Abstract
Background
Atypical teratoid/rhabdoid tumor (AT/RT) is a malignant pediatric tumor of the central nervous system (CNS) with high recurrence and low survival rates that is often misdiagnosed. MicroRNAs (miRNAs) are involved in the tumorigenesis of numerous pediatric cancers, but their roles in AT/RT remain unclear.
Methods
In this study, we used miRNA sequencing and gene expression microarrays from patient tissue to study both the miRNAome and transcriptome traits of AT/RT.
Results
Our findings demonstrate that 5 miRNAs were up-regulated, 16 miRNAs were down-regulated, 179 mRNAs were up-regulated and 402 mRNAs were down-regulated in AT/RT. qPCR revealed that hsa-miR-17-5p and MAP7 mRNA were the most significantly differentially expressed miRNA and mRNA in AT/RT tissues. Furthermore, the results from analyses using the miRTarBase database identified MAP7 mRNA as a target gene of hsa-miR-17-5p.
Conclusions
Our findings suggest that the dysregulation of hsa-miR-17-5p may be a pivotal event in AT/RT and miRNAs that may represent potential therapeutic targets and diagnostic biomarkers.
Similar content being viewed by others
Background
Atypical teratoid/rhabdoid tumor is the most common malignant embryonal central nervous system tumor in children below 12 months of age; however, the incidence of AT/RT is not yet known and its incidence rate decreases with age [1, 2]. AT/RT was first identified as one of the embryo tumors that represent approximately 1–2% of pediatric intracranial tumors [3]. Because of the lack of clinical manifestation and radiography characteristics, the early clinical diagnosis of AT/RT remains challenging [4, 5]. The treatment options for AT/RT currently include surgical resection, chemotherapy and radiotherapy [6,7,8]. However, the prognosis for pediatric patients with AT/RT is still dismal, with a median survival of 15.4 months. Studies have shown that the majority of AT/RT patients show genomic mutations in SMARCB1 (also known as INI1) [9]. However, the precise pathogenesis of this disease is unclear. The identification of novel therapeutics based on the specific mechanism of AT/RT carcinogenesis is therefore critical.
Recent studies have shown that microRNAs (miRNAs) play a vital role in CNS tumorigenesis. MiRNAs, a subtype of small non-coding RNAs, regulate gene expression through recognizing and binding to seed sequence–matching sites in the 3' untranslated regions of target mRNAs [10,11,12]. MiRNAs are involved in the pathogenesis of human malignant tumors and function as oncogenes or tumor suppressors, depending on their downstream targets [13,14,15]. Previous studies have identified abnormal miRNA levels in patients with tumors in the CNS, indicating that miRNAs may play a key role in CNS tumor development [16]. However, knowledge of the miRNA expression profile of AT/RT patients is still limited.
Previous studies revealed that some miRNAs are deregulated in AT/RT, suggesting a role of miRNAs as oncogenes or tumor suppressors [17]. The miRNA expression signatures were found to be associated with bio-molecular and prognostic characteristics in AT/RT. Roles of miRNAs in the regulation of AT/RT, through destabilization or translational repression of target mRNAs, have been widely investigated. However, a comprehensive analysis of the miRNA expression profile in pediatric AT/RT has not yet been conducted.
In this study, we determined the miRNA expression profiles of pediatric AT/RT tumors by analyzing public datasets. Our results revealed that 5 miRNAs were significantly upregulated and 16 miRNAs were significantly downregulated in tumor tissue. Furthermore, we found that abnormal hsa-miR-17-5p expression may play key functions in the tumorigenesis in AT/RT and may represent potential targets for clinical treatment.
Methods
Differential expression analysis of GEO datasets
The datasets generated and/or analysed during the current study are available in the GSE42656 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42656) and GSE42657 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42657) repository. were processed by edgeR package in RStudio (version 3.5.0), with a significant cutoff |log2FC|> 2 and P-value < 0.01 [18]. The gene expression profile GSE42656 contains eight control and five AT/RT patients. The miRNA expression dataset is derived from GSE42657, which includes seven control and five tumor tissues. Detailed information of the datasets is listed in Table 1.
Functional analysis
Based on results from the differential expression analysis, we identified the related signaling pathways using Gene Ontology (GO) enrichment analysis. GO terms have three different modules: biological process (BP), molecular function (MF), and cellular component (CC). KEGG pathway analysis was used to identify the significant pathways for dysregulated mRNAs [19,20,21]. GO and KEGG analysis were both used in cluster profiler package in R studio [22]. The P-value was calculated for each enriched function and/or pathway.
