Abstract
Background
Vaccination is effective in preventing viral respiratory infectious diseases through protective antibodies and the gut microbiome has been proven to regulate human immunity. This study explores the causal correlations between gut microbial features and serum-specific antiviral immunoglobulin G (IgG) levels.
Methods
We conduct a two-sample bidirectional Mendelian randomization (MR) analysis using genome-wide association study (GWAS) summary data to explore the causal relationships between 412 gut microbial features and four antiviral IgG (for influenza A, measles, rubella, and mumps) levels. To make the results more reliable, we used four robust methods and performed comprehensive sensitivity analyses.
Results
The MR analyses revealed 26, 13, 20, and 18 causal associations of the gut microbial features influencing four IgG levels separately. Interestingly, ten microbial features, like genus Collinsella, species Bifidobacterium longum, and the biosynthesis of L-alanine have shown the capacity to regulate multiple IgG levels with consistent direction (rise or fall). The reverse MR analysis suggested several potential causal associations of IgG levels affecting microbial features.
Conclusions
The human immune response against viral respiratory infectious diseases could be modulated by changing the abundance of gut microbes, which provided new approaches for the intervention of viral respiratory infections.
Similar content being viewed by others
Introduction
Respiratory infectious diseases are caused by pathogens like viruses, bacteria, mycoplasma, and chlamydia through respiratory secretions [1]. Pandemics of respiratory infectious diseases often affect many countries or regions and cause large numbers of deaths [2, 3]. Prevention of respiratory infectious diseases continues to be an important public health initiative and vaccines play a critical role in prevention [4]. However, the capacity of protection by vaccination varies among individuals [5, 6]. Vaccines mediate protection by inducing B cells which produce antigen-specific antibodies [7]. When the host contracts a virus or receives a virus-specific vaccine, Immunoglobulin G (IgG) antibodies are secreted from B cells and bind to a variety of pathogens or antigens to avoid infection or provide protection [8, 9]. Previous studies showed that IgG level strongly correlated with the immune response to vaccination, and the IgG level could reflect the protective function of the specific vaccine [10, 11]. Therefore, finding and intervening factors that affect individual IgG levels can improve vaccine protection function and enhance herd immunity.
Several studies have illuminated important roles for the gut microbiota in modulating B cells response that perhaps have important implications for the effects of the microbiota on specific IgG levels [12]. Moreover, the gut microbiota also produces a large number of metabolites that have the potential to adjust immune responses. Short-chain fatty acids (SCFAs) had been shown to increase acetyl-coenzyme A and regulate metabolic sensors to increase oxidative phosphorylation, glycolysis, and fatty acid synthesis in B cells to support antibody production, and had been shown to enhance the expression of genes involved in plasma cells (effector B cell) differentiation [7]. In addition, gut microbiota could be regulated in a convenient and harmless way through daily diet, probiotics, and prebiotics [13]. Thus, it is necessary to study the specific IgG level of immune response to vaccination against respiratory infectious diseases from the perspective of gut microbiota.
Influenza A, measles, rubella, and mumps caused by viruses are typical respiratory infectious diseases of concern worldwide. In European, the vaccination rates for the four infectious diseases were high, which contributed to the high seroprevalences of these four virus-specific IgG levels [14]. Based on the high seroprevalences, this research had enough sample sizes to explore the causal correlation between gut microbiota and virus-specific IgG levels.
Mendelian randomization (MR), regarding genetic variants as instrumental variables (IVs) to explore the causal correlations between risk factors and diseases, has been widely used in causal inference [15]. Two-sample MR is an MR-based research method applicable to situations where exposure and outcomes are derived from two different populations [16]. Here, we used two-sample MR to illustrate the causal relationships between gut microbiota and serum virus-specific IgG levels of viral respiratory infectious diseases.
Methods
Data collection and processing
The genome-wide association studies (GWAS) summary statistics of 412 gut microbial features (including 207 microbial taxa and 205 functional pathways) were collected from NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics), which originally came from the Dutch Microbiome Project (DMP), a sub-project of LifeLines in Netherlands, and it investigated feces and phenotype information to assess the impact of different exposures and lifestyles on gut microbial composition using 7738 LifeLines participants [17, 18]. Of these DMP participants, 58.1% were female, mean age was 48.5 years, and mean BMI value was 25.58.
