Abstract
Background
Histiocytic sarcoma is a rare disorder in humans, however it is seen with appreciable frequency in certain breeds of dogs, such as Bernese mountain dog. The purpose of this study was to fully characterize a novel canine histiocytic sarcoma cell line, and utilize it as a tool to screen for potential therapeutic drugs.
Methods
The histiocytic sarcoma cell line was characterized by expression of cellular markers as determined by immunohistochemistry and flow cytometry techniques. The neoplastic cells were also evaluated for their capability of phagocytizing beads particles, and their potential to grow as xenograft in an immunodeficient mouse. We investigated the in vitro cytotoxic activity of a panel of thirteen compounds using the MTS proliferation assay. Inhibitory effects of different drugs were compared using one-way ANOVA, and multiple means were compared using Tukey’s test.
Results
Neoplastic cells expressed CD11c, CD14, CD18, CD45, CD172a, CD204, MHC I, and vimentin. Expression of MHC II was upregulated after exposure to LPS. Furthermore, the established cell line clearly demonstrated phagocytic activity similar to positive controls of macrophage cell line. The xenograft mouse developed a palpable subcutaneous soft tissue mass after 29 days of inoculation, which histologically resembled the primary neoplasm. Dasatinib, a tyrosine kinase pan-inhibitor, significantly inhibited the growth of the cells in vitro within a clinically achievable and tolerable plasma concentration. The inhibitory response to dasatinib was augmented when combined with doxorubicin.
Conclusions
In the present study we demonstrated that a novel canine histiocytic sarcoma cell line presents a valuable tool to evaluate novel treatment approaches. The neoplastic cell line favorably responded to dasatinib, which represents a promising anticancer strategy for the treatment of this malignancy in dogs and similar disorders in humans.
Similar content being viewed by others
Background
Histiocytic sarcoma (HS) is a highly aggressive hematopoietic neoplasm in humans and animals. In humans, HS accounts for less than 1% of all hematopoietic neoplasms [1, 2], affects all ages, but predominately adults, and involves lymph nodes and/or a variety of extranodal organs including skin, bone marrow, spleen, the gastrointestinal tract and the central nervous system [3, 4]. This malignancy is often approached with a combination of various modalities of treatment, including multi-drug chemotherapeutic protocols, radiation therapy, surgery, and bone marrow transplantation [5,6,7]. However, patients with HS in general respond poorly to any form of treatment, therefore this neoplasm carries a poor prognosis and has a high mortality rate. The low number of clinical cases is a limitation for an extended understanding of the pathogenesis of this disease in people, and restricts the investigation for novel and more efficacious forms of treatment.
In dogs, a similar disorder is commonly found in certain breeds, especially the Bernese mountain dog (BMD), in which the prevalence ranges from 15 to 25% of the population [8,9,10,11,12]. The genetic susceptibility to HS in dogs has been the focus of several investigations. Current published data of a population of BMD from US and Europe, suggests deregulation of the tumor suppressor genes MTAP/CDKN2A/B located within the region homologous to human chromosome 9p21 [13, 14]. Studying HS in dogs is of high importance as, similarly to people, it is a fatal disease characterized by rapid progression and high metastatic rate [15,16,17,18]. Thus dogs, with spontaneously occurring HS, are a crucial model for development of new approaches to treat this orphan disease in people. Affected canine patients also respond poorly to treatment. The currently most effective drug is N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea (CCNU) that provides a reported response rate ranging from 29 to 46% for a median survival time of 85–96 days [16, 19]. A preliminary study using another cytotoxic drug, doxorubicin, showed a similar response rate of 46% for a median survival time of 93 days [20]. Additional alternatives of treatment have been investigated on small cohorts of dogs, including delivery of the human MHC non-restricted T-cell line TALL-104, frameless stereotactic radiosurgery, and chemotherapy using either paclitaxel or pegylated-liposomal doxorubicin; however, none has shown promising results [20,21,22,23,24].
Research groups have demonstrated the therapeutic potential of drugs as small molecule inhibitors against HS, based on in vitro studies [25,26,27]. Screening a large library of small molecules, Ito et al. successfully identified eight compounds of high potency (> 60% inhibition) at concentration of 100 nM [25]. Interestingly, only two drugs shared the same main target, showing the diversity of factors driving tumorigenesis in HS, and the potential benefit of drug combination hitting multiple targets. In humans, there is only a single report of four patients with HS treated with small molecule inhibitors, associated with the overexpression of targets for those drugs within the neoplasm, however efficacy could not be evaluated due to the small cohort of patients [28]. No published studies exist reporting the clinical use of small molecule inhibitors in dogs with HS.
We present here details of a novel HS cell line from a BMD, named the BD cell line, we have successfully established and demonstrated its utility in identification of potential novel therapeutic options [29]. Considering that to date, there is no human HS cell line available for research purposes, we believe that this cell line will provide an essential scientific tool for the study of this disease.
Methods
Origin of primary tumor
Fresh post-mortem tissue samples from both an abdominal and a pulmonary neoplasm were aseptically obtained from an 8-year-old spayed female BMD presented to the Michigan State University Veterinary Teaching Hospital. The diagnosis of HS was based on histopathology findings of the tumor and positive staining for CD18 by immunohistochemistry (Fig. 1) [8, 15, 30, 31].