Immunocyte infiltration annotation
We used the CIBERSORT approach to identify inflammatory gene expression signatures in silico to identify the characteristics of the immune response in AT/RT. CIBERSORT is a computational framework for high-throughput characterization of immune cells [23].
miRNA-mRNA pair analysis
We used miRTarBase to predict the target genes of the differentially expressed miRNAs (http://mirtarbase.mbc.nctu.edu.tw/) [24]. Differentially expressed genes (DEGs) were extracted and the putative miRNA-mRNA regulatory network was constructed using Cytoscape software (version 3.7.0). To validate the miRNA-mRNA network, we calculated the Pearson values and depicted the correlograms through R software. We evaluated the negative correlation between the key miRNA and target expression.
Reverse transcription quantitative real-time PCR (RT-qPCR)
We used a gene chip to analyze the gene expression profiles. cDNA fragments were purified with a PCR extraction kit (RR037A, Takara, China) following the manufacturer’s instructions and then enriched by RT-PCR. Total RNA was extracted using TRIzol reagent (Life Technologies, USA) and quantified using Thermo Nanodrop 2000. RNA (0.5 μg) was subjected to reverse transcription using the Script cDNA Synthesis Kit (RR037A, Takara, China). miRNA and mRNA primer sequences are listed in Tables 2 and 3.
Statistical analysis
Statistical analyses are presented as means ± SD. Comparison between two groups was performed using two-tailed Student’s t tests, and two-way ANOVAs with general linear model procedures using a univariate approach were applied for more than two groups. All statistical analyses were performed with GraphPad 8 Prism software (San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
Differential expression profiles for pediatric AT/RT
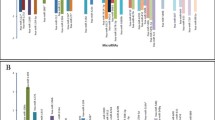
Using |log fold change|> 2 and P-value < 0.01 as a threshold, we identified a total of 581 DEGs in the tumor group compared with the control group. Among the DEGs, 179 were up-regulated and 402 were down-regulated (Fig. 1A, Supplementary Information.). In addition, 21 differentially expressed miRNAs (DEmiRNAs) were identified, including 5 up-regulated DEmiRNAs and 16 down-regulated DEmiRNAs (Table 4, Fig. 1B–C).
Differential gene expression analysis of pediatric atypical teratoid tumors. A Heat map depicting gene expression from 13 AT/RT cases and normal brain (columns; ordered automatically by hierarchical clustering). A gradient “heat spectrum” appears at the right; red indicates increased expression, whereas blue denotes decreased levels. B Heat map illustrating the expression of 50 mRNAs. C Heat map illustrating the expression of 21 differentially expressed miRNAs (fold change > 2 and P-value < 0.01)
GO enrichment analysis for DEGs
To identify the biological characteristics and signaling pathways involved in the pathogenesis of AT/RT, we next used Clusterprofile in R package to enrich DEGs. The enrichment results of the top 20 genes from the CC, MF and BP categories are shown in Fig. 2. The results indicated that many of the DEGs are closely involved in the formation of synapses. Molecular functions analysis indicated that DEGs were involved in binding to specific molecules, such as growth factor binding, calmodulin binding, and activity of passive membrane transporters. The DEGs were also involved in several critical biological progresses including the regulation of synaptic plasticity, modulation of chemical synaptic transmission, and transportation and secretion of the neurotransmitters, which are all involved in the regulation of nervous system plasticity.
GO enrichment analysis for DEGs in pediatric atypical teratoid tumors. A, C, E Barplots show the top 20 enrichment terms of CC, MF, and BP, respectively. Each bar represents a term, and the length represents the number of genes enriched. B, D, F Dotplots represent the top 20 enrichment results of CC, MF, and BP, respectively. The size of each point represents the number of genes enriched; the color represents the degree of enrichment
KEGG enrichment analysis for DEGs and the immune infiltration correlation of the expression profile
The KEGG signaling pathway results are shown in Fig. 3. DEGs are highly involved in synaptic function and neurotransmitter transmission. The top enriched pathways include the regulation of “Synaptic vesicle cycle”, “GABAergic synapse” and “Glutamatergic synapse,” which are consistent with the results of GO enrichment, indicating that impaired synaptogenesis and synaptic dysfunction could contribute to the formation and clinical manifestation of AT/RT. DEGs were also shown to modulate the “cAMP signaling pathway,” which could affect cell differentiation.
