Abstract
Background
This study aimed to evaluate the predictive power of a model combining maternal risk factors and the Quadruple screen test for late-onset preeclampsia (PE).
Methods
All pregnant women that received the Quadruple test for Down syndrome at 15+ 0-20+ 6 weeks’ gestation were recruited. Maternal serum α-fetoprotein, β-human chorionic gonadotropin, unconjugated estriol, and inhibin A were measured as multiples of the median. A logistic regression model was used to identify predictors associated with late-onset PE with severe features. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to assess the model’s predictive ability.
Results
Fifty-five of the 2,000 pregnant women had PE, and 31 of 55 women had late-onset PE. Multivariate analysis identified maternal age ≥ 35 years, inhibin A, history of previous PE, history of infertile, cardiac disease, chronic hypertension, and thyroid disease as significant risk factors. The area under the curve of the receiver operating characteristic curve was 0.78. The likelihood ratio to predict late-onset PE was 49.4 (total score > 60).
Conclusions
Our model combining serum inhibin A with maternal risk factors was useful in predicting late-onset PE. Close monitoring of these patients is recommended.
Similar content being viewed by others
Background
Preeclampsia (PE) is a complication that affects 2–8% of all pregnancies and is an important cause of maternal and perinatal morbidity and mortality worldwide [1, 2]. Over the past decade, it has become widely accepted that the pathogenesis of early-onset and late-onset PE differs [3,4,5,6,7,8], with early-onset PE (before 34 weeks) having a compromised terminal villi volume and surface area, resulting in a smaller and higher infarction of the placenta when compared with late-onset PE. For late-onset PE the thinking is that it results from an imbalance between the cardiovascular supply of pregnant woman and the metabolic demands of the fetuses, and their placenta in the third trimester period. Therefore, early-onset PE is more commonly associated with more severe adverse maternal and neonatal outcomes than late-onset PE [4, 9, 10]. Several studies have investigated first trimester gestation to predict early-onset PE via maternal characteristics, Doppler ultrasound, and maternal serum biochemical substances [11,12,13,14,15,16,17,18,19,20,21]. Early detection can identify the requirement for early prescription of low-dose aspirin, for high-risk pregnant patients before 16 weeks’ gestation. This is in accordance with the standard recommendations for the prevention of PE proposed by the National Institute for Health and Care Excellence (NICE) [22], the American College of Obstetricians and Gynecologists (ACOG) [23], and the International Federation of Gynecology and Obstetrics (FIGO) [24].
Although early-onset PE is associated with more severe maternal and perinatal.
morbidity and mortality, the vast majority of PE cases are late-onset type [25, 26]. Our institute, Songklanagarind Hospital, a referral center in southern Thailand, has a total PE incidence of 3.63%, with 1.26% and 2.37% being early- and late-onset type, respectively [27]. The morbidity and mortality associated with late-onset PE patients more commonly affect mothers than neonates. In low- and middle-income countries, where access to facilities such as specialized medical care and transportation is often limited, 10–15% of direct maternal deaths are associated with PE with severe features and eclampsia [28, 29]. Moreover, severe morbidities, including renal failure, stroke, cardiac arrest, adult respiratory distress syndrome, coagulopathy, and hepatic failure, have been reported in these patients [30, 31]. Although low-dose aspirin might not have a preventive effect on late-onset PE [23, 24], the identification of high-risk patients will result in benefits from close monitoring and early diagnosis and management in order to reduce maternal morbidity and mortality. Over the past decade, several studies have demonstrated the predictive power of combined models for late-onset PE prediction using maternal risk factors, mean arterial pressure, pregnancy-associated plasma protein A, placental growth factor, and uterine artery pulsatility index. These screening models reported a detection rate for late-onset PE of around 33.5–41.3% in the first trimester. However, there are two major concerns when using these tests in low- and middle-income countries; firstly, these tests are often beyond the capacity or accessibility of health services, and secondly, most women do not receive prenatal care until their second trimester or later in their pregnancy [32].