Histopathology and immunohistochemistry analysis of the primary neoplastic tissue. Sections of lung stained with hematoxylin and eosin (H&E) with a well-demarcated region of neoplastic cells replacing the normal pulmonary parenchyma. Neoplastic cells presented as a highly pleomorphic population of malignant histiocytic cells with marked anisocytosis and anisokaryosis. The cells contained large amounts of basophilic and typically mildly vacuolated cytoplasm. Multinucleation, megalocytosis and megalokaryosis were common features. One to several prominent nucleoli were present in most nuclei. Perimembranous expression of CD18 was strongly detected by immunohistochemistry
Treatment with various chemotherapeutic agents was attempted over a year, including CCNU, doxorubicin and prednisone. The dog responded with only short periods of tumor remission until the owners opted for humane euthanasia due to the poor physical condition of the animal. Samples were collected immediately following euthanasia under informed owner consent.
Preparation and maintenance of cell culture
The tissue samples were minced in small fragments with a surgical blade and placed in a solution of Hank’s Balanced Salt Solution with 1% collagenase (Sigma, St. Louis, MO) for cell disaggregation. After 40 min, the solution was transferred to a 70 μm cell strainer, and the filtered portion was plated in Roswell Park Memorial Institute 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA), 1% antibiotic-antimycotic 100X (Life Technologies, Carlsbad, CA), and 0.1% gentamycin (Life Technologies, Carlsbad, CA). The cell culture was then incubated at 37 °C in a humidified atmosphere of 5% CO2. For culture maintenance, the medium was thoroughly changed every 2–3 days. The cells were confirmed to be of canine origin and no mammalian interspecies contamination was detected based on results from CellCheck Plus test (IDEXX BioResearch, Columbia, MO). A genetic profile using a panel of microsatellite markers for genotyping is available and can be used in future monitoring, the detailed genotype is presented in Additional file 1.
Cytology and immunohistochemistry
Concentrated cytospin slide of cell suspension was prepared, and stained with Diff-Quik for morphologic characterization by a board certified veterinary clinical pathologist. For immunohistochemistry analysis of the cell line, the cell pellet was fixed in 10% formalin for up to 24 h and transferred to 70% ethanol until embedding in paraffin. Sections from the paraffin block containing the cell pellet were deparaffinized in xylene and rehydrated in ethanol at different concentrations. Hydrogen peroxide 3% was used to neutralize endogenous peroxidases.
Antigen retrieval of formalin-fixed, paraffin embedded tissues was performed on the PT Link (Dako North America), using the EnVision FLEX Target Retrieval Solution, Low pH (Dako North America) for 20 min. For immunolabeling with the primary antibodies (CD3, CD18, CD79a and CD204), sections were processed with the Autostainer Link 48 (Dako North America, Carpinteria, CA) using the EnVision Flex+ detection system (Dako North America), and the immunoreaction was visualized with 3,3′-diaminobenzidine substrate (DAB) (Dako North America) and sections were counterstained with haematoxylin. For immunolabeling with antibody vimentin, the Discovery Ultra Automated Staining system (Ventana Medical Systems, Inc., Tucson, Arizona) was used with UltraMap alkaline phosphatase red detection system (Ventana Medical Systems, Inc) with a red chromogen. Antigen retrieval was achieved using the Ventana medical System antigen retrieval solution CC1 (Ventana Medical Systems, Inc., Tucson, Arizona) for 60 mins. Details of the antibodies are available in Additional file 2.
Flow cytometry
BD cells harvested from cell culture were labeled with the following monoclonal antibodies: anti-canine CD3 (CA17.2A12, Serotec), anti-canine CD11c (CA11.6A1, UC Davis/NIH NeuroMab Facility), anti-bovine CD14 (MM61A, WSU MAC), anti-canine CD21 (CA2.1D6, Serotec), anti-canine CD45 (YKIX716.13, Serotec), anti-canine CD79a (HM57, LS Bio), anti-bovine CD172a (DH59B, WSU MAC), anti-canine MHC II (YKIX334.2, eBioscience), and anti-bovine MHC I (MHC CL I, WSU MAC). Isotype matched control antibodies were used to exclude non specific binding. The flow cytometers BD LSR II and BD Accuri C6 (BD Bioscience, Bedford, MA) were used for analysis. For the stimulation studies, BD cells were treated with either LPS 50 ng/ml (Sigma Aldrich, St. Louis, MO) or IFNγ 50 ng/ml (Peprotech, Rocky Hill, NJ), for 24 and 48 h. Details of antibodies are listed in Additional file 2.
Phagocytosis assay
The phagocytic and endocytic properties of the established cell line were evaluated using 2% pHrodo™ E. coli Bioparticles® (Life Technologies, Carlsbad, CA). Using a 24-well plate, 100,000 cells were plated per well and left overnight. Culture medium was removed and replaced by 2% pHrodo™ E. coli Bioparticles® diluted in Live Cell Imaging Solution (Life Technologies, Carlsbad, CA) for 1.5–2 h before imaging. Confocal images were obtained using Leica TCS SPE confocal system (Leica Microsystems, Buffalo Grove, IL) on excitation wavelength of 460 nm. Commercially available murine macrophage cell line J774.A (ATCC® TIB-67™), a canine HS cell line DH82, derived from a macrophage derived sarcoma, hemophagocytic HS (ATCC® CRL-10389™), and canine fibroblasts isolated from the tunica albuginea were used for functional comparison purposes.
Neoplastic cell growth and characterization in a xenograft mouse
In order to evaluate the ability of the cells to form tumor in vivo, 1 × 106 neoplastic cells were injected into one ten-week old female mouse of NOD scid gamma strain (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, The Jackson Laboratory, Bar Harbor, ME). One million cells were suspended in 100 μl of Dulbecco’s Modified Eagle Medium (Life Technologies, Carlsbad, CA) with 10% FBS, and mixed with BD Matrigel™ Matrix HC in 1:1 ratio (BD Biosciences, San Jose, CA). The cell suspension was then inoculated subcutaneously into the left flank of the mouse under anesthesia.