KEGG enrichment analysis for DEGs and the immune infiltration correlation of the expression profile in pediatric AT/RT. A Barplot shows the top 20 enrichment results from KEGG. The length represents the number of genes enriched. B Dotplot presents the top 20 enrichment results from KEGG. The size of each point represents the number of genes enriched. C Bar charts summarize immune cell subset proportions against AT/RT p-value by study
We also analyzed the correlation between the expression profile and immune infiltration pathways to identify the association between immune cell types and AT. The proportions of certain immune cells such as memory T cells, resting dendritic cells, neutrophils, and neutrophils were relatively lower in tumor tissues compared with levels in normal tissues. B cells, activated NK cells, and T follicular helper cells showed no difference between tumor tissues and normal tissues.
Construction of the AT/RT-associated miRNA-mRNA correlation and network
To clarify the potential roles of significantly dysregulated miRNAs and to further explore miRNA-mRNA regulatory mechanisms in AT/RT, we identified the potential targets of DEmiRNAs and the genes that were inversely co-expressed with DEmiRNAs using the previously shown gene expression profile. The 581 DEmRNAs and 21 mature DEmiRNAs were analyzed using the miRTarBase database (http://mirtarbase.mbc.nctu.edu.tw/). A total of 17 DEmiRNAs were found to negatively regulate at least one of the targets in DEmRNAs. Detailed information for each miRNA-mRNA targeting pair is shown in Table 5. The co-expression network of DEmiRNAs and DEmRNAs was constructed and visualized using Cytoscape software; the results are shown in Fig. 4. miR-17a-5p appeared to play the central role in the DEG network; therefore, miR-17A-5p was selected for further analysis.
Correlation analysis and network of AT/RT-associated miRNA-mRNA. A The correlation analysis of miR-17A-5p and 14 downregulated mRNAs by Pearson coefficient. B The subnetworks revealed the molecular pathways that were altered by miR-17a-5p. C The co-expression network of differentially expressed miRNAs and mRNAs was constructed
We next examined the regulatory relationship of miR-17a-5p. The subnetworks shown in Fig. 4 revealed the molecular pathways that were altered by miR-17a-5p. There were 15 mRNAs downregulated by miR-17a-5p. In addition, correlation analysis by Pearson coefficient revealed that KIF5C and DPYSL2 had the highest correlation with miR-17a-5p (Fig. 4, Table 6).
Validation of related miRNA expression levels in AT/RT using qRT-PCR
Previous studies have shown that miRNAs play a vital role in tumor progression in AT/RT. We next evaluated the performance of the seven candidate miRNAs (hsa-miR-17-5p, has-miR-18a-5p, hsa-miR-488-5p, hsa-miR-128-3p, hsa-miR-495-3p, hsa-miR-668-3p, hsa-miR-874-3p) in diagnosing AT/RT. The data demonstrated higher expression of miR-17-5p and miR-18a-5p in AT/RT compared expressions in with normal brain tissues by qPCR (Fig. 5). In addition, the expression of miR-874-3p was lower in AT/RT compared with levels in normal brain tissues.
The related miRNA expression level using quantitative real-time PCR in AT/RT. A–G The relative expression levels of hsa-miR-17-5p, has-miR-18a-5p, hsa-miR-488-5p, hsa-miR-128-3p, hsa-miR-495-3p, hsa-miR-668-3p, and hsa-miR-874-3p (*P < 0.05, compared with control; **P < 0.01, compared with control; ***P < 0.001 compared with control)
Verification for related mRNA expression levels using qRT-PCR
To investigate the potential function and underlying mechanism of miR-17-5p in AT/RT, we used bioinformatics algorithms and mRNA profiling from AT/RT patients to identify potential target genes of miR-17-5p. The binding of a miRNA to its target mRNA can induce translational silencing or degradation, leading to inhibition or enhancement of gene expression. Various studies have performed expression profiling to identify the roles of miRNAs in AT/RT. Our results indicated that MAP7, PRKCB, CDK1, PPP3R1, CCND1, HDAC1 and CDC20 mRNAs were differentially expressed in AT/RT (Fig. 6). Notably, our study showed that MAP7 plays an important regulatory role in AT/RT.
