Summary
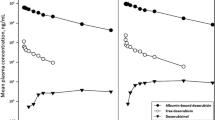
The plasma pharmacokinetics of orally administered CCNU (130 mg/m2) were studied in four patients using reversed-phase high-performance liquid chromatography (HPLC) analysis. Parent CCNU was not detected in the plasma of any of the patients, probably due to complete conversion to monohydroxylated metabolites during the ‘first pass’ through liver and gut. However, two monohydroxylated metabolites, trans-4-hydroxy CCNU and cis-4-hydroxy CCNU, were found at high concentrations, the relative amounts being about 6 : 4. Peak concentrations of the metabolites were reached 2–4 h after administration and were remarkably similar for all four patients, the total being 0.8–0.9 μg/ml. The metabolites were also detected in a tumour biopsy. Plasma clearance half-lives of the two metabolites were similar in each patient but showed a two-fold variation between patients, from 1.3 to 2.9 h. These results suggest that the antitumour activity and systemic toxicity of CCNU when given orally are due mainly to its monohydroxylated metabolites. Finally, comparison with data obtained in vitro and in mice showed that the nitrosourea exposures in these patients were at the lower limit of those required for significant antineoplastic activity.
Similar content being viewed by others
References
Ames MM, Powis G (1983) Pharmacokinetics of nitrosoureas. In: Ames MM, Powis G, Kovach JS (eds) Pharmacokinetics of anticancer agents in humans. Elsevier, Amsterdam, pp 113–134
Erickson LC, Bradley MO, Ducore JM, Ewig RAG, Kohn KW (1980) DNA crosslinking and cytotoxicity in normal and transformed human cells treated with antitumour nitrosoureas. Proc Natl Acad Sci USA 77: 467
Hilton J, Walker MD (1975) Hydroxylation of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Biochem Pharmacol 24: 2153
Kari P, McConnell WR, Finkel JM, Hill DL (1980) Distribution of Bratton-Marshall-positive material in mice following intravenous injections of nitrosoureas. Cancer Chemother Pharmacol 4: 243
Lee FYF, Workman P (1983) Modification of CCNU pharmacokinetics by misonidazole — A major mechanism of chemosensitisation in mice. Br J Cancer 47: 659
Lee FYF, Workman P (1984a) Misonidazole and CCNU: Further evidence for a pharmacokinetic mechanism of chemosensitisation and therapeutic gain. Br J Cancer 49: 579
Lee FYF, Workman (1984b) Nitroimidazoles as modifiers of nitrosourea pharmacokinetics. Int J Radiat Oncol Phys (in press)
Levin VA (1981) Clinical pharmacology of nitrosoureas. In: Prestakyo AW, Crooke ST, Baker LH, Carter SK, Schein PS (eds) Nitrosoureas, current status and new developments. Academic, New York, pp 171–180
Levin VA, Stearns J, Byrd A, Weinkam RJ (1979) The effect of phenobarbital pretreatment on the antitumour activity of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) and 1-(2-chloroethyl)-3-(2,6-dioxo-3-piperidyl-1-nitrosourea (PCNU), and on plasma pharmacokinetics and biotransformation of CCNU. J Pharmacol Exp Ther 208: 1
Loo TL, Dion RL (1965) Colorimetric method for the determination of 1,3-bis-(2-chloroethyl)-1-nitrosourea. J Pharm Sci 54: 809
Montgomery JA, Johnson PJ, Thomas HJ, Piper JR, Temple CJr (1977) The use of microparticulate reversed-phase packing in high-pressure liquid chromatography of compounds of biological interest. Adv Chromatogr 15: 169
Mulcahy RT, Dembs NL, Ublacker GA (1984) Enhancement of nitrosourea cytotoxicity by misonidazole in vitro: Correlation with carbamoylating potential. Br J Cancer 49:307
Muller PJ, Tator CH, Bloom ML (1978) The effect of phenobarbital on the toxicity and tumoricidal activity of 1-(2-chloroethyl)-1-nitrosourea (CCNU) in a murine brain tumour model. J Neurosurg 49: 579
Muller PJ, Tator CH, Bloom ML (1983) Use of phenobarbital and high doses of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea in the treatment of brain tumour-bearing mice. Cancer Res 43: 2068
Oliverio VT, Vietzke WM, Williams MK, Adamson RH (1970) The absorption, distribution, excretion, and biotransformation of the carcinostatic 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea in animals. Cancer Res 30: 1330
Reed DJ (1981) Metabolism of nitrosoureas. In: Prestayko AW, Crooke ST, Baker LH, Caster SK, Schein P (eds) Nitrosoureas: Current status and new developments. Academic, London, pp 51–67
Reeve JR, Wright KA, Twentyman PR (1983) Response to X-radiation and cytotoxic drugs of clonal subpopulations of different ploidy and metastatic potential isolated from RIF-1 mouse sarcoma. Br J Cancer 47: 841
Schabel FM (1976) Nitrosoureas: a review of experimental antitumour activity. Cancer Treat Rep 60: 665
Selker RG, Moore P, LoDolce D (1978) Bone-marrow depression with cimetidine plus carmustine. N Engl J Med 299: 834
Siemann DW (1981) In vivo combination of misonidazole and the chemotherapeutic agent CCNU. Br J Cancer 43: 367
Siemann DW (1982) Response of murine tumours to combinations of CCNU with misonidazole and other radiation sensitizers. Br J Cancer 45: 272
Siemann DW (1983) Effect of pretreatment with phenobarbital or SKF 525A on the toxicity and antitumour activity of lomustine. Cancer Treat Rep 67: 259
Siemann DW, Morrissey S, Wolf K (1983) In vivo potentiation of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea by the radiation sensitizer benznidazole. Cancer Res 43: 1010
Skipper HE, Schabel FM Jr, Mellett LB, Montgomery JA, Wilkoff LJ, Lloyd HH, Brockman RW (1970) Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep 54: 431
Sponzo RW, DeVita VT, Oliverio VT (1973) Physiologic disposition of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) and 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (meCCNU) in man. Cancer 31: 1154
Steel GG, Courtenay VD, Peckham M (1983) The response to chemotherapy of a variety of human tumour xenografts. Br J Cancer 47: 1
Twentyman PR, Bleehen NM (1975) Changes in sensitivity to cytotoxic agents occuring during the life history of monolayer cultures of a mouse tumour cell line. Br J Cancer 31: 417
Twentyman PR, Workman P (1983) Chemosensitization by lipophilic nitroimidazoles. Br J Cancer 48: 17
Walker MD, Hilton J (1976) Nitrosourea pharmacodynamics in relation to the central nervous system. Cancer Treat Rep 60: 725
Weiss RB, Issell BF (1982) The nitrosoureas carmustine (BCNU) and lomustine (CCNU). Cancer Treat Rep 9: 313
Wheeler GP, Johnston TP, Bowdon BJ, McCaleb GS, Hill DL, Montgomery JA (1977) Comparison of the properties of metabolites of CCNU. Biochem Pharmacol 26: 2331
Wheeler KT, Levin VA, Deen DF (1978) The concept of drug dose for in vitro studies with chemotherapeutic agents. Radiat Res 76: 441
Workman P, Twentyman P (1982) Structure/activity relationships for the enhancement by electron-affinic drugs of the antitumour effect of CCNU. Br J Cancer 46: 249
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, F.Y.F., Workman, P., Roberts, J.T. et al. Clinical pharmacokinetics of oral CCNU (Lomustine). Cancer Chemother. Pharmacol. 14, 125–131 (1985). https://doi.org/10.1007/BF00434350
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00434350