Abstract
Background
Serum CGRP has been found to increase during migraine attack. However, whether CGRP can identify MA with PFO subtypes in MA remains unknown. This study aimed to investigate the differential expression of calcitonin gene-related peptide (CGRP) between migraine (MA) patients with and without patent foramen ovale (PFO), and to evaluate the predictive value of CGRP for MA with PFO.
Methods
A total of 153 patients with MA, 51 patients with PFO and 102 patients without. Venous blood was drawn and HIT-6 score was calculated during the onset of MA, and blood routine, inflammatory indexes and serum CGRP were detected. The differences in serum markers and HIT-6 scores were compared between the two groups, and the risk factors of MA with PFO were determined by univariate and multivariate logistics regression. Furthermore, the correlation between CGRP level with right-to-left shunt (RLS) grades and headache impact test-6 (HIT-6) score in MA patients with PFO were assessed. Independent risk factors were screened out by multivariate Logistic regression analysis. We used the receiver operating characteristic (ROC) curve to analyze the diagnostic value of these risk factors in MA complicated with PFO.
Results
The serum CGRP level and HIT-6 scores in the MA with PFO group were significantly higher than those in the MA group (P < 0.001). Multivariate regression analysis showed that CGRP was an independent risk factor for MA with PFO (OR = 1.698, 95% CI = 1.325–2.179, P < 0.001). CGRP values increased with the increase of RLS grade(Spearmen rho = 0.703, P < 0.001). Furthermore, a positive correlation between CGRP and HIT-6 scores was found (Spearmen rho = 0.227; P = 0.016). ROC curve showed that the optimal cut-off value for diagnosing MA with PFO was 79 pg/mL, the area under the curve (AUC) for predicting MA with PFO was 0.845, with 72.55% sensitivity and 78.43% specificity.
Conclusions
MA patients with PFO have higher serum CGRP level. elevated CGRP concentration was associated with higher RLS grade and increased HIT-6 score. Higher serum CGRP level has certain clinical value in predicting PFO in MA patients.
Trial registration
This study was approved by the Ethics Committee of Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine (Ethics batch number: 20,201,215,005).
Similar content being viewed by others
Introduction
The number of migraine (MA) patients worldwide has reached 1.1 billion in 2019, becoming the second most disabling disease [1]. Until now, MA treatment focuses on symptomatic pain relief, which is only effective in 30–50% of the MA population [2]. To be noted, it is reported that patients with MA with patent foramen ovale (PFO) can achieve a radical cure of headache by blocking PFO [3]. Given that the prevalence rate of MA with PFO varies between 14.6 and 66.5% in different studies, there is a possibility of missed diagnosis of MA with PFO [4]. Moreover, current methods [contrast transcranial Doppler(c-TCD), transthoracic echocardiography (TTE), and transesophageal echocardiography (TEE)] for diagnosing PFO depend on the physicians to recognize cardiac abnormality, and their diagnostic criteria are not unified [5]. Therefore, it is crucial to find biomarkers that can distinguish PFO subtypes from MA, in addition to improving the diagnostic level of cardiac color ultrasound physicians in PFO.
Calcitonin gene-related peptide (CGRP) is a bioactive peptide widely distributed in the central and peripheral nervous system. It can cause MA attacks by expanding blood vessels via the trigeminal pathway, controlling neuron excitability, sensitizing the peripheral and central nervous systems via degranulating mast cells, and enhancing pain perception [6, 7]. CGRP has been found to increase during migraine attack [8, 9]. It is suggested that CGRP could be used as a non-invasive marker for migraine [10]. Many vasoactive substances are usually excreted or metabolized through pulmonary circulation, but venous blood can be shunted into arterial blood through the PFO channel without circulation in the lungs. Some chemicals and hormones, such as 5-HT and CGRP, can bypass the pulmonary circulation and cross the blood-brain barrier directly, stimulating the trigeminal nerve and causing migraines [11, 12].Therefore, we speculate that the concentration of serum CGRP is higher in patients with MA. However, it is not clear whether CGRP, as the most potential biomarker of migraine, can identify MA with PFO subtypes in MA remains unknown.
