mortality among older women with breast cancer. Breast Cancer Res Treat [electronic article]. 2021;189(3):769–79. https://doi.org/10.1007/s10549-021-06323-3.
Yang Z, Li K, Qiu P, et al. Research on the cutoff tumor size of omitting radiotherapy for BCSS after breast conserving surgery in women aged 65 years or oder with low-risk invasive breast carcinoma: Results based on the SEER database. Breast. 2021;60:287–94.
Yuan C, Xie Z, Bian J, et al. Outcomes of primary endocrine therapy in elderly women with stage I-III breast cancer: a SEER database analysis. Breast Cancer Res Treat. 2020;180(3):819–27.
Zanuso V, Fregoni V, Gervaso L. Side effects of adjuvant chemotherapy and their impact on outcome in elderly breast cancer patients: a cohort study. Future Sci OA [electronic article]. 2020;6(9):FSO617. https://doi.org/10.2144/fsoa-2020-0076.
Zhao M, Sanz J, Rodríguez N, et al. Weekly radiotherapy in elderly breast cancer patients: a comparison between two hypofractionation schedules. Clin Transl Oncol. 2021;23(2):372–7.
Zhi X, Yang X, Pan T, et al. Correlation of radiotherapy with prognosis of elderly patients with hormone receptor-positive breast cancer according to immunohistochemical subtyping. Chin J Cancer Res. 2019;31(3):471–80.
Zhong Y, Xu Y, Zhou Y, et al. Omitting radiotherapy is safe in breast cancer patients ≥ 70 years old after breast-conserving surgery without axillary lymph node operation. Sci Rep. 2020;10(1):19481.
Zhong Y, Xu Y, Zhou Y, et al. Breast-conserving surgery without axillary lymph node surgery or radiotherapy is safe for HER2-positive and triple negative breast cancer patients over 70 years of age. Breast Cancer Res Treat. 2020;182(1):117–26.
Zhou M, Peddi P, Chu QD. Radiation therapy for positive surgical margins in women ≥70 years with stage I, estrogen receptor-positive breast cancer. Anticancer Res. 2018;38(9):5253–60.
De Vries NM, Staal JB, Van Ravensberg CD, et al. Outcome instruments to measure frailty: A systematic review. Ageing Res Rev. 2011;10(1):104–14.
Wang S, Yang T, Qiang W, et al. The prevalence of frailty among breast cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2022;30(4):2993–3006.
O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104.
Collard RM, Boter H, Schoevers RA, et al. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J Am Geriatr Soc. 2012;60(8):1487–92.
Baijal P, Periyakoil V. Understanding Frailty in Cancer Patients. The Cancer Journal. 2014;20(5):358–66.
Kim DH. Measuring Frailty in Health Care Databases for Clinical Care and Research. Ann Geriatr Med Res. 2020;24(2):62–74.
Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–60.
Anand A, Tew YY, Chan JH, et al. 29 Predicting Unplanned Readmission and Death After Hospital Discharge: How Do Frailty Tools Compare to Electronic Health Record Frailty Markers? Age Ageing. 2021;50(Supplement_1):i7–i11.
Brundle C, Heaven A, Brown L, et al. Convergent validity of the electronic frailty index. Age Ageing. 2019;48(1):152–6.
Oviedo-Briones M, Laso ÁR, Carnicero JA, et al. A Comparison of Frailty Assessment Instruments in Different Clinical and Social Care Settings: The Frailtools Project. J Am Med Dir Assoc. 2021;22(3):607.e7–607.e12.
Li D, Sun C-L, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021;7(11): e214158.
Soo WK, King MT, Pope A, et al. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open-label, randomised controlled trial. Lancet Healthy Longev. 2022;3(9):e617–27.
Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer–Alliance for Clinical Trials in Oncology-International Society Of Geriatric Oncology position article. J Clin Oncol. 2013;31(29):3711–8.
Acknowledgements
We would like to thank the liaison librarians at the University of Zu¨rich who helped to design the search strategy (Martina G. Eichenberger and Jacqueline Huber).
Declaration of generative AI in scientific writing
During the preparation of this work the author(s) used ChatGPT in order to provide suggestions for sentence structure, grammar, or vocabulary. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Funding
DM received funding by the University of Zürich Postdoc Grant, grant no. FK-22–053. The funding source played no role in the study's design, data collection, analysis, interpretation, or in the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
DNS: Conceptualization, Investigation, Data curation, Writing – original draft, Writing – review and editing, Visualization, Project administration. MGMD: Investigation, Writing – review and editing. JAV: Investigation, Writing – review and editing. DM: Investigation, Writing – review and editing. JEAP: Investigation, Writing – review and editing. FVdB: Investigation, Writing – review and editing. EB: Conceptualization, Investigation, Writing – review and editing, Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sanchez, D.N., Derks, M.G.M., Verstijnen, J.A. et al. Frequency of use and characterization of frailty assessments in observational studies on older women with breast cancer: a systematic review. BMC Geriatr 24, 563 (2024). https://doi.org/10.1186/s12877-024-05152-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05152-5