Abstract
Background
Mean perfusion pressure (MPP) has recently emerged as a potential biomarker for personalized management of tissue perfusion in critically ill patients. However, its association with the occurrence of acute kidney injury (AKI) in septic patients and the optimal MPP range remain uncertain. Therefore, this study aims to investigate the relationship between MPP and AKI in critically ill patients with sepsis.
Methods
We identified 5867 patients with sepsis from the MIMIC-IV database who met the inclusion and exclusion criteria. The exposure variable was the first set of MPP measured within 24 h after ICU admission with invasive hemodynamic monitoring. The primary outcome was the incidence of AKI at 7 days following ICU admission according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. Secondary outcomes included in-hospital mortality, lengths of ICU, and hospital stay. Optimal cut-off point for MPP were determined using the Youden index, and multivariable logistic regression was employed to examine the association between MPP and AKI. Subgroup analyses were conducted to enhance result robustness. Kaplan-Meier survival analysis was utilized to evaluate in-hospital mortality rates categorized by MPP.
Results
A total of 5,867 patients with sepsis were included in this study, and the overall incidence of AKI was 82.3%(4828/5867). Patients were categorized into low MPP (< 63 mmHg) and high MPP (≥ 63 mmHg) groups using the optimal ROC curve-derived cut-off point. The incidence of AKI in the low MPP group was higher than that in the high MPP group (87.6% vs. 78.3%, P < 0.001). Multivariable logistic regression analysis adjusted for confounding factors revealed that each 1 mmHg increase in MPP as a continuous variable was associated with a 2% decrease in AKI incidence within 7 days of ICU admission (OR:0.98, 95%CI:0.97–0.99, P < 0.001). When MPP was used as a categorical variable, patients in the high MPP group had a lower risk of AKI than those in the low MPP group (OR:0.71, 95%CI:0.61–0.83, P = 0.001). Subgroup analyses demonstrated a consistent association between MPP and AKI risk across all variables assessed (P for interaction all > 0.05). Kaplan-Meier curve analysis demonstrated a higher survival rate during hospitalization in the high MPP group compared to the low MPP group (Log-rank test, P < 0.0001).
Conclusions
Lower levels of MPP are associated with an increased incidence of AKI at 7 days in critically ill patients with sepsis.
Similar content being viewed by others
Background
Sepsis is characterized by life-threatening organ dysfunction resulting from a dysregulated host response to infection, and it remains a significant cause of death among critically ill patients [1, 2]. According to surveys, the global incidence of sepsis cases reached 48.9 million in 2017, with an estimated 11 million sepsis-related deaths [3]. Approximately one-third of sepsis patients develop acute kidney injury (AKI), often referred to as sepsis-associated acute kidney injury (SA-AKI) [4, 5]. Numerous studies have demonstrated that SA-AKI carries a poor prognosis, with high mortality rates and an independent association with prolonged hospital stays [6,7,8,9,10]. Previous research indicates that renal injury within the initial 48 h may primarily be functional, stemming from renal vasoconstriction and reduced renal blood flow leading to ischemia [11,12,13]. Furthermore, the interaction between renal hemodynamic alterations and inflammation likely contributes to AKI occurrence [14]. Thus, early monitoring of renal hemodynamics is crucial for improving SA-AKI outcomes.
Regulation of renal perfusion hemodynamics significantly influences renal function. Early experiments confirmed that renal blood flow autoregulation occurs within an arterial blood pressure range of 60–100 mmHg [15]. Factors such as inflammation, sepsis, and volume depletion can impair renal blood flow autoregulation, leading to marked fluctuations in glomerular filtration rate and contributing to AKI [5, 16, 17]. The Save Sepsis Campaign guidelines recommend maintaining a minimum mean arterial pressure (MAP) of ≥ 65 mmHg for adequate organ perfusion; however, MAP may not reliably reflect organ perfusion[ [18]. Prior research indicates that elevated central venous pressure (CVP) independently correlates with a higher incidence of AKI, especially post-sepsis resuscitation, due to increased resistance to renal venous return and subsequent venous congestion [19,20,21]. Therefore, a management strategy of lower CVP in the clinic may contribute to the maintenance of renal perfusion. However, relying solely on MAP or CVP may be inadequate, requiring a comprehensive approach. Currently, there is a lack of methods for directly monitoring or predicting renal blood flow and renal perfusion pressure. Recent studies suggest using mean perfusion pressure (MPP), calculated as MPP = MAP – CVP, as an alternative to MAP and CVP for estimating renal perfusion pressure [22]. A previous study on post-cardiac surgery patients demonstrated an association between decreased MPP and AKI [23]. However, research on the predictive value of MPP for AKI in septic patients is limited, and the optimal range of MPP is still undetermined.