This study aims to identify the correlation between plasma CGRP levels and MA with PFO attacks during the attack of MA, and to provide some evidence for the clinical identification of MA with PFO subtypes.
Materials and methods
Study setting and participants
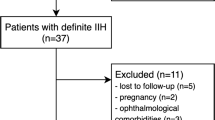
From January 2020 to January 2023, 51 patients diagnosed with MA and PFO by c-TCD foaming test combined with TEE examination in Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine were selected. The data of MA patients with PFO and those without PFO with the same bed number were collected according to the 1:2 pairing principle(Fig. 1). All patients were systematically diagnosed by neurologists according to the guidelines [13]. This study as conducted in accordance with the guidelines of the World Medical Association Declaration of Helsinki (2000) and was approved by the Ethics Committee of Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine (Ethics batch number: 20,201,215,005). All participants provided written informed consent.
Inclusion and exclusion criteria
Inclusion criteria included: (1) the diagnostic criteria for migraine refer to the third edition of the International Headache Classification [14]; (2) positive c-TCD, TEE clearly diagnosed PFO and right-to-left shunt (RLS), the degree of shunt in TEE angiography is quantified in accordance with the microbubbles detected in the left atrium: Grade 0, no microbubbles are negative; Grade I, a small amount, 1–10 microbubbles; Grade II Moderate volume, 11–25 microbubbles; Grade III, large amount, more than 25 microbubbles or left atrium almost filled with microbubbles or left atrium opacity) [15]; (3) aged 18–80 years old; (4) able to cooperate with the completion of follow-up with complete and valid information; (5) electrocardiogram and other laboratory tests are normal.
Exclusion criteria included: (1) with headaches caused by other definite reasons; (2) pregnant or breastfeeding; (3) severe liver and kidney dysfunction, abnormal blood coagulation, autoimmune diseases, malignant tumors, or severe physical diseases, (4) cannot cooperate with the examination because of abnormal mental behavior or poor physical fitness; (4) incomplete follow-up data; (5) pulmonary arteriovenous fistula; (6) participate in other clinical research at the same time.
Clinical information
General clinical data of the subjects were collected, including age, gender, basic medical history (essential hypertension, diabetes, coronary heart disease, atrial fibrillation), medication history, migraine aura, and smoking and drinking hobbies. All patients completed the head CT/MRI, ECG and foaming test. If the foaming test was positive, TEE was performed.
Clinical and laboratory parameters
Headache scale score
Headache impact test-6 (HIT-6) [16]: Evaluate the negative evaluation of daily life caused by the onset headache of the patient, with a score ranging from 36 to 78 points. The score is positively correlated with the degree of disability impact, and a score above 60 means severe impact, 56–59 means significant impact, 50–55 means moderate impact, 36–49 means slight or no impact.
Collection and processing of blood samples
Fasting peripheral venous blood in the morning was drawn for detection.
General blood indicators: Complete blood cell counts were measured using the ethylenediaminetetraacetic acid blood sample method on a Toshiba analyzer (Hitachi High-Tech, Tokyo, Japan). Serum C-reactive protein (CRP), uric acid (UA) total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL) were measured with a Hitachi LST008 automatic biochemical analyzer. Fibrinogen (FIB) was determined using an automated coagulation analyzer (ACL-TOP-700, Wolfen, Spain).
CGRP collection and measurement: CGRP level was detected during the migraine attack period with EDTA anticoagulant tubes, followed by the centrifugation at a speed of 1000 r/min and at a temperature below 4 °C for 10 min. The separated supernatant was stored in a -80 °C refrigerator. Finally, radioimmunoassay was used to measure the concentration of plasma CGRP following the instructions of the kit (Human CGRP ELISA kit (ml026014), Shanghai Enzymatic Biology Co., Ltd.).