In Thailand, the Quadruple (Quad) test is widely used for Down syndrome screening in the second trimester of gestation. The test consists of four biochemical hormones, namely inhibin A, alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), and unconjugated estriol (uE3). Several previous studies [33,34,35,36,37,38,39] have reported that abnormal Quad test results could predict the detection of high-risk PE patients. Although abnormal serum levels in the Quad test might be associated to PE, neither abnormal serum levels nor maternal risk factors alone resulted in a high detection rate of late-onset PE [22,23,24, 27, 32].
To our knowledge, no previous studies have used both maternal risk factors and the serum Quad test to improve the detection of PE patients, especially in those with late-onset type, in low- and middle-income countries with limited access to high-technology healthcare, funds, and specialists. Therefore, this study aimed to evaluate the efficacy of combined serum biochemical hormones (the Quad test) and maternal risk factors during the second trimester of pregnancy in predicting late-onset PE.
Methods
This retrospective cohort study was conducted between January 2015 and November 2018 in Songklanagarind Hospital. This study received approval from the Human Research Ethics Committee of the Faculty of Medicine at Prince of Songkla University (REC.63-004-12-4). The ethics committee (Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University) waived the requirement for informed consent to participate. We reviewed the medical records of all pregnant women who underwent the Quad test for Down syndrome screening at 15+ 0–20+ 6 weeks’ gestation by extracting data from the hospital’s database of computerized medical records. Pregnancies that were electively terminated at less than 24 weeks’ gestation, such as those with serious maternal diseases or an abnormal fetus, were excluded (Fig. 1).
Preeclampsia was diagnosed according to the ACOG criteria [40], defined as hypertension and proteinuria diagnosed after 20 weeks of gestation with a systolic blood pressure ≥ 140 mmHg and a diastolic blood pressure ≥ 90 mmHg in two separate measurements at least 4–6 h apart. Preeclampsia with severe features consisted of (1) systolic blood pressure > 160 mmHg or diastolic pressure > 110 mmHg, (2) serum creatinine > 1.1 mg/dl, (3) thrombocytopenia (platelets < 100,000/µL), (4) serum transaminase twice the normal concentration, (5) persistent headache or other cerebral or visual disturbance, (6) persistent epigastric pain, and (7) pulmonary edema. Late-onset PE was defined as onset at more than 34 weeks’ gestation.
Our model to predict late-onset preeclampsia with severe features was developed using maternal baseline characteristics and the values of the four serum markers assessed in the Quad test. All maternal factors thought to be associated with PE were recorded [22,23,24], including maternal age, gravidity, parity, gestational age at delivery, body mass index (BMI), history of preeclampsia, history of infertility, history of gestational diabetes mellitus (GDM), history of abortion, previous history of preterm birth, previous history of fetal growth restriction, underlying disease (e.g., chronic hypertension, diabetes mellitus, thyroid, and cardiac disease), as well as antepartum complications such as gestational diabetes mellitus, and fetal growth restriction. The values of maternal serum AFP, hCG, uE3, and inhibin A were measured as multiples of the median (MoM).
Statistical analysis was performed using R software v. 4.1.1 and STATA release 14.2 (Prince of Songkla University, Thailand). All demographic data were summarized and compared between PE and non-PE patients. Differences between the groups were evaluated using the chi-square (χ2) test or rank sum test. A receiver operating characteristic (ROC) curve was constructed and the area under the curve (AUC) was examined for each variable’s ability to predict PE. To develop a predictive model for late-onset PE with severe features, all maternal characteristics and median MoM values of the serum Quad test were entered into a logistic regression model in R software. The best-fitting model, as determined by the minimum value of the Akaike Information Criterion, was extracted. The model was then further reduced by sequentially removing variables that had only weak evidence (p > 0.05) of association with late-onset PE with severe features, as determined by the change of log-likelihood. The scores were integrated by coefficients of this model. Each predictor level was assigned an integer value score so that the ratios of the scores approximated those among of the coefficients. We then constructed an overall score to predict late-onset PE with severe features from the summary of the individual variable level score.