The tumor growth in the inoculated mouse was monitored daily using calipers, until the tumor measured close to 10 mm in diameter as this was one of our humane endpoints. The mouse was sacrificed using carbon dioxide gas, and a full necropsy evaluated the presence of metastases into other organs. Tissues that had macroscopic changes were fixed in 10% formalin, routinely processed, and embedded in paraffin wax. Paraffin sections were stained with H&E for microscopic examination. For further characterization of the neoplasm, immunohistochemistry for CD18 was performed on paraffin sections.
Drug-screening assays
For the drug-screening assays, we used both the BD cell line, and the DH82 (CRL-10389™ - ATCC®) cell line, established from a golden retriever with hemophagocytic HS. In total, 13 drugs (Table 1) were tested from stock solutions prepared with the appropriate solvent as indicated, stored at − 20 °C, and protected from light. Serial dilutions of each drug were made from the stock solutions in culture medium immediately before adding to the cells in such a way that the solvent concentration was always < 1%. Each compound was tested at different concentrations in order to bracket the corresponding IC50 (concentration of drug necessary to inhibit the cell growth by 50%).
Viability assay and data analysis
For viability assay, DH82 and BD cell lines were seeded on 96-well plates with 7500 cells/well and 12,000 cells/well, respectively. After a 24-h incubation time, the cell culture medium was replaced with 100 μl of complete medium with drug, and cells were then incubated for 72 h. Subsequently, the viability of the cells was analyzed using a CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS) (Promega, Madison, WI). The formazan product was measured using EnVision® Multimode Plate Reader (PerkinElmer, Waltham, MA) at a wavelength of 490 nm. Each experiment was run in triplicate.
The background absorbance was subtracted from the absorbance values generated by the cells exposed to drugs. The effect on cell viability caused by each drug was calculated as follows: viability (%) = [1-(A-B)/(C-B)] × 100, where A is the response with drug, B is the background response with no drug, and C is the response with vehicle. The absorbance generated by the “cells alone” control was denoted as AbsIC100 (100%) and the absorbance generated by water control was denoted as AbsIC0 (0%). The calculated percentage at each (log10) drug concentration was then plotted using GraphPad Prism 5 software nonlinear regression curve fitting (PRISM 5, GraphPad Software, La Jolla, CA). The IC50 was determined as the drug concentration corresponding to the value of the mean between 0 and 100% viability. “Cells alone” control was treated with the vehicle DMSO 1%, the same concentration used to the cells exposed to the drugs.
Drug combination assays
Both HS cell lines were counted and seeded in 96-well plates as described in Viability assay and data analysis section. Each cell line was incubated with 5 different concentrations of dasatinib with or without doxorubicin. The concentrations of dasatinib were defined to bracket the IC50 for each cell line, and the concentration of doxorucibin was fixed with the concentration necessary to inhibit cell viability by 70%. Cell viability was measured by MTS proliferation assay after 72 h of incubation. Each experiment was run in triplicate.
Western blotting
BD cells were treated with either vehicle (0.1%) or dasatinib for 4 h. Cells were lysed using CellLytic M (Sigma-Aldrich) supplemented with 1% protease inhibitor and phosphatase cocktail inhibitor (P8340/P5726 – Sigma-Aldrich). A total of 40 μg of protein per lane were separated by Novex NuPAGE SDS-PAGE (4–12%), and transferred to polyvinylidene difluoride membrane. Membranes were blocked with 5% BSA in TBS buffer, and probed overnight with monoclonal rabbit anti-human antibodies against phospho-SRC (44-660G, Thermo Fisher) or SRC (2109, Cell Signaling), and monoclonal mouse anti-human β-actin (8H10D10, Cell Signaling). Secondary antibodies include IRDye 800CW goat anti-mouse and 680RD goat anti-rabbit (LI-COR). Infrared fluorescence was detected using Odyssey Imaging System (LI-COR), and analyzed using Image Studio™ Lite software (LI-COR).
Statistical analysis
Comparison of means of the cell viability rate among groups of different drug concentrations was done by one-way ANOVA, followed by Tukey’s multiple means comparison test. In order to determine whether the addition of dasatinib to doxorubicin increased the anti-proliferative effect, two-way ANOVA was utilized. Statistical analyses were performed using GraphPad Prism 5 software (PRISM 5, GraphPad Software, La Jolla, CA). Differences were considered statistically significant when p < 0.05.
Results
Characteristics of BD cell line
Neoplastic cells in culture grew satisfactorily in 10% FBS without the addition of specific growth factors with the vast majority of the population growing as adherent and non-clustering cells. BD cell line was maintained in tissue culture for a minimum of 50 passages over 12 months, with a doubling time of approximately 36 h. Cytospins stained with Diff-Quik from cells harvested from cell culture showed the presence of significant cellular pleomorphism, marked anisocytosis and anisokaryosis. Giant cells and multinucleation were frequently observed (Fig. 2).
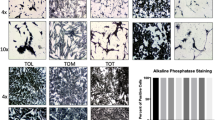
The established cell line showed lack of expression of both CD3 and CD79a, and positive expression of CD18, CD204 and vimentin on immunohistochemistry, consistent with features observed initially in the primary neoplasm (Fig. 3). The positive expression of myeloid marker CD45 and the lack of expression of lymphoid markers CD3, CD21 and CD79a were confirmed by flow cytometry. BD cell line expressed CD11c, CD14, CD172a and MHC I at a high level, and MHC II at a lower level (Fig. 4). MHC II expression was not affected by the stimulation with IFNγ, however it was significantly increased under the stimulation with LPS over time.
