Abstract
Purpose
The aim of this study is to search for new therapeutic targets for atypical teratoid–rhabdoid tumors (ATRT).
Methods
To achieve this, we compared the expression of 365 microRNAs among ATRT, medulloblastomas, and normal brain.
Results
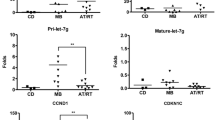
MiR-221 and miR-222 were within the top differentially expressed microRNAs. The deregulated expression of miR221/222 was demonstrated to inhibit the expression of the tumor suppressor and inhibitor of cell cycle p27Kip1. Here, we demonstrated the negative regulation of p27Kip1 by miR-221/222 in ATRT using microarray, real-time reverse transcriptase polymerase chain reaction, and immunohistochemistry.
Conclusion
As anti-miR therapy was recently proposed as an alternative treatment for cancer, these findings suggest that anti-miR-221/222 therapy might have therapeutic potential in ATRT.

Similar content being viewed by others
References
Strother D (2005) Atypical teratoid rhabdoid tumors of childhood: diagnosis, treatment and challenges. Expert Rev Anticancer Ther 5:907–915
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Wurdinger T, Costa FF (2007) Molecular therapy in the microRNA era. Pharmacogenomics J 7:297–304
Oyharcabal-Bourden V, Kalifa C, Gentet JC, Frappaz D, Edan C, Chastagner P, Sariban E, Pagnier A, Babin A, Pichon F, Neuenschwander S, Vinchon M, Bours D, Mosseri V, Le Gales C, Ruchoux M, Carrie C, Doz F (2005) Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. J Clin Oncol 23:4726–4734
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33(20):e179
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Judkins AR, Mauger J, Ht A, Roke LB, Biegel JA (2004) Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol 28:644–650
Ishizaki Y (2006) Control of proliferation and differentiation of neural precursor cells: focusing on the developing cerebellum. J Pharmacol Sci 101:183–188
Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, Rorke-Adams LB, Fisher MJ, Janss A, Mazewski C, Goldman S, Manley PE, Bowers DC, Bendel A, Rubin J, Turner CD, Marcus KJ, Goumnerova L, Ullrich NJ, Kieran MW (2009) Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 27:385–389
Koff A (2006) How to decrease p27Kip1 levels during tumor development. Cancer Cell 9:75–76
le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, Farace MG, Agami R (2007) Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J 26:3699–3708
Zhang C, Kang C, You Y, Pu P, Yang W, Zhao P, Wang G, Zhang A, Jia Z, Han L, Jiang H (2009) Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27kip1 in vitro and in vivo. Int J Oncol 34:1653–1660
Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG (2005) Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun 334:1351–1358
Gillies JK, Lorimer IA (2007) Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 6:2005–2009
Schiappacassi M, Lovat F, Canzonieri V, Belletti B, Berton S, Di Stefano D, Vecchione A, Colombatti A, Baldassarre G (2008) p27Kip1 expression inhibits glioblastoma growth, invasion, and tumor-induced neoangiogenesis. Mol Cancer Ther 7:1164–1175
Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, Negrini M (2008) MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27:5651–5661
Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM, Fusco A (2007) MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer 14:791–798
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafré SA, Farrace MG (2007) miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282:23716–23724
Mercatelli N, Coppola V, Bonci D, Miele F, Costantini A, Guadagnoli M, Bonanno E, Muto G, Frajese GV, De Maria R, Spagnoli LG, Farace MG, Ciafrè SA (2008) The inhibition of the highly expressed miR-221 and miR-222 impairs the growth of prostate carcinoma xenografts in mice. PLoS ONE 3(12):e4029
Acknowledgments
This project was supported by the Dr. Ralph and Marian C. Falk Medical Research Trust, the Rally Foundation for Childhood Cancer Research, and the Maeve McNicholas Memorial Foundation.
Ethical standards
This study has been approved by the Children’s Memorial Hospital Institutional Review Board (IRB#2009-13778) and had therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sredni, S.T., Bonaldo, M.d.F., Costa, F.F. et al. Upregulation of mir-221 and mir-222 in atypical teratoid/rhabdoid tumors: potential therapeutic targets. Childs Nerv Syst 26, 279–283 (2010). https://doi.org/10.1007/s00381-009-1028-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-009-1028-y