Results
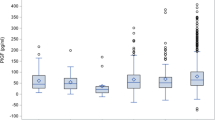
Of the 2000 pregnant women enrolled, 55 (2.75%) developed PE, of whom 31 had late-onset PE with severe features. The median gestational age at delivery was 37.3 weeks (interquartile range: 35.6–38.4). A comparison of baseline clinical characteristics showed that the PE group had significantly higher BMI; earlier gestational age at delivery; longer hospital stay; and a higher rate of history of preeclampsia, history of GDM, previous preterm birth, previous fetal growth restriction, chronic hypertension, diabetes mellitus, renal disease, thyroid disease, GDM, fetal growth restriction, and preterm labor than the non-PE group (Table 1). Furthermore, a higher percentage of perinatal morbidities was observed in the PE group than in the non-PE group (p < 0.001). Among the four serum markers of the Quad test, only hCG and inhibin A were significantly associated with PE (Table 1), with inhibin A having the highest AUC in the ROC for predicting late-onset PE with severe features (Fig. 2).
Table 2 shows the multivariate analysis of the factors associated with late-onset PE with severe features. The significant factors were maternal age ≥ 35 years, history of preeclampsia, history of infertility, cardiac disease, chronic hypertension, thyroid disease, and an inhibin A. Pregnant women with a history of infertility and who received infertility treatment had the highest odds ratio (OR), at 17.50 (95% CI 3.47–88.33), for PE. In particular, pregnant women with thyroid diseases (one patient was hyperthyroid and three were hypothyroid) had increased risk for late-onset PE with severe features (OR 4.89, 95% CI 1.02–23.36). From the logistic regression model, the ROC curve of the combination of maternal factors and MoM of inhibin A was 0.775 for predicting late-onset preeclampsia with severe features (Fig. 3). The score allocation derived from the multivariable logistic regression analysis (Table 3) was used to predict late-onset PE with severe features. The scores were classified into three levels (Table 4). The likelihood ratio to predict late-onset PE with severe features was 49.4 (total score > 60).
Discussion
In this study, late-onset PE patients comprised the majority of PE patients at our institute, and almost all patients were at late preterm to term gestation and still had a higher percentage of perinatal morbidities than non-PE patients. The logistic regression model demonstrated that a combination of maternal risk factors (maternal age, history of preeclampsia, history of infertility, cardiac disease, chronic hypertension, and thyroid disease) and inhibin A levels from the Quad test had a good predictive ability for late-onset PE with severe features.
Late-onset PE has a pathogenesis that is different from that of early-onset PE. Late-onset PE patients usually have a normal placental weight, and the fetuses frequently have appropriate birth weight for gestational age and an umbilical artery Doppler velocimetry generally within normal range [5,6,7, 41]. Late-onset PE is thought to result from an imbalance between the cardiovascular supply of the pregnant woman and the metabolic demands of the fetuses and their placenta in the third trimester period [6, 42]. Patients who develop late-onset PE had a high BMI, increased cardiac output, and relatively unchanged total vascular resistance [6]. Previous reports have focused on predicting late-onset PE (severe and non-severe) and have reported that maternal risk factors alone result in an overall detection rate of 33.5–62.9% [20, 27] using the criteria proposed by the NICE and ACOG. The detection rate was slightly improved when combined with Doppler ultrasound, serum pregnancy-associated plasma protein A, and placental growth factor in the first trimester gestation [20]. Although these combined tests might have a high detection rate for late-onset PE, these are often beyond the capacity of health services in developing countries. Our study was specifically aimed at predicting late-onset PE with severe features, which, to our knowledge, has not been reported in any previous work, and showed that not all maternal risk factors of PE increased the risk of late-onset PE with severe features.