Statistical analysis
Data conforming to normal distribution was represented by mean ± standard deviation, and the t test was used for comparison between groups. Data not conforming to the normal distribution Otherwise, data was represented by median (Q25, Q75), and the Mann-Whitney U test was used for comparison between groups. The count data were expressed as cases (%), and the chi-square test or Fisher’s exact test was used for comparison between groups. Univariate and multivariable logistic regression models were used to assess the association between dependent variable (MA with POA (yes/no)) and variables. Correlation analysis was performed on CGRP and HIT-6 score as well as PFO diameter in MA patients with PFO. Pearson correlation analysis is carried out for the data in accordance with normal distribution, otherwise spearman correlation analysis is carried out. The area under the curve (AUC) of the receiver operator characteristic curve (ROC) was calculated and Youden Index was used to obtain the optimal cut-off value. P < 0.05 means significant difference. Statistical analysis was analyzed using GraphPad Prism 8 and SPSS 23.0.
Results
Comparison of general data and serum CGRP levels
A total of 153 patients were included in this study: 102 MA patients (MA group), and 51 MA patients with PFO (Table 1). There was no difference between the two groups in terms of demographic data, including gender, BMI, coronary heart disease, atrial fibrillation, hypertension, diabetes, and medication history (Table 1).
Serum CGRP levels in the MA with PFO group were significantly higher than those in the MA group (83(78,88) vs. 64(52,68), P < 0.001). Serum levels of RBC, MPV, CRP, FIB, and TC in the MA with PFO group were higher than those in the MA group (Table 1). In addition, the HIT-6 score of the MA with PFO group was higher than that of the MA group (P < 0.001) (Fig. 2 A-C).
Risk factors for MA with PFO
Univariate logistic regression analysis showed that RBC, MPV, CRP, FIB, TC, CGRP and HIT-6 were associated with MA with PFO (Table 2, P < 0.05). After adjusting for significant variables in univariate logistic regression model, multivariate logistic regression analysis revealed that CGRP was an independent risk factor for MA with PFO (P < 0.001; OR = 1.698; 95%CI = 1.325–2.179).
Relationship between CGRP value and RLS diversion level
In order to further explore the characteristics of CGRP in MA patients with PFO, we divided MA patients with PFO into 3 groups according to RLS classification (Grade 1, Grade 2, Grade 3, Table 3). Our results suggested that serum CGRP levels increased as the RLS grade increased (78 (73.25,81.5) vs. 83.25 (6.37) vs. 91 (87,92), all P < 0.05). (Fig. 2D)
Correlation analysis between CGRP and HIT-6 score, PFO diameter
There was a positive correlation between CGRP and RLS grade (Spearmen rho = 0.703, P < 0.001) (Fig. 3 A). Furthermore, we found that there was a positive correlation between CGRP and HIT-6 score in patients with MA with PFO (Spearmen rho = 0.227; P = 0.016), while there was no correlation between CGRP and PFO diameter (P = 0.083). (Fig. 3 B).
ROC analysis of CGRP value predicting PFO in MA patients
According to the ROC curve analysis, the optimal cut-off value for diagnosing MA with PFO was 79 pg/mL, and the area under the curve (AUC) for predicting RD was 0.845, with 72.55% sensitivity and 78.43% specificity (Fig. 4).
Discussion
Our results showed that the CGRP in MA with PFO group was significantly higher than that in MA group, and increased CGRP level was associated with elevated RLS grade and HIT-6 score. Furthermore, CGRP value was independently associated with the risk of MA with PFO. Moreover, the cutoff of CGRP in ROC analysis was 79, with the sensitivity 72.55%, and the specificity 78.43%. As far as we know, this study is the first one to explore the correlation between CGRP and MA with PFO, and to use CGRP as the biomarker to evaluate whether MA patients are accompanied by PFO.