Specifically, the gut microbial taxa were divided according to Taxonomy, using “s_”, “g_”, “f_”, “o_”, “c_”, “p_”, and “k_” to represent species, genus, family, order, class, phylum, and kingdom, respectively. The pathways were identified from the MetaCyc Metabolic Pathways Database (https://metacyc.org/ ), presenting in gut microbes and involving primary and secondary metabolism, as well as associated metabolites, reactions, enzymes, and genes [19]. Due to the lengthy names of functional pathways, we chose to use specific abbreviations, the full names of which were given in Supplementary Table S1. We regarded standard deviation (s.d.) as the unit of change of gut microbial features (Supplementary Table S2).
In addition, we estimated the heritability (h2) of all 412 gut microbiome features using linkage disequilibrium score regression (LDSC) (v1.0.1), with the 503 European individuals from the 1000 Genome Project as the reference panel for analysis [20].
We collected four virus-specific immunoglobulins G (IgG) levels datasets (anti-IAV IgG, anti-measles virus IgG, anti-rubella virus IgG, and anti-mumps virus IgG levels) focusing on long-term immunity also from the NHGRI-EBI GWAS Catalog, and the datasets originally were from 1,000 healthy individuals of the Milieu Interieur (MI) cohort in France [21]. This cohort consisted of 500 males and 500 females, with an age range between 20 and 70 years old. For the four mentioned IgG levels, Elisa or multiplex EIA techniques were used for quantitative detection, and the seroprevalence rate were 77.7%, 88.5%, 93.5%, and 91.2% separately [14]. We also used s.d. as the unit of change.
Then, we filtered the single nucleotide polymorphisms (SNPs) in the abovementioned 416 data for subsequent analyses [22, 23]. We retained the SNPs following: (i) on the autosomes (1–22); (ii) minimum allele frequency (MAF) larger than 0.001; (iii) more than 70% of observers had these specific SNPs.
MR analysis
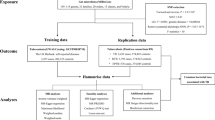
Two-sample MR is a method to estimate the causal effect of an exposure on an outcome using only summary statistics from GWAS [24, 25]. We treated the gut microbial features as exposures and the four anti-virus IgG levels as outcomes to conduct a two-sample MR study. Based on the datasets, we followed a strict screening procedure to select IVs in other previous MR studies [26, 27] (Fig. 1). First, we used the suggestive threshold P < 1E-5 to select genetic variations associated with each particular gut microbial feature. Second, we performed the clumping process [linkage disequilibrium (LD) r2 < 0.1, sliding window of 1 Mb] in the reference panel and retained identified LD-independent SNPs. Third, for each respiratory infectious disease, we screened the GWAS database to exclude SNPs associated with a specific IgG level to avoid potential pleiotropy and removed palindromic SNPs. Specifically, if the number of IVs was three or less, we excluded the microbial feature.
To explore the potential causal effects of gut microbial features on the four IgG levels, we performed two-sample MR analyses using the four methods including fixed-effects (IVW (fe)) and multiplicative random-effects inverse variance weighting (IVW (mre)), weighted median (WM), and MR-Egger regression methods in the TwoSampleMR (v0.5.6) R package [28,29,30]. Importantly, we prefer to interpret the results based on the IVW (fe) model without heterogeneity or pleiotropy of IVs [31]. The MR-Egger regression model, where its intercept was used to evaluate the directional pleiotropy of instruments, is preferred in the presence of pleiotropy while the WM method is preferred to account for it in the presence of heterogeneity [32, 33]. As a result, IVW (fe) and MR-Egger regression methods were mainly used to estimate their causal effects with P < 0.05. We also performed false discovery rate (FDR) to adjust the false positive rate.
Sensitivity analysis
First, we used Cochran’s Q test to conduct a heterogeneity test to examine the differences between IVs. We used the P value of Q statistics < 0.05 as the significant level. Second, we performed a pleiotropy test. When there was a statistical difference between the intercept and zero (P < 0.05), horizontal pleiotropy existed. In addition, we performed Mendelian Randomization Pleiotropy RESidual Sum and Outlier analysis (MR-PRESSO) to detect and correct the effects from outliers [34].
Reverse-direction MR analysis
We were also concerned about whether these four IgG levels affect the abundance of gut microbial features in humans. We used the same settings as the above-mentioned MR analysis (P = 1.0E-5, r2 = 0.1, and window size = 1 Mb) to select IVs of four antiviral IgG levels (Fig. 1). In addition, we computed the sum of values of variance in phenotype explained (PVE) to assess the explanatory power of IVs, and computed the F statistics following reference to judge whether IVs are strong instruments [35].