Therefore, we conducted a retrospective cohort study utilizing the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database to investigate the association between MPP and AKI in septic patients, aiming to assess the predictive validity of MPP for AKI in critically ill patients with sepsis.
Methods
Data source
Participants in this study were obtained from the publicly available MIMIC-IV Database (v2.2), developed and maintained by the Laboratory of Computational Physiology at the Massachusetts Institute of Technology (MIT) (https://physionet.org/content/mimiciv/2.2/). The MIMIC-IV data is de-identified, thus exempting the need for informed consent or ethical approval. From 2008 to 2019, the database accumulated data from over 70,000 critically ill patients at Beth Israel Deaconess Medical Center (BIDMC). Data extraction was performed by the first author (Ling Li), who had full database access (certification number: 12187573). This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) declaration [24].
Study population
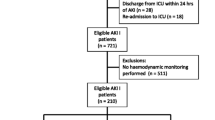
The MIMIC-IV database comprises data on 299,712 critically ill patients. We included patients over 18 years old who met the sepsis-3 criteria or had a sepsis diagnosis in the discharge diagnosis in the ICD code. Sepsis-3 is defined as an increase of ≥ 2 points in the sequential organ failure assessment (SOFA) score and suspected or confirmed infection [1]. We excluded patients with ICU stays < 24 h and those who had missing MAP and CVP data within the first 24 h of ICU admission. In addition, we also excluded patients with MAP and CVP measurements longer than 3 h. For patients with multiple ICU admissions, we only included the data from their first ICU admission. Ultimately, 5,867 patients with sepsis were included in the study.
Data extraction
Baseline patient information was extracted from the MIMIC-IV database using Structured Query Language (SQL). Vital signs included initial measurements of MAP, CVP, systolic blood pressure (SBP), and diastolic blood pressure (DBP) during the first 24 h of ICU stay. Laboratory tests covered first measurements of hemoglobin, platelets, white blood cell count (WBC), red blood cell count (RBC), blood urea nitrogen (BUN), and creatinine at ICU admission. Comorbidities comprised myocardial infarction, congestive heart failure, chronic pulmonary disease, liver disease, diabetes, renal disease, malignant cancer, and hypertension. Treatment details encompassed mechanical ventilation, renal replacement therapy, and vasopressor use within the initial 24 h of ICU admission. The severity of illness at admission was assessed using SOFA [25] and simplified acute physiology score II (SAPS II).
Exposure and outcomes
The exposure variable was MPP. We obtained the levels of MAP and CVP in the first 24 h after ICU admission in the first set of invasive hemodynamic monitoring, i.e., MPP = MAP-CVP [22]. The primary outcome was the occurrence of AKI at 7 days after ICU admission. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [26], AKI was diagnosed if serum creatinine was ≥ 1.5 times baseline, increased by ≥ 0.3 mg/dL within 48 h, or urine output was < 0.5 mL/(kg·h) for ≥ 6 h. The first serum creatinine measurement at ICU admission served as the baseline value [27]. Secondary outcomes included in-hospital mortality, length of ICU, and hospital stay.
Statistical analysis
Continuous variables were denoted as mean [standard deviation (SD)] or median [interquartile range (IQR)] and tested using the Student t-test for independent samples or the Mann-Whitney U test. Quantitative and percentage data were provided for categorical variables, and the χ2 or Fisher exact test was used to compare groups. We calculated the receiver operating characteristic (ROC) curve to determine the optimal cut-off point for MPP using the Youden index. Patients were then categorized into two groups: a high MPP(≥ 63mmHg) group and a low MPP(< 63mmHg) group based on this cut-off point (Supplementary Material: Fig. S1). Univariate logistic regression analyses identified variables with P < 0.1, which were included in multivariate logistic regression models to assess the association between MPP and AKI in septic patients. Models included unadjusted Model 1 (no adjustment for covariates), minimally adjusted Model 2 (adjusted only for sex and age), and fully adjusted Model 3 (adjusted for all covariates with P < 0.1). Next, we conducted subgroup analyses to assess the consistency of MPP with the risk of occurring AKI in different populations and tested for interactions using the log-likelihood ratio test. In-hospital survival was evaluated using Kaplan–Meier survival curves for MPP groups and analyzed with the Log-Rank test.