This study found that maternal age, history of PE, history of infertility, cardiac disease, chronic hypertension, and thyroid disease were all significant risk factors for late-onset PE with severe features. Pregnant women with a history of infertility and who underwent infertility treatment were found to have the highest risk for PE. Previous studies [43,44,45,46] have also reported that infertility status and infertility treatment confer an increased risk of hypertensive disorders during pregnancy. In addition to advanced age, high estradiol levels in the IVF cycles potentially increase the risk for PE due to abnormal placentation and ultimately uteroplacental vascular insufficiency in infertile pregnant women [44, 45]. Furthermore, a recent report showed a comorbid relationship between infertility and other pathologies and highlighted shared genes and molecular pathways related to hypertensive disorders in pregnant women [43]. We found that pregnant women with thyroid diseases had increased risk for late-onset PE with severe features, despite receiving treatment and having no symptoms of thyroid dysfunction. Previous reports [47, 48] have also found that thyroid dysfunction, especially hypothyroidism, is associated with risk of PE, which had increase in endothelial cell dysfunction. In addition to some specific maternal risk factors related to late-onset PE with severe features, we found that serum inhibin A during the second trimester had an additive value for improving the prediction of PE.
Inhibin A is a glycoprotein hormone of the transforming growth factor-β superfamily produced by the placenta during pregnancy [48]. Inhibin A can regulate embryo implantation and differentiation; affect the normal permeability, integrity of maternal blood vessels, and adaptability of the maternal cardiovascular system to pregnancy; reduce placental blood flow; and aggravate placental ischemia and metabolic disorders [49]. Previous reports [33,34,35,36,37,38,39] have reported an association between developing preeclampsia, especially in early-onset type [35, 37, 50], and a high inhibin A level. A systematic review demonstrated that the best predictor for PE among pregnant women who underwent the Quad test for Down syndrome screening was inhibin A, with a positive likelihood ratio of 19.52 (95%CI 8.33–45.79) [34]. Our study revealed that inhibin A had the highest AUC (0.708) for predicting late-onset PE with severe features, and when we combined inhibin A with maternal risk factors, the AUC improved to 0.775, with a high predictive value among patients with a score > 60. This predictive model with both maternal risk factors and inhibin A also had a higher predictive ability than the model using maternal risk factors alone, proposed by the NICE and ACOG guidelines (AUC 0.591 [0.538–0.644] and 0.695 [0.604–0.714], respectively), in predicting any PE at < 37 weeks’ gestation in the Asian population [27].
According to the pathogenesis, late-onset PE is different from early-onset PE, which is a defective placentation in origin, so as the use of low-dose aspirin might have a limited value for prevention of this condition [23, 24]. However, patients that develop late-onset PE have a high BMI, increased cardiac output, and relatively unchanged total vascular resistance [6]; therefore, some researchers are also interested in behavioral modifications to prevent PE. There is evidence that a healthy diet, appropriate weight gain, exercise, and stress reduction can reduce the risk of PE, without significant differences in birth weight or small for gestational age [51, 52]. Therefore, lifestyle modifications should be recommended for patients who have a risk of late-onset PE (for primary prevention purposes). Furthermore, the monitored blood pressure and abnormal symptoms by themself in the third trimester period are also suggested for early detection and diagnosis (especially severe features) in order to reduce maternal morbidities and mortality (for secondary prevention purposes). Currently, there is an increase in ongoing research that focus on new drugs for pregnant woman during late second to early thrid trimester periods, such as pravastatin, proton-pump inhibitors, and metformin, that act against both placental and maternal vascular diseases; which can reduce circulating soluble fms-like tyrosine kinase-1 (sFlt-1) and up-regulate nitric oxide synthase. These reduce antiangiogenic factors, and the proinflammatory state might have an effect on prevention and treatment of late-onset PE patients [53].
A strength of our study is that it is the first study to develop a model of predictive risk factors for late-onset PE with severe features using both maternal risk factors and the Quad test in pregnant women in the second trimester with a good predictive ability. This predictive model would benefit patients by resulting in close monitoring of those at a high risk of developing late-onset PE with severe features and assist in early diagnosis in order to reduce maternal morbidities and mortality from this condition, particularly in developing countries where the majority of pregnant women usually only receive antenatal care in the second trimester. However, an important limitation of our study was its retrospective design and limited number of patients who may have had other maternal risk factors that might be associated with this condition.