Migraine and PFO
With the development of clinical medicine, accumulating studies have confirmed that PFO is related to MA [5]. It was found that PFO is more common in MA patients with aura [17]. Consistently, aura migraine accounted for 41% of patients with MA with PFO in our study [18]. It is unclear why PFO is associated with migraine. A hypothesis is that many vasoactive substances are usually excreted or metabolized through pulmonary circulation, but venous blood can be shunted directly into arterial blood through the PFO channel without circulation in the lungs. Chemicals and hormones, such as serotonin or CGRP, could bypass the pulmonary circulation, cross the blood-brain barrier directly and stimulate the trigeminal nerve, causing migraines [19, 20].In addition, tiny emboli in the venous circulation can pass and directly enter the arterial system through PFO, triggering low perfusion or cortical spread inhibition, and leading to migraine attacks. Further, Cao et al. provides neuroimaging evidence and new insights into the correlation between PFO and migraine. They found that in migraine patients with PFO, the presence of PFO may affect the structure of the cerebral cortex and the integrity of white matter, which is mainly locked in subcortical, deep white matter, and posterior circulation, and may lead to changes in brain function, such as cerebellum and colliculus, which are involved in the processing and transmission of pain [21].
CGRP and MA with PFO
The important role of CGRP in the pathophysiology of MA has been proven in many studies. First, CGRP exists in the peripheral sensory nerves of the meninges, and the neurons originating from the meningeal sensory pathway are related to migraine [22]. Secondly, CGRP is associated with pain transmission and inflammation promotion, and exogenous CGRP administration leads to migraine-like headache symptoms in patients with migraine [23]. Moreover, all anti-CGRP agents were more effective than placebo in migraine prevention [24]. Third, CGRP regulates the activity of neurons, glial cells and immune cells in the trigeminal nervous system to promote peripheral and central sensitization and persistent hypervigilance associated with migraine phenotypes [25].
Serum CGRP is a vasoactive polypeptide with a strong expansion effect. Several studies found that serum CGRP levels increased during migraine attacks [26, 27]. Our study found that CGRP level of the MA with PFO group was significantly higher than those without PFO, indicating that less CGRP in MA with PFO patients was metabolized in the pulmonary circulation. In addition, the HIT-6 score of MA with PFO group was significantly higher than that of MA, indicating that patients with MA with PFO suffered more severe headaches.
We further found that higher concentration of CGRP was associated with increased RLS grade. These results suggest that the more RLS shunt flow, the more CGRP bypassing the pulmonary circulation and directly crossing the blood-brain barrier to cause severer migraine attacks in MA patients with PFO.
Some studies have found that RLS grades are related to the severity of migraine [28], but our study did not find a correlation between RLS and HIT-6 scores. The possible reason may be that the sample size of our study is small.
FIB, MPV and MA with PFO
Moreover, our study found that patients in MA with PFO group had higher levels of MPV and CRP than those in MA group, which was consistent with previous studies. Previos study found that increased level of platelet activation and enhanced release response was common in the attack of MA [29], and the blood MPV in MA patients was significantly higher than that in healthy controls [30].
In addition, our study found that the FIB of MA with PFO group was higher than that of MA group. According to literature, MPV is closely related to platelet activity and participates in inflammation and thrombosis [31], and FIB is associated with thrombus. Our study indicates that MA patients with PFO had increased degree of platelet activation, and slower velocity of venous blood flow, therefore, platelets were more likely to aggregate in the vein to form microemboli, resulting in increased FIB reactivity [32]. This is also consistent with the hypothesis of MA caused by PFO, because RLS leads to decreased blood oxygen saturation and hypoxia, thus increasing the expression of plasminogen activator, such as FIB, which increases the possibility of microembolism, leads to cortical spread inhibition, and leads to MA attack [33].
Limitation
Our study has some certain limitations. Firstly,, this study is a retrospective study, which can not draw a conclusion on causality, and the popularization of the conclusion still needs to be cautious. Secondly, the short time of this study, strict inclusion of subjects, small sample size and only collecting CGRP during MA attack may have a certain deviation to the results. Thirdly, the onset of MA is affected by many factors, which were not included in this test. Therefore, multicenter experiments with expanded sample, rigorous inclusion and more risk factors are required in the future.
