Abstract
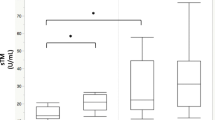
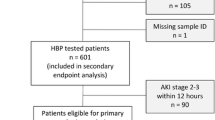
The incidence of sepsis-associated acute kidney injury (AKI) is on the rise. Recent studies have found a correlation between antithrombin III and AKI. We established a predictive model for sepsis-associated AKI based on plasma ATIII levels. A prospective study (March 2018–January 2020) was conducted in sepsis patients admitted to the Critical Care Medicine Department at Shanghai General Hospital. ATIII levels were obtained within 48 h after admission to the ICU and before the diagnosis of sepsis-associated AKI was recorded. Renal function was assessed by measuring serum creatinine levels and urine volume. Male sex, other cardiovascular disease, and low ATIII levels were identified as independent risk factors for AKI. Age, immune disease, and low ATIII levels were identified as independent risk factors for death. Plasma ATIII levels in the non-AKI group were higher than those in the AKI group, plasma ATIII levels were higher in the survival group than in the non-survival group, plasma ATIII levels in the non-CRRT group were higher than those in the CRRT group, and plasma ATIII levels in the non-CKD group were higher than those in the CKD group. ATIII was significantly higher in the group with pulmonary infection than in the group without pulmonary infection. ATIII was significantly lower in the celiac infection group than in the nonceliac infection group. There was no statistically significant difference between the ATIII in the gram-positive group and the gram-negative group. ATIII was significantly higher in medical patients than in surgical patients. The predictive model of sepsis-associated AKI established based on ATIII was ln[P/(1 − p)] = −1.211 × sex − 0.017 × ATIII + 0.022 × Cr + 0.004 × BUN − 2.8192. The model goodness-of-fit test (p = 0.000) and the area under the ROC curve of the model (0.9862) suggested that the model has a high degree of discrimination and calibration. ATIII reduction was closely related to the prognosis of patients with sepsis. ATIII reduction was an independent risk factor for sepsis-associated AKI and an independent risk factor for mortality in patients with sepsis. ATIII reduction could predict sepsis-associated AKI. Low ATIII predicted a poor prognosis.









Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II score
- SOFA:
-
Sepsis-related organ failure assessment
- COPD:
-
Chronic obstructive pulmonary disease
- ICU:
-
Intensive care unit
- AKI:
-
Acute kidney injury
- CRRT:
-
Continuous renal replacement therapy
- ATIII:
-
Antithrombin III
- KDIGO:
-
Kidney Disease Improving Global Outcomes
References
Godin M, Murray P, Mehta RL. Clinical approach to the patient with AKI and sepsis. Semin Nephrol. 2015;35(1):12–22.
Liarte S, Chaves-Pozo E, Abellán E, García-Ayala A. 17β-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev Comp Immunol. 2011;35(1):19–27.
Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab (TEM). 2007;18(10):371–8.
Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;41(Suppl 7):S490–7.
George RL, Mcgwin G, Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19(1):28–32.
Angstwurm MW, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–93.
Fourrier F, Jallot A, Leclerc L, et al. Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ Shock. 1994;43:171–8.
Christeff N, Carli A, Benassayag C, Bleichner G, Vaxelaire JF, Nunez EA. Relationship between changes in serum estrone levels and outcome in human males with septic shock. Circ Shock. 1992;36:249–55.
Gomez H, Ince C, De Backer D, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11.
Wen X, Peng Z, Kellum JA. Pathogenesis of acute kidney injury: effects of remote tissue damage on the kidney. Contrib Nephrol. 2011;174:129–37.
Post EH, Kellum JA, Bellomo R, Vincent JL. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 2016;91:45–60.
Regueira T, Andresen M, Mercado M, Downey P. Physiopathology of acute renal failure during sepsis. Med Intensiva. 2011;35(7):424–32.
Kaifei W, Sheling X, Kun X, Yan P, He W, Xie L. Biomarkers of sepsis-induced acute kidney injury. Biomed Res Int. 2018;2018:1–7.
Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–74.