Conclusions
In conclusion, a model combining serum inhibin A with maternal risk factors was useful in predicting late-onset PE with severe features. Therefore, close monitoring of these high-risk patients for PE is recommended for the early diagnosis and secondary prevention of maternal morbidity and mortality related to PE.
(total number of cases = 31)
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACOG:
-
The American College of Obstetricians and Gynecologists
- AFP:
-
Alpha Fetoprotein
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- FIGO:
-
Federation of Gynecology and Obstetrics
- GDM:
-
Gestational diabetes mellitus
- hCG:
-
Human chorionic gonadotropin
- MoM:
-
Multiples of the median
- NICE:
-
National Institute for Health and Care Excellence
- PE:
-
Preeclampsia
- ROC:
-
Receiver operating characteristic
- uE3:
-
Unconjugated estriol
References
Wu P, van den Berg C, Alfirevic Z, O’Brien S, Röthlisberger M, Baker PN, et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. Int J Mol Sci. 2015;16:23035–56.
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, Paris Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–8.
Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–6.
Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99.
Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113:580–9.
Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–80.
van der Merwe JL, Hall DR, Wright C, Schubert P, Grové D. Are early and late preeclampsia distinct subclasses of the disease–what does the placenta reveal? Hypertens Pregnancy. 2010;29:457–67.
Phillips JK, Janowiak M, Badger GJ, Bernstein IM. Evidence for distinct preterm and term phenotypes of preeclampsia. J Matern Fetal Neonatal Med. 2010;23:622–6.
Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy. 2000;19:221–31.
Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–9.
Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–8.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161e1–11.
Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, et al. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol. 2010;202:40e1–7.
Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35:662–70.
Youssef A, Righetti F, Morano D, Rizzo N, Farina A. Uterine artery Doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11 + 0 to 13 + 6 weeks in the prediction of late (> 34 weeks) pre-eclampsia. Prenat Diagn. 2011;31:1141–6.
Rolnik DL, Wright D, Poon LCY, Syngelaki A, O’Gorman N, de Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–5.
Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217:585e1–5.
O’Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49:756–60.
Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an asian population. Am J Obstet Gynecol. 2019;221:650e1–16.
Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;52:186–95.
De Kat AC, Hirst J, Woodward M, Kennedy S, Peters SA. Prediction models for preeclampsia: a systematic review. Pregnancy Hypertens. 2019;16:48–66.
National Institute for Health and Care Excellence.Hypertension in pregnancy:diagnosis and management [NICE website]. ; 2019.www.nice.org.uk/guidance/ng133. Accessed 30 Nov 2021.
ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–52.
Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145:1–33.
Scazzocchio E, Figueras F, Crispi F, Meler E, Masoller N, Mula R, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. Am J Obstet Gynecol. 2013;208:203e1–10.
Scazzocchio E, Crovetto F, Triunfo S, Gratacós E, Figueras F. Validation of a first-trimester screening model for pre-eclampsia in an unselected population. Ultrasound Obstet Gynecol. 2017;49:188–93.
Phumsiripaiboon P, Suksai M, Suntharasaj T, Geater A. Screening for pre-eclampsia: performance of National Institute for Health and Care Excellence guidelines versus American College of Obstetricians and Gynecologists recommendations. J Obstet Gynaecol Res. 2020;46:2323–31.
Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–53.
Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74.
Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307–12.
Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The magpie trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–90.
Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2022;226:1071–S1097. e2.
Rocha RS, Alves JAG, Maia E, Holanda Moura SB, Araujo Júnior E, Peixoto AB, Santana EFM, et al. Simple approach based on maternal characteristics and mean arterial pressure for the prediction of preeclampsia in the first trimester of pregnancy. J Perinat Med. 2017;45:843–9.