Conclusions
In summary, this study illustrates that the serum of CGRP in MA patients with PFO is significantly higher than that in MA patients. Furthermore, CGRP is positively associated with RLS grade and HIT-6 score. Serum CGRP has diagnostic predictive value for MA with PFO, which is worthy of further study.Higher serum CGRP level has certain clinical value in predicting PFO in MA patients.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- CGRP:
-
calcitonin gene-related peptide
- MA:
-
migraine
- PFO:
-
patent foramen ovale
- ROC:
-
receiver operating characteristic
- AUC:
-
area under the curve
- c-TCD:
-
contrast transcranial Doppler
- TTE:
-
transthoracic echocardiography
- TEE:
-
transesophageal echocardiography
- RLS:
-
right-to-left shunt
- HIT-6:
-
headache impact test-6
- CRP:
-
C-reactive protein ()
- UA:
-
uric acid
- TC:
-
total cholesterol
- TG:
-
triglycerides
- HDL:
-
high-density lipoprotein cholesterol
- LDL:
-
low-density lipoprotein cholesterol
- FIB:
-
fibrinogen
References
Tepper SJ, Cirillo J, Kim E, L’Italien G, Tweedie JM, Lodaya K, Riley D, Pathan F, Antaki N, Nathanson BH, et al. The temporal trend of placebo response in migraine prevention from 1990 to 2021: a systematic literature review and meta-analysis with regression. J Headache Pain. 2023;24(1):54.
Agboola F, Atlas SJ, Touchette DR, Borrelli EP, Rind DM, Pearson SD. The effectiveness and value of novel acute treatments for migraine. J Manag Care Spec Pharm. 2020;26(11):1456–62.
Zhang QQ, Lu JJ, Yan MY, Hu XW, Qin YR, Wang DP, Jiang JH, Fang Q, Zhao HR. The Efficacy of Percutaneous Patent Foramen Ovale Closure on Migraine: a Meta-Analysis of Randomized Controlled Trials and Observational Studies. Biomed Res Int 2021, 2021:6643266.
Tariq N, Tepper SJ, Kriegler JS. Patent Foramen Ovale and Migraine: closing the Debate–A review. Headache. 2016;56(3):462–78.
Kumar P, Kijima Y, West BH, Tobis JM. The connection between patent Foramen Ovale and Migraine. Neuroimaging Clin N Am. 2019;29(2):261–70.
Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301–14.
Kilinc E, Tore F, Dagistan Y, Bugdayci G. Thymoquinone inhibits neurogenic inflammation underlying migraine through modulation of calcitonin gene-related peptide release and Stabilization of Meningeal Mast Cells in Glyceryltrinitrate-Induced Migraine Model in rats. Inflammation. 2020;43(1):264–73.
Cho S, Chu MK. Serological biomarkers of chronic migraine. Curr Pain Headache Rep; 2023.
Edvinsson L. The Trigeminovascular pathway: role of CGRP and CGRP receptors in Migraine. Headache. 2017;57(Suppl 2):47–55.
Iljazi A, Ashina H, Zhuang ZA, Lopez Lopez C, Snellman J, Ashina M, Schytz HW. Hypersensitivity to calcitonin gene-related peptide in chronic migraine. Cephalalgia. 2021;41(6):701–10.
Paredes S, Cantillo S, Candido KD, Knezevic NN. An Association of Serotonin with Pain disorders and its modulation by Estrogens. Int J Mol Sci 2019, 20(22).
Wang HL. The Clinical Curative Effect and Safety of Percutaneous Closure of Patent Foramen Ovale. Master’s Thesis, Jilin University, Changchun, China, 2016.
Charles A. Migraine. N Engl J Med. 2017;377(6):553–61.