Immunohistochemistry of BD cell line showing strong expression of CD18, vimentin, and CD204, while being negative for CD3 and CD79a expression (left column). Middle and right columns are negatitve control and positive control sections, respectively. For CD18, CD204, CD3 and CD79a antibodies, DAB chromogen was used; and for vimentin antibody, alkaline phosphatase red chromogen. Calibration bar: 50 μm
Phagocytic properties of BD cell line
For the phagocytosis assays, the majority of cells from BD cell line were capable of phagocytizing the bioparticles, demonstrated by fluorescent intracellular signal on most of the cells (Fig. 5a). Both the murine macrophage cell line J774A.1 and the canine HS cell line DH82 demonstrated a high capability of phagocytosis, as expected (Fig. 5b and c). In contrast, we observed the presence of the bioparticles inside only a few canine fibroblasts (Fig. 5d).
Experiment with xenograft mouse
The xenograft mouse developed a palpable soft tissue mass of 5 mm of diameter detected at the injection site 29 days after injection. The tumor increased rapidly in size over 6 days until it reached 10 mm of diameter, when the mouse was humanely euthanized. A full necropsy revealed a large lobulated subcutaneous mass at the injection site measuring 25 × 15 × 10 mm, showed in more detail in Additional file 3. The neoplasm had locally invaded the musculature of the abdominal wall dorsally to the tumor. No other significant macroscopic changes were observed in other organs.
Evaluation of inhibitory effects of drugs on the growth of canine HS cells
The inhibition of cell growth by the drugs was variable between the two HS cell lines, BD and DH82. Dose-response curves were generated based on the cell viability at various concentrations of drug, from which IC50 values were determined. The results of IC50 (Table 2) values indicated that across 13 drugs tested, only dasatinib and doxorubicin were capable of inhibiting the growth of HS cells, within a pharmacologically relevant concentration. Both drugs exhibited statistically significant inhibitory effects on cell growth in a dose dependent manner using one-way ANOVA analysis (p < 0.0001). The IC50s of dasatinib were 10 and 9 nM, and of doxorubicin were 90 and 412 nM for BD and DH82 cell lines, respectively (Fig. 6). These concentrations were within the known tolerable plasma concentration values (Table 2). The maximum achievable plasma concentrations of the drugs were based on the plasma concentration values of the no-observed-adverse-effect level (NOAEL), or on the level of the therapeutic dosage described in the veterinary and human medicine literature. When available, the plasma concentration values encountered for dogs were used over those for humans.
Results of IC50 (nM) values of drugs for each HS cell line, and associated values of achievable plasma concentration described in the veterinary and human medicine literature.
Exploring synergistic combinations of drugs
We next assessed the resulting cytostatic effect caused by increasing concentrations of dasatinib combined to doxorubicin at a fixed concentration close to the correspondent cell line’s IC50. When BD cell line was treated with the drug combination for 72 h, there was no significant increase in the antiproliferative activity in comparison to the cells treated with dasatinib alone. On the other hand, dasatinib and doxorubicin combination significantly increased the inhibitory effect on the growth of DH82 cells (p = 0.0003) (Fig. 7).
Effect of dasatinib on SRC activity
The expression of total SRC and phopho-SRC was evaluated on cells treated with escalating concentrations of dasatinib. We observed a decrease in phospho-SRC but not in total SRC in both cell lines, BD and DH82, after 4 h of treatment. Expression of phosphor-SRC was minimal at the concentration of 100 nM of dasatinib (Fig. 8).
Discussion
The use of the naturally occurring histiocytic sarcoma in the dog as a translational model for human HS represents a valuable opportunity to further understand this malignancy, and to find better tools for treatment. In this article we have presented the initial characterization of a novel HS cell line - BD cell line - and investigated potential novel treatment approaches.
Histiocytic sarcomas are frequently associated with a high level of cellular pleomorphism, therefore, the definitive diagnosis is determined by a pattern of expression of markers specific for histiocytes. Although, several markers have been used to characterize human HS including CD163, CD68, CD11c, lysozyme, and CD14 [4, 32, 33], two different markers were validated for HS tumors in dogs: CD18 (integrin beta chain beta 2) and CD204 (class A macrophage scavenger receptor) [8, 15, 30, 31, 34]. The novel BD cell line showed positive expression of CD18 and CD204, consistent with the diagnosis of HS. The negative expression of CD3, CD21 and CD79a excluded lymphoid malignancies, while positive vimentin, a type III intermediate filament protein, confirmed the mesenchymal origin, and positive CD45 confirmed the myeloid origin. Interestingly, the stimulation of BD cells with LPS, a lipopolysaccharide component of Gram-negative bacteria walls, resulted in increased expression of MHC class II. A similar increase in MHC Class II expression was reported when murine dendritic cells were exposed to LPS, including CD14 positive dendritic cells [35, 36]. CD14 expression is mainly present in macrophages and monocytes, however it can also be found in DCs, where it has been proposed to play a role on the immunologic responses in DCs [37]. The same effect was not observed when the cells were stimulated with interferon-γ (IFNγ). Due to the fact that the majority of BD cells expressed MHC I and MHC II, we could confirm an antigen presenting cell profile. Both macrophages and DCs are antigen presenting cells that, despite their differences in biological functions, are only distinguished by a few surface markers. Candidate markers that have an increased specificity for DC and DC subsets have been identified in several studies [38,39,40,41]. Two DC-specific markers were tested in the present study, CD11c and CD172a, for which the vast majority of BD cells were positive. Together, these findings indicated a pattern of expression associated with a dendritic cell origin.