Discussion
AT/RT is an aggressive pediatric tumor of the CNS. Because of the limited available treatments and poor prognosis of AT/RT, there is an urgent need to identify novel therapeutic targets and develop innovative treatment strategies for this disease [25,26,27]. Mutations and/or deletions of the SMARCB1 (BAF47/INI1/SNF5) gene are hallmarks of AT/RT tumors, and so far no other recurrent genetic abnormalities have been identified [2, 28]. Previous studies showed that HMGA2, LIN28, RPL5, RPL10 and SUN2 are crucial regulators in AT/RT [29, 30]. However, the precise molecular mechanism of AT/RT remains largely unknown.
miRNAs play crucial roles in regulating gene expression at the transcriptional, post-transcriptional and epigenetic levels. Previous studies have established that miRNAs participate in a wide variety of biological processes including genomic imprinting, cell cycle, cell differentiation, invasion and migration [31,32,33,34]. Hsiehet et al. showed that miR-221/222 represents a promising new target in AT/RT [30]. Our multi-omics analysis identified five upregulated miRNAs (hsa-miR-301a-3p, hsa-miR-18a-5p, hsa-miR-335-3p, hsa-miR-18b-5p, hsa-miR-17-5p) and 16 downregulated miRNAs (hsa-miR-129–1-3p, hsa-miR-128-3p, hsa-miR-656-3p, hsa-miR-329-3p, hsa-miR-1224-5p, hsa-miR-668-3p, hsa-miR-488-5p, hsa-miR-29c-5p, etc.) in AT/RT. Hsa-miR-129–1-3p was the most-downregulated in AT/RT while hsa-miR-17-5p was the most up-regulated miRNA in AT/RT.
We further found that 179 mRNAs were up-regulated and 402 mRNAs were down-regulated, which could be the result of the dysregulated miRNA networks in AT/RT, as miRNAs regulate the levels and functions of their target mRNAs. GO analyses revealed that these mRNAs are involved in critical pathways such as the regulation of synaptic plasticity, modulation of chemical synaptic transmission, neurotransmitter transportation and secretion. KEGG pathway analysis showed that “Synaptic vesicle cycle,” “GABAergic synapse” and “Glutamatergic synapse” were related to the DEGs, which is consistent with GO enrichment analysis. These findings suggest that altered synaptogenesis and synaptic dysfunction could contribute to the formation and clinical manifestation of AT/RT. Additionally, DEGs were involved in the canonical pathways such as cAMP signaling pathway, which may contribute to the stemness of the AT/RT tumor cells.
Several recent studies have analyzed the influence of the host immune system on cancer prognosis [35]. We performed analyses using CIBERSORT, a computational method for high-throughput characterization of different types of immune cells in complex tissues. Our results demonstrated there was no difference in immune-related cells in AT/RT.
MiRTarBase database is a database that predict targets for miRNAs [36]. Seventeen DEmiRNAs were found to have at least one negatively regulated miRNA-mRNA pair in the DEmRNAs. Notably, over 30 mRNAs were predicted to be regulated by hsa-miR-17-5p. To further probe the negative correlations between hsa-miR-17-5p and its target mRNAs, we calculated the Pearson values using R software. A total of 15 mRNAs were negatively correlated with hsa-miR-17-5p. In addition to the protein–protein interaction networks constructed between DEmiRNAs and DEmRNAs, we also further verified the expression of hsa-miR-17-5p, hsa-miR-18a-5p, hsa-miR-488-5p, hsa-miR-128-3p, hsa-miR-495-3p, hsa-miR-668-3p, and hsa-miR-874-3p using qPCR [37]. These results further demonstrated the importance of hsa-miR-17-5p in AT/RT.
Zeng et al. previously reported that miRNA-17-5p expression is upregulated in glioblastoma and is a potential marker for the proneural subtype [38]. However, the mechanisms by which miRNA-17-5p expression regulates tumorigenesis are not well elucidated. We screened and identified possible targets of miRNA-17-5p and the results suggested that CCND1, THBS1, WEE1, SIRPA, SOX4, UBE2C, MDK, KIF5C, PTBP1, GPM6A, DPYSL2, PTTG1, TPRG1L, KIAA0513, SCAMP5, RAPGEF4, NRIP3, MAP7, RAB11FIP1, BTG3, MELK, TSPAN6, PEA15, PPP3R1, PGM2L1, LAMC1, IER3, GABBR1, CD47, and ABCA1 genes may play important roles in the pathogenesis of AT/RT. qPCR experiments verified that the expressions of MAP7, CDK1, PPP3R1, PRKC1, CCND1 and HDAC1 genes were indeed altered in AT/RT tumor tissue. Interestingly, CCND1, which encodes a crucial regulator of the cell cycle [39], was upregulated in AT/RT. We hypothesize that miRNA-17-5p promotes tumorigenesis in AT/RT by promoting CCND1 expression and cell cycle entry and progression. In addition, we reported that MAP7 mRNA showed the greatest down-regulation in AT/RT among all identified mRNAs. Together, these studies point to a potential role of miR-17-5p in AT/RT tumorigenesis.