Missing values for all variables were below 5% and were imputed using the mean or median. The Supplementary Material presents the missing rates for each variable (Table S1). Statistical analysis was conducted using R 4.3.2 (http://www.Rproject.org; The R Foundation) and Free Statistics software version 1.8 (https://www.clinicalscientists.cn/). A significance level of P < 0.05 (two-tailed) was used for all analyses.
Results
The MIMIC-IV database includes data on 299,712 critically ill patients, 33,177 of whom were diagnosed with sepsis. According to the inclusion and exclusion criteria, our study included 5,867 patients with sepsis (Fig. 1). We used the Youden index of the ROC curve to determine that the optimal cut-off point for MPP is 63 mmHg. Then the study population was categorized into two groups based on the cut-off, i.e., the low MPP(< 63mmHg) group and the high MPP(≥ 63mmHg) group (Supplementary Material: Fig. S1).
Baseline characteristics
In the entire cohort, the low MPP group comprised 2,535 patients with sepsis, while the high MPP group included 3,332. The median age was 68.0 (IQR 59.0, 77.0) years and 65.6% of patients were male. The incidence of AKI at 7days in the cohort was 82.3%. Patients in the high MPP group exhibited lower BUN, creatinine, SAPS II, and SOFA scores. Additionally, patients in the low MPP group were likely to be treated with mechanical ventilation, renal replacement therapy, and vasopressors, and more likely to have comorbidities of myocardial infarct, congestive heart failure, chronic pulmonary disease, liver disease, diabetes, and renal disease than the high MPP group (Table 1).
Primary outcome
We constructed three multivariate logistic regression models to assess the relationship between MPP and the incidence of AKI at 7 days in septic patients, and the results are shown in Table 2. In the unadjusted model 1, we found that for each 1 mmHg increase in MPP, the risk of AKI was reduced by 3% (OR: 0.97, 95% CI: 0.96–0.97, P < 0.001). After fully adjusting for confounders (Model 3), the risk of AKI at 7 days decreased by 2% for each 1 mmHg increase in MPP (OR: 0.98, 95% CI: 0.97–0.99, P < 0.001). To further explore sensitivity, MPP was categorized from a continuous to a categorical variable, with low MPP groups serving as the baseline reference. In Model 3, patients in the high MPP group exhibited lower AKI risk compared to those in the low MPP group (OR: 0.71, 95% CI: 0.61–0.83, P = 0.001).
Subgroup analyses
We conducted subgroup analyses to investigate the trend of association between MPP and the risk of 7 days AKI occurrence across different demographic subgroups(Fig. 2). Our results indicate that we did not observe any significant association between MPP and the risk of 7-day AKI occurrence, irrespective of patient age, sex, or presence of comorbidities such as myocardial infarct, congestive heart failure, chronic pulmonary disease, liver disease, diabetes, renal disease, malignant cancer and hypertension (P for interaction > 0.05).
Secondary outcomes
The median length of ICU stay was 3.3 days (IQR: 1.9-7.0) in the low MPP group and 2.2 days (IQR 1.3–4.3) in the high MPP group. The median length of hospital stay was 8.8 days (IQR 5.7–14.6) in the low MPP group and 7.2 days (IQR 5.1–12.0) in the high MPP group. Patients in the low MPP group had significantly longer ICU stays and hospital stays compared to the high MPP group (P < 0.001) (Table 1). Kaplan-Meier survival curves showed that patients with sepsis in the high MPP group were significantly more likely to survive hospitalization than those in the low MPP group (log-rank test: P < 0.0001) (Fig. 3).