Dendritic cells are professional phagocytes that have an important role in processing antigens for adaptive immune recognition [42]. The majority of cells from the newly established cell line were able to phagocytize pHrodo E. coli bioparticles when coincubated for 2 h. In this assay, the bioparticles would only emit fluorescence once they are inside the phagosomes of the cells, where the pH is low. Therefore, this pH-sensitive fluorescent method permits a clear discrimination of where the particles are located in respect to the cells [43]. As a representation of positive controls for this assay, the two macrophage cell lines, J774.A and DH82, showed a high level of phagocytosis. In contrast, we could appreciate phagocytic activity in only a very small number of canine fibroblasts, which were used as negative control.
When transplanted to an immunodeficient xenograft mouse, BD cell line successfully grew as a palpable soft tissue mass after 29 days, a time-frame comparable to other tumor xenograft studies [44]. We were able to confirm that BD cell line is a tumorigenic cell line, and could potentially be useful for in vivo studies as an experimental tool to study histiocytic sarcoma. Further studies with a larger number of animals are warranted to truly establish the transplantability and metastatic potential. A few xenograft mouse models of canine HS have been successfully established and were used for the evaluation of their metastatic potential, and to study the phenomenon of resistance to CCNU-based treatment [45, 46]; however, none of these cell lines had originated from BMDs, the most frequently affected breed. To the best of our knowledge, across the many existent canine HS cell lines, only two others were reported to be originated from tumors of BMDs [47]; and these two were not part of their drug response report.
Results from the in vitro drug screening experiment showed a variable response between the different drugs, and across the cell lines. Across a total of 13 compounds, dasatinib and doxorubicin effectively inhibited the cell growth of both HS cell lines within a clinically tolerable and achievable plasma concentration according to the veterinary and human literature.
Doxorubicin was the only conventional chemotherapeutic drug that elicited a favorable response against the HS cell lines. Several studies have reported the use of doxorubicin for the treatment of histiocytic disorders, including HS [5, 6, 33, 48,49,50,51]. Due to the low number of cases, the effectiveness of doxorubicin could not be determined in those studies, especially as single agent, as the drug was invariably used in a drug combination protocol. Our results agree with previous in vitro studies that considered doxorubicin an effective drug against canine HS [20, 52]. The potential of doxorubicin for the treatment of dogs with HS was suggested by a preliminary clinical study, where the response to doxorubicin was comparable to the response to the drug with the best response reported to date, CCNU [20].
Our positive results with dasatinib are in accordance with results from a study by Ito et al., where dasatinib was effective against 4 out of 7 canine HS cell lines derived from various breeds. The calculated IC50 values from that study varied from 5.4 to 54.5 nM, while the average IC50 value in our study was 9.5 nM, clearly within their range [25]. Dasatinib is an multi tyrosine kinase inhibitor with multiple main targets, including the SRC family kinases (SRC, LCK, YES, and FYN), the BCR-ABL, and to a lesser extent, c-KIT, PDGFRβ and EPHA2 [53]. We demonstrated that dasatinib inhibited the activation of SRC, as revealed by decrease of p-SRC (phospho-SRC) in both cell lines, BD and DH82. We hypothesize that the downregulation of p-SRC could be associated with the inhibitory effect of dasatinib on the HS cell lines, however, as a multi-kinase inhibitor, many other molecular targets might also be affected. However, the SRC pathway is a major oncogenic driver involved in HS against which dasatinib and other novel compounds may be used. Although dasatinib has been investigated in many human cancers, its clinical therapeutic value for HS has never been documented. [54,55,56,57,58]. However, the use of other small inhibitors for the treatment of HS has been reported in one study where human patients were treated with imatinib, sorafenib and bevacizumab, based on the pattern of expression of key molecular targets [28]. Another set of studies reported two patients carrying a HS associated with a mutation in BRAFV600E gene, most commonly seen in melanomas in humans [50, 59]. Neoplasms that are driven by this mutation are suitable for vemurafenib-based treatment, a B-Raf small molecule inhibitor. In fact, a human patient from one of the studies was treated with vemurafenib and experienced therapeutic response for a couple of months [59]. Although the efficacy of these drugs could not be evaluated due to the small cohort of patients, the identification of druggable targets in HS shows the value of targeted therapy for this disease.
We demonstrated that the combination of dasatinib and doxorubicin resulted in a favorable additive effect against one of the two HS cell lines, DH82 cell line. The association of small inhibitors with chemotherapeutic drugs has been strategized as a treatment with broader spectrum, which resulted in synergistic anticancer activity for many tumors [26, 60, 61]. Often the increased efficiency is accompanied with a dose reduction of the drugs, and consequently decreased treatment related side effects. The combination of drugs with different mechanisms of action should be considered as a relevant strategy to optimize the therapeutic effect of each inhibitor against HS.