This study has several limitations Future studies are required to determine the effect of miRNA-17-5p in AT/RT cell lines. RNA-seq and brain imaging using large-scale sample sizes would fully represent AT/RT tumorigenesis. Additional studies on the mechanism related to miRNA-17-5p will provide a better understanding of AT/RT therapy.
Conclusions
The results of this study indicate that upregulated/downregulated miRNAs and mRNAs have potential clinical value as prognostic biomarkers in AT/RT, in particular showing great potential as prognostic molecular markers. We demonstrated that miRNA-17-5p promotes tumorigenesis in AT/RT by promoting CCND1 expression and cell cycle entry and is associated with disease progression. To the best of our knowledge, this is the first study to find that the miRNA-17-5p/CCND1 axis regulates AT/RT. Further large scale cohort studies focusing on these miRNAs are recommended to verify the clinical utility of these markers individually and/or in combination. These findings provide a better understanding of the pathogenesis and development of AT/RT and may be an important implication for future therapy of the AT/RT.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the GSE42656 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42656) and GSE42657 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42657) repository.
Abbreviations
- AT/RT:
-
Atypical teratoid/rhabdoid tumor
- CNS:
-
Central nervous system
- qPCR:
-
Quantitative Real-time PCR
- KEEG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Ontology
- BP:
-
Biological process
- MF:
-
Molecular function
- CC:
-
Cellular component
- DEGs:
-
Differentially Expressed Genes
- RT-qPCR:
-
Reverse transcription quantitative Real-time PCR
- DEmiRNAs:
-
Differentially expressed miRNAs
References
Biegel JA, Zhou J-Y, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Can Res. 1999;59:74–9.
Biswas A, Kashyap L, Kakkar A, Sarkar C, Julka PK. Atypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer management and research. 2016;8:115.
Gigante L, Paganini I, Frontali M, Ciabattoni S, Sangiuolo FC, Papi L. Rhabdoid tumor predisposition syndrome caused by SMARCB1 constitutional deletion: prenatal detection of new case of recurrence in siblings due to gonadal mosaicism. Fam Cancer. 2016;15:123–6.
Morgenstern DA, Gibson S, Brown T, Sebire NJ, Anderson J. Clinical and pathological features of paediatric malignant rhabdoid tumours. Pediatr Blood Cancer. 2010;54:29–34.
Phi JH, Sun C-H, Lee S-H, Lee S, Park I, Choi SA, et al. NPM1 as a potential therapeutic target for atypical teratoid/rhabdoid tumors. BMC Cancer. 2019;19:1–12.
Paolini MA, Kipp BR, Sukov WR, Jenkins SM, Barr Fritcher EG, Aranda D, et al. Sellar Region Atypical Teratoid/Rhabdoid Tumors in Adults: Clinicopathological Characterization of Five Cases and Review of the Literature. J Neuropathol Exp Neurol. 2018;77:1115–21.
Wang R-f, Guan W-b, Yan Y, Jiang B, Ma J, Jiang M-w, et al. Atypical teratoid/rhabdoid tumours: clinicopathological characteristics, prognostic factors and outcomes of 22 children from 2010 to 2015 in China. Pathology. 2016;48:555–563.
Thatikunta M, Mutchnick I, Elster J, Thompson MP, Huang MA, Spalding AC, et al. Neoadjuvant chemotherapy for atypical teratoid rhabdoid tumors: case report. J Neurosurg Pediatr. 2017;19:546–52.
Alimova I, Pierce A, Danis E, Donson A, Birks DK, Griesinger A, et al. Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo. Int J Cancer. 2019;144:1983–95.
Hwang H, Mendell J. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776.
Nana-Sinkam S, Croce C. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104.
Ferreira HJ, Esteller M. Non-coding RNAs, epigenetics, and cancer: tying it all together. Cancer Metastasis Rev. 2018;37:55–73.
Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704.
Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27:5848.
Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–91.
Ahir BK, Elias NM, Lakka SS. SPARC overexpression alters microRNA expression profiles involved in tumor progression. Genes Cancer. 2017;8:453.
Sredni ST, Bonaldo Mde F, Costa FF, Huang CC, Hamm CA, Rajaram V, et al. Upregulation of mir-221 and mir-222 in atypical teratoid/rhabdoid tumors: potential therapeutic targets. Childs Nerv Syst. 2010;26:279–83.
McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–97.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–51.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics J Integr Biol. 2012;16:284–7.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453.
Hsu S-D, Lin F-M, Wu W-Y, Liang C, Huang W-C, Chan W-L, et al. miRTarBase: a database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2010;39:D163–9.
Eaton KW, Tooke LS, Wainwright LM, Judkins AR, Biegel JA. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56:7–15.
Gump JM, Donson AM, Birks DK, Amani VM, Rao KK, Griesinger AM, et al. Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun. 2015;3:30.
Zhang Y, Xu J. IDENTIFICATION OF microRNA-BASED PROGNOSTIC BIOMARKERS AND CANDIDATE THERAPEUTIC AGENTS FOR ATYPICAL TERATOID/RHABDOID TUMOR. Neuro Oncol. 2020;22:275–276.
Lechler MB. CRISPR/Cas9-mediated genome engineering of the SMARCB1 gene locus. Darmstadt. 2021;1:1–147
Ren Y, Tao C, Wang X, Ju Y. Identification of RPL5 and RPL10 as novel diagnostic biomarkers of Atypical teratoid/rhabdoid tumors. Cancer Cell Int. 2018;18:190.
Hsieh T-H, Chien C-L, Lee Y-H, Lin C-I, Hsieh J-Y, Chao M-E, et al. Downregulation of SUN2, a novel tumor suppressor, mediates miR-221/222-induced malignancy in central nervous system embryonal tumors. Carcinogenesis. 2014;35:2164–74.
Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–609.
Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet. 2012;81:303–11.
Sastre D, Baiochi J, de Souza Lima IM, Canto de Souza F, Corveloni AC, Thomé CH, et al. Focused screening reveals functional effects of microRNAs differentially expressed in colorectal cancer. BMC Cancer. 2019;19:1239.
Endzeliņš E, Berger A, Melne V, Bajo-Santos C, Soboļevska K, Ābols A, et al. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730.
Schmidt M, Böhm D, Von Törne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Can Res. 2008;68:5405–13.
Chou C-H, Chang N-W, Shrestha S, Hsu S-D, Lin Y-L, Lee W-H, et al. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2015;44:D239–47.
Lee Y-Y, Yang Y-P, Huang M-C, Wang M-L, Yen S-H, Huang P-I, et al. MicroRNA142-3p promotes tumor-initiating and radioresistant properties in malignant pediatric brain tumors. Cell Transplant. 2014;23:669–90.
Zeng A, Yin J, Wang Z, Zhang C, Li R, Zhang Z, et al. miR-17–5p-CXCL14 axis related transcriptome profile and clinical outcome in diffuse gliomas. Oncoimmunology. 2018;7:e1510277.
Gennaro VJ, Stanek TJ, Peck AR, Sun Y, Wang F, Qie S, et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci. 2018;115:E9298–307.
Acknowledgements
Not applicable.
Funding
This research was funded by grants from the National Natural Science Foundation Committee of China (No.81572497; 81873739; 82102690) and the Guangdong Provincial Department of Science and Technology, China (No.2017A030313487). Guangzhou Municipal Science and Technology Bureau, China (No.202002030197), Guangzhou Municipal Health Commission, and China (No.20201A011034).
Author information
Authors and Affiliations
Contributions
FCL conceived and designed the study. XKX, HYY, CC, and WC performed the experiments, analyzed the data and wrote the manuscript. YL, HYY, and PJP performed the analysis using bioinformatics. HYY and YL assisted in performing the research, and YL and PJP provided language help and assisted in analyzing data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Guangzhou Women and Children's Medical Center, and written informed consent was obtained from each patient (approval number: 201932200). All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, X., Yuan, H., Pan, J. et al. The identification of miRNA and mRNA expression profiles associated with pediatric atypical teratoid/rhabdoid tumor. BMC Cancer 22, 499 (2022). https://doi.org/10.1186/s12885-022-09549-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09549-6