Discussion
Key findings
In this retrospective cohort study, we utilized data from the large-scale MIMIC-IV database to explore the association between MPP and the risk of AKI at 7 days in septic patients admitted to the ICU. The current study’s findings suggested lower levels of MPP are associated with an increased incidence of AKI at 7 days in critically ill patients with sepsis. Notably, even after accounting for possible confounding variables, this association is still statistically significant. This association was constant across subgroup analyses and sensitivity analyses, demonstrating the robustness of the finding. Additionally, the current study also highlights the significant association of MPP with in-hospital mortality, length of ICU, and hospital stay among critically ill patients with sepsis.
Relation with previous evidence
It has been shown that hemodynamic instability may lead to circulatory compromise and renal insufficiency and that maintaining large-vessel hydrostatic pressure parameters such as MAP and CVP at a certain level maintains renal perfusion and reduces the risk of AKI [10, 15, 28]. Two prior retrospective studies investigated the impact of MPP on AKI in critically ill patients [17, 29]. Among those, one large-scale retrospective investigation indicated that an increase in mean perfusion pressure variability (MPPV) in critically ill patients was related to an increased risk of eventual worsening in renal function after controlling for relevant variables [29]. Another study indicated that low MPP is a risk factor for the progression of AKI in critically ill patients, with MPP < 60 mmHg being independently associated with the progression from AKI I to AKI III [17]. Maintaining stable mean perfusion pressure may decrease the risk of renal function impairment. A retrospective observational study by Wong et al. suggested that patients with septic shock who developed severe AKI exhibited lower MPP levels during the initial 24 h of ICU admission in comparison to patients without severe AKI [30]. In a study by Saito et al. [15], post-cardiac surgery patients were analyzed and categorized based on AKI progression. The study found no significant differences in pressure indicators between the groups initially. However, retrograde calculation analysis revealed that the AKI progression group had a significantly higher MPP retrograde value compared to the non-progression group, indicating a relatively lower renal perfusion status. Furthermore, a multicenter retrospective cohort study by Kotani et al. also indicated that in post-cardiac surgery patients, lower MPP was associated with the progression of AKI [31]. The aforementioned studies emphasize the significance of individualized blood pressure management, considering underlying illnesses and pre-existing conditions. This approach is crucial to maintain adequate renal perfusion pressure and prevent the occurrence of AKI. In this study, we found that higher levels of MPP (≥ 63mmHg) were associated with a lower incidence of AKI, further supporting the potential utility of MPP as a target variable for hemodynamic management in this critically ill septic patient population. Our research findings validate these previous study results in the large-scale MIMIC-IV database. We strengthened the reliability of our research findings by performing multivariable logistic regression analysis, which showed consistent results in subgroup analyses. This emphasizes the importance of utilizing MPP as a valuable indicator for blood pressure restoration and tissue perfusion in critically ill septic patients. Furthermore, we also observed a correlation between higher MPP levels and lower in-hospital mortality rates. In conclusion, these findings support the association between MPP and the risk of AKI in critically ill patients with sepsis.
Clinical implications
MPP is an indicator of the stable perfusion pressure maintained by blood in the arterial system [32]. Targeting high MPP levels in the early stages of patients with sepsis may have potential benefits in preventing renal injury. Previous studies have illustrated that lower levels of MPP may result in inadequate blood supply to the kidneys [33]. Hypotension resulting from factors such as fluid loss and systemic inflammatory response can compromise kidney perfusion, leading to impaired renal function in septic patients [12, 14]. In addition, inflammation can infiltrate and damage inflammatory cells in renal tissues, thereby impairing glomerular filtration function. This impairment can contribute to the development of glomerulonephritis and AKI [4, 5]. Previous animal studies have adequately demonstrated that renal ischemia leads to long-term loss of renal autoregulation [34]. Therefore, for septic patients, maintaining an appropriate level of MPP is crucial to ensure adequate blood flow perfusion to the kidneys. The current study’s findings underline the need for closer monitoring of renal perfusion and more active care in critically ill patients, particularly those with sepsis. They support the potential for interventions based on MPP to improve the prognosis of septic patients at risk of AKI. Clinicians can integrate MPP assessment into clinical practice to improve risk stratification, personalize patient care, identify high-risk individuals for AKI, and develop corresponding management strategies. However, the current literature on target MPP levels remains scarce, highlighting the need for additional research and prospective studies to validate these findings and establish standardized protocols for MPP monitoring and management.