Headache Classification Committee of the International Headache Society (IHS). The International classification of Headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Liu F, Kong Q, Zhang X, Li Y, Liang S, Han S, Li G. Comparative analysis of the diagnostic value of several methods for the diagnosis of patent foramen ovale. Echocardiography. 2021;38(5):790–7.
Houts CR, Wirth RJ, McGinley JS, Cady R, Lipton RB. Determining thresholds for meaningful change for the Headache Impact Test (HIT-6) total and item-specific scores in Chronic Migraine. Headache. 2020;60(9):2003–13.
Takizawa T, Ayata C, Chen SP. Therapeutic implications of cortical spreading depression models in migraine. Prog Brain Res. 2020;255:29–67.
Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, Seiler C. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. 2005;65(9):1415–8.
Alpert JS. Strange bedfellows: Migraine Headache and Patent Foramen Ovale. Am J Med. 2021;134(11):1307–8.
Close LN, Eftekhari S, Wang M, Charles AC, Russo AF. Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia. 2019;39(3):428–34.
Cao W, Shen Y, Zhong J, Chen Z, Wang N, Yang J. The patent Foramen Ovale and Migraine: Associated mechanisms and perspectives from MRI evidence. Brain Sci 2022, 12(7).
Ji Y, Rizk A, Voulalas P, Aljohani H, Akerman S, Dussor G, Keller A, Masri R. Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus. Neurobiol Pain. 2019;6:100031.
Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–86.
Sun W, Cheng H, Xia B, Liu X, Li Y, Wang X, Liu C. Comparative efficacy and safety of five anti-calcitonin gene-related peptide agents for Migraine Prevention: A Network Meta-analysis. Clin J Pain. 2023;39(10):560–9.
Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache. 2019;59(5):659–81.
Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019;18(8):795–804.
Al-Khazali HM, Ashina H, Wiggers A, Rose K, Iljazi A, Christensen RH, Schytz HW, Amin FM, Ashina M. Calcitonin gene-related peptide causes migraine aura. J Headache Pain. 2023;24(1):124.
Ling Y, Wang M, Pan X, Zhao H. Clinical features of right-to-left shunt in the different subgroups of migraine. Brain Behav. 2020;10(3):e01553.
Sarchielli P, Alberti A, Coppola F, Baldi A, Gallai B, Floridi A, Floridi A, Capocchi G, Gallai V. Platelet-activating factor (PAF) in internal jugular venous blood of migraine without aura patients assessed during migraine attacks. Cephalalgia. 2004;24(8):623–30.
Turan E, Kilic SS. Retrospective view of primary Raynaud’s phenomenon in childhood. Reumatol Clin (Engl Ed). 2019;15(6):e92–5.
Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): New perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074.
Ranucci M, Ranucci M, Laddomada T, Baryshnikova E, Nano G, Trimarchi S. Plasma viscosity, functional fibrinogen, and platelet reactivity in vascular surgery patients. Clin Hemorheol Microcirc. 2015;61(3):417–27.
Khasiyev F, Arsava EM, Topcuoglu MA. Cerebral vasomotor reactivity in migraine: effect of patent foramen ovale and aerogenic microembolism. Neurol Res. 2020;42(9):795–804.
Acknowledgements
Not applicable.
Funding
This work was supported by Zhuhai Science and Technology Innovation Bureau (ZH22036201210133PWC).
Author information
Authors and Affiliations
Contributions
Conceptualization, Chaojie Li.; Data curation, Kehang Xie and Donghui Huang; Formal analysis, Yu Yu.; Investigation, Donghui Huang; Resources, Yana Yin; Software, Lianjun Zhang and Donghui Huang; Visualization, Ningning Li; Writing original draft, Chaojie Li; Writing review and editing, Kehang Xie.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine (Ethics batch number: 20201215005). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, C., Yu, Y., Li, N. et al. Calcitonin gene-related peptide: a possible biomarker in migraine patients with patent foramen ovale. BMC Neurol 24, 126 (2024). https://doi.org/10.1186/s12883-024-03615-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03615-1