Smith LE, Smith DK, Blume JD, Siew ED, Billings FT. Latent variable modeling improves AKI risk factor identification and AKI prediction compared to traditional methods. BMC Nephrol. 2017;18(1):55.
Koyner JL, Adhikari R, Edelson DP, Churpek MM. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol. 2016;11:1935–43.
Kalisvaart M, Schlegel A, Umbro I, et al. The AKI-predict-score: a new prediction model for acute kidney injury after liver transplantation. Transplantation. 2018;102:S414.
Rhodes Andrew, Evans Laura E, Levy MM, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Crit Care Med. 2017;45(3):1.
Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819–25.
Hafez T. Modification of diet in renal disease (MDRD) estimated glomerular filtration rate (eGFR) formula. Am J Cardiol. 2007;99(4):584–90.
Okusa MD, Davenport A. Reading between the (guide)lines—the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int. 2014;85(1):39–48.
Lourenço DM, Noguti MA, Juliano L. Antithrombin III dosage using the chromogenic substrate Tos-Gly-Pro-Arg-NAN, in several pathological situations. Rev Assoc Med Bras. 1992;41(6):373–8.
Alharbi A, Thompson JP, Brindle NP, Stover CM. Ex vivo modelling of the formation of inflammatory platelet-leucocyte aggregates and their adhesion on endothelial cells, an early event in sepsis. Clin Exp Med. 2019;19(3):321–37.
Vavrova L, Rychlikova J, Mrackova M, Novakova O, Zak A, Novak F. Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: a correlation with low HDL cholesterol and albumin. Clin Exp Med. 2016;16(4):557–69.
Allingstrup M, Wetterslev J, Ravn FB, et al. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensiv Care Med. 2016;42(4):505–20.
Katayama S, Nunomiya S, Koyama K, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care. 2017;21(1):229.
Yin J, Wang F, Kong Y, et al. Antithrombin III prevents progression of chronic kidney disease following experimental ischaemic-reperfusion injury. J Cell Mol Med. 2017;21(12):3506–14.
Samejima T, Yamashita T, Takeda Y, Adachi T. Low antithrombin levels accompanied by high urine protein/creatinine ratios are predictive of acute kidney injury among CS patients with preeclampsia. J Matern Fetal Neonatal Med. 2019;1:1–241.
Kong Y, Yin J, Cheng D, et al. Antithrombin III attenuates AKI following acute severe pancreatitis. Shock. 2017;49:1.
Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891.
Tsushida K, Tanabe K, Masuda K, et al. Estrogen-related receptor α is essential for maintaining mitochondrial integrity in cisplatin-induced acute kidney injury. Biochem Biophys Res Commun. 2018;498:918–24.
Kang K, Lee J, Lee A, et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep. 2014;9(6):2061–8.
Ikeda M, Swide T, Vayl A, Lahm T, Anderson S, Hutchens MP. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit Care. 2015;19:332.
Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166.
Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104(5):1404–10.
Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340–3.
Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–14.
Liu L, Benten WP, Wang L, et al. Modulation of Leishmania donovani infection and cell viability by testosterone in bone marrow-derived macrophages: signaling via surface binding sites. Steroids. 2005;70:604–14.
Funding
The present study was supported by grants from Shanghai Science and Technology Committee Scientific and Technological Support Project (Grant Nos. 18411950600 and 18411950602, respectively), Clinical Research Innovation Plan of Shanghai General Hospital (Grant No. CTCCR-2016B01) Wu Jieping Medical Foundation (Grant No. 320.6750.18546), and Songjiang district science and technology Project (19SJKJGG92).
Author information
Authors and Affiliations
Contributions
YX, YZ, and RT were involved in conception and design; RW, RT, and ZZ were involved in administrative support; WJ and JD were involved in provision of study materials or patients; YX was involved in collection and assembly of data; YZ was involved in data analysis and interpretation; all authors were involved in manuscript writing. All authors were involved in final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval was obtained from Shanghai General Hospital Institutional Review Board [Approval No. (2018)KY004].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, Y., Zhang, Y., Tian, R. et al. A prediction model of sepsis-associated acute kidney injury based on antithrombin III. Clin Exp Med 21, 89–100 (2021). https://doi.org/10.1007/s10238-020-00656-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00656-x