Conclusions
The present study was able to establish and fully characterize a DC-subtype HS cell line from a tumor in a BMD. This novel HS cell line represents a model available not only for the investigation of potential therapeutic drugs, but also for the studies of gene expression and genetic variability associated with HS. The existence of cancer cell lines has been a critical tool for the understanding of cancer biology and response to therapy. For that matter, panels of human cancer cell lines such as NCI60 and GDSC have become available for researches, so that the collective use of these resources would generate more efficiently information including genomics and drug sensitivity of these cell lines [62, 63]. Nevertheless, it is valuable to mention that cell lines are prone to genetic changes over time, and early passages should be a better model of the tumor of origin. More recently, a panel of canine cancer cell lines has been created covering the most important cancers seen in canine patients [64]. Due to the rarity of HS in humans, studies based on spontaneous HS in dogs can provide important and relevant translational understanding of this malignancy. Moreover, we also propose BD cell line as a useful system for studies where a cell line of dendritic cell origin can be of value.
Abbreviations
- BMD:
-
Bernese mountain dog
- CCNU:
-
N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea
- CD:
-
cluster of differentiation
- DAB:
-
3,3′-diaminobenzidine
- DC:
-
Dendritic cell
- H&E:
-
Hematoxylin and eosin
- HS:
-
Histiocytic sarcoma
- IFNγ:
-
Interferon-γ
- MHC:
-
Major histocompatibility complex
References
Jaffe ES. Histiocytoses of lymph nodes: biology and differential diagnosis. Semin Diagn Pathol. 1988;5(4):376–90.
Jaffe ES, World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001.
Soria C, Orradre JL, Garcia-Almagro D, Martinez B, Algara P, Piris MA. True histiocytic lymphoma (monocytic sarcoma). Am J Dermatopathol. 1992;14(6):511–7.
Vos JA, Abbondanzo SL, Barekman CL, Andriko JW, Miettinen M, Aguilera NS. Histiocytic sarcoma: a study of five cases including the histiocyte marker CD163. Mod Pathol. 2005;18(5):693–704.
Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: A pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88(2):253–71.
Soriano AO, Thompson MA, Admirand JH, Fayad LE, Rodriguez AM, Romaguera JE, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82(8):725–8.
Abidi MH, Tove I, Ibrahim RB, Maria D, Peres E. Thalidomide for the treatment of histiocytic sarcoma after hematopoietic stem cell transplant. Am J Hematol. 2007;82(10):932–3.
Abadie J, Hedan B, Cadieu E, De Brito C, Devauchelle P, Bourgain C, et al. Epidemiology, pathology, and genetics of histiocytic sarcoma in the Bernese mountain dog breed. J Hered. 2009;100(Suppl 1):S19–27.
Dobson J, Hoather T, McKinley TJ, Wood JL. Mortality in a cohort of flat-coated retrievers in the UK. Vet Comp Oncol. 2009;7(2):115–21.
Erich SA, Rutteman GR, Teske E. Causes of death and the impact of histiocytic sarcoma on the life expectancy of the Dutch population of Bernese mountain dogs and flat-coated retrievers. Vet J. 2013;198(3):678–83.
Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006;43(5):632–45.
Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48(10):1041–3.
Hedan B, Thomas R, Motsinger-Reif A, Abadie J, Andre C, Cullen J, et al. Molecular cytogenetic characterization of canine histiocytic sarcoma: a spontaneous model for human histiocytic cancer identifies deletion of tumor suppressor genes and highlights influence of genetic background on tumor behavior. BMC Cancer. 2011;11:201.
Shearin AL, Hedan B, Cadieu E, Erich SA, Schmidt EV, Faden DL, et al. The MTAP-CDKN2A locus confers susceptibility to a naturally occurring canine cancer. Cancer Epidemiol. 2012;21(7):1019–27.
Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39(1):74–83.
Rassnick KM, Moore AS, Russell DS, Northrup NC, Kristal O, Bailey DB, et al. Phase II, open-label trial of single-agent CCNU in dogs with previously untreated histiocytic sarcoma. J Vet Intern Med. 2010;24(6):1528–31.
Craig LE, Julian ME, Ferracone JD. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol. 2002;39(1):66–73.
Fidel J, Schiller I, Hauser B, Jausi Y, Rohrer-Bley C, Roos M, et al. Histiocytic sarcomas in flat-coated retrievers: a summary of 37 cases (November 1998-march 2005). Vet Comp Oncol. 2006;4(2):63–74.
Skorupski KA, Clifford CA, Paoloni MC, Lara-Garcia A, Barber L, Kent MS, et al. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Intern Med. 2007;21(1):121–6.
Higuchi T, Dervisis N, Kitchell B. Efficacy of doxorubicin for histiocytic sarcoma in dogs. Abstracts presented at the 30th Annual VCS Conference, San Diego, CA, USA, 29 October-1 November 2010. Vet Comp Oncol. 2010;9:e1-e49.
Visonneau S, Cesano A, Tran T, Jeglum KA, Santoli D. Successful treatment of canine malignant histiocytosis with the human major histocompatibility complex nonrestricted cytotoxic T-cell line TALL-104. Clin Cancer Res. 1997;3(10):1789–97.
Vail DM, Kravis LD, Cooley AJ, Chun R, MacEwen EG. Preclinical trial of doxorubicin entrapped in sterically stabilized liposomes in dogs with spontaneously arising malignant tumors. Cancer Chemother Pharmacol. 1997;39(5):410–6.
Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18(2):219–22.
Mariani CL, Schubert TA, House RA, Wong MA, Hopkins AL, Barnes Heller HL, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol. 2013;13(4):409–23.
Ito K, Kuroki S, Kobayashi M, Ono K, Washizu T, Bonkobara M. Identification of dasatinib as an in vitro potent growth inhibitor of canine histiocytic sarcoma cells. Vet J. 2013;196(3):536–40.