Strengths and limitations
This study evaluated the relationship between early-recorded MPP and early-onset AKI in a large cohort of septic patients using the extensive MIMIC-IV database. The inclusion of a high number of cases aimed to capture real-world scenarios as much as possible. However, the study still has the following limitations. First, this study was a retrospective observational design and, therefore could not determine a causal association between MPP and the occurrence of AKI. Second, the data were gathered from the MIMIC IV database, and despite the inclusion of multivariate adjustments and subgroup analyses, the results were influenced by residual bias and unmeasured potential confounders. Third, in the extracted data, some of the relevant indexes with more missing data failed to be included in the study, and we did not take into account advanced hemodynamic data like the renal resistance index or peripheral vascular resistance. Lastly, to validate our findings, future research should incorporate stronger evidence, such as prospective cohort studies.
Conclusions
In summary, lower levels of MPP are associated with an increased incidence of AKI at 7 days in critically ill patients with sepsis. This study highlights the importance of MPP as a potential predictive factor for the occurrence of AKI in severe septic patients.
Data availability
Data is provided within the manuscript or supplementary information files.The datasets are available in the physionet (https://physionet.org/content/ mimic iv/2.2/).
Abbreviations
- MPP:
-
Mean perfusion pressure
- MAP:
-
Mean arterial pressure
- CVP:
-
Central venous pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- SAPS II:
-
Simplified acute physiology score II
- SOFA:
-
Sequential organ failure assessment
- WBC:
-
White blood cell
- RBC:
-
Red blood cell
- BUN:
-
Blood urea nitrogen
- AKI:
-
Acute kidney injury
- LOS:
-
Length of stay
- CHF:
-
Congestive heart failure
- CPD:
-
Chronic pulmonary disease
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA-J AM MED ASSOC. 2016;315(8):801–10.
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, Allegranzi B, Reinhart K. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. INTENS CARE MED. 2020;46(8):1552–62.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of Disease Study. Lancet. 2020;395(10219):200–11.
Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. KIDNEY INT. 2019;96(5):1083–99.
Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ-BRIT MED J. 2019;364:k4891.
Moman RN, Ostby SA, Akhoundi A, Kashyap R, Kashani K. Impact of individualized target mean arterial pressure for septic shock resuscitation on the incidence of acute kidney injury: a retrospective cohort study. ANN INTENSIVE CARE. 2018;8(1):124.
Angus DC, van der Poll T. Severe sepsis and septic shock. NEW ENGL J MED. 2013;369(9):840–51.
Xie Y, Zhang Y, Tian R, Jin W, Du J, Zhou Z, Wang R. A prediction model of sepsis-associated acute kidney injury based on antithrombin III. CLIN EXP MED. 2021;21(1):89–100.
Peters E, Antonelli M, Wittebole X, Nanchal R, Francois B, Sakr Y, Vincent JL, Pickkers P. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from the Intensive Care Over Nations audit. CRIT CARE. 2018;22(1):188.
Suarez J, Busse LW. New strategies to optimize renal haemodynamics. CURR OPIN CRIT CARE. 2020;26(6):536–42.
Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, May CN, Lankadeva YR. Sepsis-induced acute kidney injury: a disease of the microcirculation. Microcirculation. 2019;26(2):e12483.
John S. Lessons learned from kidney dysfunction: preventing organ failure. MED KLIN-INTENSIVMED. 2020;115(Suppl 1):21–7.
Vijayan A, Abdel-Rahman EM, Liu KD, Goldstein SL, Agarwal A, Okusa MD, Cerda J. Recovery after critical illness and acute kidney Injury. CLIN J AM SOC NEPHRO. 2021;16(10):1601–9.
Beloncle F, Rousseau N, Hamel JF, Donzeau A, Foucher AL, Custaud MA, Asfar P, Robert R, Lerolle N. Determinants of Doppler-based renal resistive index in patients with septic shock: impact of hemodynamic parameters, acute kidney injury and predisposing factors. ANN INTENSIVE CARE. 2019;9(1):51.