Morris RK, Cnossen JS, Langejans M, Robson SC, Kleijnen J, Ter Riet G, et al. Serum screening with Down’s syndrome markers to predict pre-eclampsia and small for gestational age: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2008;8:33.
Ree PH, Hahn WB, Chang SW, Jung SH, Kang JH, Cha DH, et al. Early detection of preeclampsia using inhibin A and other second-trimester serum markers. Fetal Diagn Ther. 2011;29:280–6.
Jacquemyn Y, Zemtsova O. Risk factors and prediction of preeclampsia. Acta Clin Belg. 2010;65:1–12.
Olsen RN, Woelkers D, Dunsmoor-Su R, Lacoursiere DY. Abnormal second-trimester serum analytes are more predictive of preterm preeclampsia. Am J Obstet Gynecol. 2012;207:228e1–7.
Singnoi W, Wanapirak C, Sekararithi R, Tongsong T. A cohort study of the association between maternal serum inhibin-A and adverse pregnancy outcomes: a population-based study. BMC Pregnancy Childbirth. 2019;19:124.
Yaron Y, Cherry M, Kramer RL, O’Brien JE, Hallak M, Johnson MP, et al. Second-trimester maternal serum marker screening: maternal serum alpha fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999;181:968–74.
American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–31.
Eskild A, Romundstad PR, Vatten LJ. Placental weight and birthweight: does the association differ between pregnancies with and without preelcampsia? Am J Obstet Gynecol. 2009;201:595e1–5.
Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302.
Tarín JJ, García-Pérez MA, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod Biol Endocrinol. 2015;13:31.
Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary syndrome: results from a randomized trial. Fertil Steril. 2018;109:324–9.
Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–9.
Monseur BC, Morris JR, Hipp HS, Berghella V. Hypertensive disorders of pregnancy and infertility treatment: a population-based survey among United States women. J Assist Reprod Genet. 2019;36:1449–56.
Gui J, Xu W, Zhang J. Association between thyroid dysfunction and perinatal outcomes in women with gestational hypertension: a retrospective study. BMC Pregnancy Childbirth. 2020;20:119.
Birdsall M, Ledger W, Groome N, Abdalla H, Muttukrishna S. Inhibin A and activin A in the first trimester of human pregnancy. J Clin Endocrinol Metab. 1997;82:1557–60.
Yue CY, Zhang CY, Ni YH, Ying CM. Are serum levels of inhibin A in second trimester predictors of adverse pregnancy outcome? PLOS ONE. 2020;15:e0232634.
Zeeman GG, Alexander JM, McIntire DD, Byrd W, Leveno KJ. Inhibin-A levels and severity of hypertensive disorders due to pregnancy. Obstet Gynecol. 2002;100:140–4.
Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088.
Roberts JM, Balk JL, Bodnar LM, Belizan JM, Bergel E, Martinez A. Nutrient involvement in preeclampsia. J Nutr. 2003;133:1684s–92s.
Stephen T, Tu’uhevaha J, Kaitu’u-Lino, Roxanne H, Fiona B, Catherine C, Natalie H. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. Am J Obstet Gynecol. 2022;226:1157–70.
Acknowledgements
The authors thank the Faculty of Medicine, Prince of Songkla University, Thailand.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
PB was involved in project development, data collection, data analysis, and manuscript writing. NP aided with project development, data analysis, and manuscript writing. CS aided with manuscript editing. AG performed data analysis and helped with, manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Faculty of Medicine, Prince of Songkla University (REC.63-004-12-4) in March 2020. To access and use the medical records of all participants in this study were allow by the medical director of Songklanagarind Hospital. The ethics committee (Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University) waived the requirement for informed consent to participate. All human data have been performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bunyapipat, P., Pruksanusak, N., Suwanrath, C. et al. Combined maternal risk factors and the Quadruple test to predict late-onset preeclampsia in pregnant Thai women. BMC Pregnancy Childbirth 23, 277 (2023). https://doi.org/10.1186/s12884-023-05623-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05623-4