Thamm DH, Rose B, Kow K, Humbert M, Mansfield CD, Moussy A, et al. Masitinib as a chemosensitizer of canine tumor cell lines: a proof of concept study. Vet J. 2012;191(1):131–4.
Kisseberth WC, Murahari S, London CA, Kulp SK, Chen CS. Evaluation of the effects of histone deacetylase inhibitors on cells from canine cancer cell lines. Am J Vet Res. 2008;69(7):938–45.
Schlick K, Aigelsreiter A, Pichler M, Reitter S, Neumeister P, Hoefler G, et al. Histiocytic sarcoma - targeted therapy: novel therapeutic options? A series of 4 cases. Onkologie. 2012;35(7–8):447–50.
Takada M, Parys M, Gregory-Bryson E, Yuzbasiyan-Gurkan V. A novel canine histiocytic sarcoma cell line provides a potential path to effective treatments with relevance for translational and comparative studies in humans. Cancer Res. 2014;74(19 Supplement):3931.
Klahn SL, Kitchell BE, Dervisis NG. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J Am Vet Med Assoc. 2011;239(1):90–6.
van Kuijk L, van Ginkel K, de Vos JP, Brearley MJ, Butinar J, Gielen I, et al. Peri-articular histiocytic sarcoma and previous joint disease in Bernese Mountain dogs. J Vet Intern Med. 2013;27(2):293–9.
Skoog L, Tani E. Histiocytic and dendritic neoplasms. Monogr Clin Cytol. 2009;18:56–9.
Lauritzen AF, Delsol G, Hansen NE, Horn T, Ersboll J, Hou-Jensen K, et al. Histiocytic sarcomas and monoblastic leukemias. A clinical, histologic, and immunophenotypical study. Am J Clin Pathol. 1994;102(1):45–54.
Kato Y, Murakami M, Hoshino Y, Mori T, Maruo K, Hirata A, et al. The class a macrophage scavenger receptor CD204 is a useful immunohistochemical marker of canine histiocytic sarcoma. J Comp Pathol. 2013;148(2–3):188–96.
Casals C, Barrachina M, Serra M, Lloberas J, Celada A. Lipopolysaccharide up-regulates MHC class II expression on dendritic cells through an AP-1 enhancer without affecting the levels of CIITA. J Immunol. 2007;178(10):6307–15.
Mahnke K, Becher E, Ricciardi-Castagnoli P, Luger TA, Schwarz T, Grabbe S. CD14 is expressed by subsets of murine dendritic cells and upregulated by lipopolysaccharide. Adv Exp Med Biol. 1997;417:145–59.
Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460(7252):264–8.
Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkotter HJ. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur J Immunol. 2005;35(5):1418–27.
Mansouri-Attia N, Oliveira LJ, Forde N, Fahey AG, Browne JA, Roche JF, et al. Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle. Biol Reprod. 2012;87(5):123.
Kaisho T. Pathogen sensors and chemokine receptors in dendritic cell subsets. Vaccine. 2012;30(52):7652–7.
Schutz F, Hackstein H. Identification of novel dendritic cell subset markers in human blood. Biochem Biophys Res Commun. 2014;443(2):453–7.
Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–56.
Beletskii A, Cooper M, Sriraman P, Chiriac C, Zhao L, Abbot S, et al. High-throughput phagocytosis assay utilizing a pH-sensitive fluorescent dye. BioTechniques. 2005;39(6):894–7.
Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2(2):247–50.
Thongtharb A, Uchida K, Chambers JK, Nakayama H. Variations in histiocytic differentiation of cell lines from canine cerebral and articular histiocytic sarcomas. Vet Pathol. 2017;54(3):395–404.
Yamazaki H, Takagi S, Hosoya K, Okumura M. Survivin suppressor (YM155) enhances chemotherapeutic efficacy against canine histiocytic sarcoma in murine transplantation models. Res Vet Sci. 2015;99:137–44.
Azakami D, Bonkobara M, Washizu T, Iida A, Kondo M, Kato R, et al. Establishment and biological characterization of canine histiocytic sarcoma cell lines. J Vet Med Sci. 2006;68(12):1343–6.
Soslow RA, Davis RE, Warnke RA, Cleary ML, Kamel OW. True histiocytic lymphoma following therapy for lymphoblastic neoplasms. Blood. 1996;87(12):5207–12.
Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the international lymphoma study group based on 61 cases. Histopathology. 2002;41(1):1–29.
Michonneau D, Kaltenbach S, Derrieux C, Trinquand A, Brouzes C, Gibault L, et al. BRAFV600E mutation in a histiocytic sarcoma arising from hairy cell leukemia. J Clin Oncol. 2014;32(35):e117-21.
Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004;28(9):1133–44.
Hafeman SD, Varland D, Dow SW. Bisphosphonates significantly increase the activity of doxorubicin or vincristine against canine malignant histiocytosis cells. Vet Comp Oncol. 2012;10(1):44–56.
Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29(11):2289–308.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123–9.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70.
Benjamini O, Dumlao TL, Kantarjian H, O'Brien S, Garcia-Manero G, Faderl S, et al. Phase II trial of hyper CVAD and dasatinib in patients with relapsed Philadelphia chromosome positive acute lymphoblastic leukemia or blast phase chronic myeloid leukemia. Am J Hematol. 2014;89(3):282–7.
Spreafico A, Chi KN, Sridhar SS, Smith DC, Carducci MA, Kavsak P, et al. A randomized phase II study of cediranib alone versus cediranib in combination with dasatinib in docetaxel resistant, castration resistant prostate cancer patients. Investig New Drugs. 2014;32(5):1005–16.