Sato R, Luthe SK, Nasu M. Blood pressure and acute kidney injury. CRIT CARE. 2017;21(1):28.
Morales-Alvarez MC. Nephrotoxicity of antimicrobials and antibiotics. ADV CHRONIC KIDNEY D. 2020;27(1):31–7.
Ostermann M, Liu K, Kashani K. Fluid Management in Acute kidney Injury. Chest. 2019;156(3):594–603.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. INTENS CARE MED. 2021;47(11):1181–247.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. CRIT CARE MED. 2011;39(2):259–65.
Chen CY, Zhou Y, Wang P, Qi EY, Gu WJ. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: a meta-analysis. CRIT CARE. 2020;24(1):80.
Ihara K, Ishigami J, Inoshita S. Predictors of withdrawal from renal replacement therapy among patients with acute kidney injury requiring renal replacement therapy. CLIN EXP NEPHROL. 2019;23(6):814–24.
Ostermann M, Hall A, Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients - a retrospective analysis. BMC NEPHROL. 2017;18(1):151.
Saito S, Uchino S, Takinami M, Uezono S, Bellomo R. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. CRIT CARE. 2016;20:74.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ-BRIT MED J. 2007;335(7624):806–8.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-related problems of the European Society of Intensive Care Medicine. INTENS CARE MED. 1996;22(7):707–10.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. NEPHRON. 2012;120(4):c179–84.
Sun R, Guo Q, Wang J, Zou Y, Chen Z, Wang J, Zhang Y. Central venous pressure and acute kidney injury in critically ill patients with multiple comorbidities: a large retrospective cohort study. BMC NEPHROL. 2022;23(1):83.
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. CRIT CARE. 2013;17(6):R278.
Peng Y, Wu B, Xing C, Mao H. Increased mean perfusion pressure variability is associated with subsequent deterioration of renal function in critically ill patients with central venous pressure monitoring: a retrospective observational study. Ren Fail. 2022;44(1):1976–84.
Wong BT, Chan MJ, Glassford NJ, Martensson J, Bion V, Chai SY, Oughton C, Tsuji IY, Candal CL, Bellomo R. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J CRIT CARE. 2015;30(5):975–81.
Kotani Y, Yoshida T, Kumasawa J, Kamei J, Taguchi A, Kido K, Yamaguchi N, Kariya T, Nakasone M, Mikami N, et al. The impact of relative hypotension on acute kidney injury progression after cardiac surgery: a multicenter retrospective cohort study. ANN INTENSIVE CARE. 2021;11(1):178.
Panwar R, Tarvade S, Lanyon N, Saxena M, Bush D, Hardie M, Attia J, Bellomo R, Van Haren F. Relative hypotension and adverse kidney-related outcomes among critically ill patients with shock. A Multicenter, prospective cohort study. AM J RESP CRIT CARE. 2020;202(10):1407–18.
Panwar R, Lanyon N, Davies AR, Bailey M, Pilcher D, Bellomo R. Mean perfusion pressure deficit during the initial management of shock–an observational cohort study. J CRIT CARE. 2013;28(5):816–24.
Post EH, Vincent JL. Renal autoregulation and blood pressure management in circulatory shock. CRIT CARE. 2018;22(1):81.
Acknowledgements
The authors sincerely thank all the participants in this cohort study and the Computational Physiology Laboratory team from the Massachusetts Institute of Technology for providing the raw data.
Funding
This research is supported by Project for Enhancing Young and Middle-aged Teacher’s Research Basis Ability in Colleges of Guangxi(2023KY0131).
Author information
Authors and Affiliations
Contributions
L.L. and D.B.H. designed the study and drafted the manuscript. X.H.L. and L.L. extracted the data from the MIMIC-IV database. L.L. analyzed and interpreted the data. S.W.Q., M.J.X., and L.Y.H. guided the literature review. L.L., D.B.H., X.H.L., S.W.Q., M.J.X., and L.Y.H. revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (The data for this study came from the MIMIC public database).
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Qin, S., Lu, X. et al. Association between the mean perfusion pressure and the risk of acute kidney injury in critically ill patients with sepsis: a retrospective cohort study. BMC Infect Dis 24, 806 (2024). https://doi.org/10.1186/s12879-024-09706-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09706-1