Safdari Y, Khalili M, Farajnia S, Asgharzadeh M, Yazdani Y, Sadeghi M. Recent advances in head and neck squamous cell carcinoma - a review. Clin Biochem. 2014;47(13–14):1195–202.
Idbaih A, Mokhtari K, Emile JF, Galanaud D, Belaid H, de Bernard S, et al. Dramatic response of a BRAF V600E-mutated primary CNS histiocytic sarcoma to vemurafenib. Neurology. 2014;83(16):1478–80.
Gleixner KV, Rebuzzi L, Mayerhofer M, Gruze A, Hadzijusufovic E, Sonneck K, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Exp Hematol. 2007;35(10):1510–21.
Robat C, London C, Bunting L, McCartan L, Stingle N, Selting K, et al. Safety evaluation of combination vinblastine and toceranib phosphate (palladia(R)) in dogs: a phase I dose-finding study. Vet Comp Oncol. 2012;10(3):174–83.
Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6(10):813–23.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7.
Fowles JS, Dailey DD, Gustafson DL, Thamm DH, Duval DL. The Flint animal cancer center (FACC) canine tumour cell line panel: a resource for veterinary drug discovery, comparative oncology and translational medicine. Vet Comp Oncol. 2016;15(2):481–92.
Doriano F. 25 years of small molecular weight kinase inhibitors: potentials and limitations. Mol Pharmacol. 2014;87(5):766–75.
Sun L, Tran N, Liang C, Hubbard S, Tang F, Lipson K, et al. Identification of substituted 3-[(4,5,6, 7-tetrahydro-1H-indol-2-yl)methylene]-1,3-dihydroindol-2-ones as growth factor receptor inhibitors for VEGF-R2 (Flk-1/KDR), FGF-R1, and PDGF-Rbeta tyrosine kinases. J Med Chem. 2000;43(14):2655–63.
Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–7.
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–6.
Lee FY, Workman P, Roberts JT, Bleehen NM. Clinical pharmacokinetics of oral CCNU (lomustine). Cancer Chemother Pharmacol. 1985;14(2):125–31.
Saven A, Piro L. Newer purine analogues for the treatment of hairy-cell leukemia. N Engl J Med. 1994;330(10):691–7.
Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258.
Kamath AV, Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008;61(3):365–76.
Scientific discussion for the approval of Erlotinib [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000618/WC500033991.pdf]. Accessed 28 Feb 2017.
Assessment report for Iressa [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001016/WC500036361.pdf]. Accessed 28 Feb 2017.
Scientific discussion for the approval of Imatinib [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000406/WC500022203.pdf]. Accessed 28 Feb 2017.
Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, Legendre A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22(6):301–9.
Scientific discussion for the approval of Nilotinib [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000798/WC500034398.pdf]. Accessed 28 Feb 2017.
Zavodovskaya R, Liao AT, Jones CL, Yip B, Chien MB, Moore PF, et al. Evaluation of dysregulation of the receptor tyrosine kinases kit, Flt3, and met in histiocytic sarcomas of dogs. Am J Vet Res. 2006;67(4):633–41.
Blanchet B, Billemont B, Cramard J, Benichou AS, Chhun S, Harcouet L, et al. Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal. 2009;49(4):1109–14.
Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13(5):1367–73.
Traynor AM, Hewitt M, Liu G, Flaherty KT, Clark J, Freedman SJ, et al. Phase I dose escalation study of MK-0457, a novel aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67(2):305–14.
Scientific discussion for the approval of Cladribine [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000504/WC500041660.pdf]. Accessed 28 Feb 2017.
Gustafson DL, Thamm DH. Pharmacokinetic modeling of doxorubicin pharmacokinetics in dogs deficient in ABCB1 drug transporters. J Vet Intern Med. 2010;24(3):579–86.
Acknowledgements
We thank Marc Baillie, DVM, PhD for helpful suggestions. We thank the Berner-Garde Foundation for their efforts to advance research on disorders affecting Bernese mountain dogs.
Funding
Michigan State University College of Veterinary Medicine Endowed Research Funds, including the Foster-Smith Oncology Fund, were used for the research. MT was supported in part by CVM Endowed Research Funds and Michigan State University Graduate School Fellowship Funds. EGB was supported in part by the NIH T32 RR018411 post-doctoral fellowship grant from NIH. The funding bodies did not have any role in the design, conduct of the study or in analysis of the results or the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
MT, MP and VYG designed the study. PVS’s provided the tumor sample and clinical characterization and care of the affected dog. EGB contributed to sample collection, cells isolation and culture. MK provided histological interpretation. MT and VYG drafted the manuscript, and all authors contributed critically, revised and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
The xenograft study described in the present paper was approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC) (AUF# 07/12–127-00). Tumor tissue was used to establish the cell line from a client owned dog at post-mortem examination, with owner’s written consent. The use of left over specimens was approved by IACUC, through an IACUC exemption form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Genetic profile of BD cell line genotyped using of a panel of microsatellite markers. (XLSX 31 kb)
Additional file 2:
Description of primary and secondary antibodies. (XLSX 27 kb)
Additional file 3:
Xenograft tumor in a mouse. On necropsy, a large lobulated subcutaneous mass was present at the site of injection of tumor cells 35 days after transplantation (black arrow). (TIFF 7833 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Takada, M., Parys, M., Gregory-Bryson, E. et al. A novel canine histiocytic sarcoma cell line: initial characterization and utilization for drug screening studies. BMC Cancer 18, 237 (2018). https://doi.org/10.1186/s12885-018-4132-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4132-0