Results
Heritability of gut microbial features
Heritability is the proportion of variation in a given gut microbial feature that can be attributed to genetic factors. For all 412 gut microbial features, the median heritability of all features was 5.25%, for example, 22.29% of species Alistipes senegalensis and 19.48% of Aspartate superpathway, while only 0.02% of family Prevotellaceae (Supplementary Table S1). The relative abundance variance of five genera could be explained over 10.00% by their corresponding independent genetic variants, including genus Bifidobacterium (13.05%), Barnesiella (13.00%), Bacteroidales noname (12.08%), Oscillibacter (12.26%) and Subdoligranulum (10.39%) (Fig. 2).
MR analyses
We calculated the causal effects of the remained various gut microbial features with four anti-virus IgG levels respectively.
Anti-IAV IgG level
It was shown that diverse microbial taxa or functional pathways have different effects on a particular IgG, which manifested as positive or negative causal associations with different effect values. The number of gut microbial features causally associated with anti-IAV IgG level was the largest including 12 taxa and 14 pathways (P < 0.05). The taxa all belonged to the four phyla of Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria, which were consistent with the fact that these four phyla were the largest of the nine identified phyla of human gut microbiota [36].
Of these 12 microbial taxa, nine had the potential to elevate serum anti-IAV IgG concentration, including genus Bilophila (\( \beta \)=0.081, P = 0.04), species Bilophila unclassified (\( \beta \)=0.078, P = 0.013), genus Collinsella (\( \beta \)=0.076, P = 0.026), genus Ruminococcus (\( \beta \)=0.066, P = 0.049), family Veillonellaceae (\( \beta \)=0.066, P = 0.010), species Bifidobacterium longum (\( \beta \)=0.061, P = 0.035), phylum Bacteroidetes (\( \beta \)=0.062, P = 0.023), class Bacteroidia (\( \beta \)=0.062, P = 0.023), and order Bacteroidales (\( \beta \)=0.062, P = 0.023). The results of the last three taxa were similar perhaps because of their affiliations. While three other taxa may decrease anti-IAV IgG level, including genus Lachnospiraceae noname (\( \beta \)=-0.103, P = 0.013), genus Coprococcus (\( \beta \)=-0.068, P = 0.022), and species Lachnospiraceae bacterium 3_1_46FAA (\( \beta \)=-0.065, P = 0.035) (Fig. 3A; Supplementary Table S3).
Causal effects of gut microbial features on anti-IAV IgG and anti-measles virus IgG levels. The forest plots represent the MR estimates beta and 95%CI values of the odds ratio of gut microbial features on different serum anti-IAV IgG (A, B) and anti-measles virus IgG levels (C, D), as estimated using the fixed effect (IVW) two-sample MR or MR-Egger methods
Similar to taxa, seven pathways had positive causal associations with anti-IAV IgG level, for example, the higher functional capacity for inosine 5’-phosphate degradation (PWY-5695) contributed to a higher level of anti-IAV IgG (\( \beta \)=0.087, P = 0.007). Nevertheless, an increase functional capacity of seven pathways may lower anti-IAV IgG, such as the capacity of pantothenate and coenzyme A biosynthesis (PANTOSYN-PWY, \( \beta \)=-0.115, P = 0.020) with the largest negative effect (Fig. 3B; Supplementary Table S3).
Anti-measles virus IgG
The number of gut microbial features causally associated with anti-measles IgG level was 13, involving five taxa and eight pathways (Fig. 3, C-D; Supplementary Table S4). Not entirely consistent with anti-IAV IgG level, these five taxa did not belong to phylum Bacteroidetes. Four taxa including species Bifidobacterium longum (\( \beta \)=0.117, P = 0.007), genus Collinsella (\( \beta \)=0.105, P = 0.039), species Desulfovibrio piger (\( \beta \)=0.104, P = 0.025), and genus Ruminococcaceae noname (\( \beta \)=0.050, P = 0.028) had the potential to increase anti-measles virus IgG level. Only species Coprococcus catus may decrease the titer of anti-measles virus IgG (\( \beta \)=-0.114, P = 0.041).
For pathways, the higher functional capacity for L-alanine biosynthesis (PWY0-1061, \( \beta \)=0.098, P = 0.024), NAD salvage (PYRIDNUCSAL-PWY, \( \beta \)=0.084, P = 0.036), and glycerol degradation to 1,3-propanediol (GOLPDLCAT-PWY, \( \beta \)=0.069, P = 0.030) may elevate the level of anti-measles virus IgG. In addition, an increase functional capacity of five pathways could lower anti-measles virus IgG including ppGpp biosynthesis (PPGPPMET-PWY, \( \beta \)=-0.744, P = 0.026), purine nucleobases degradation (P164-PWY, \( \beta \)=-0.288, P = 0.042), N-acetylneuraminate degradation (P441-PWY, \( \beta \)=-0.101, P = 0.031), hexitol fermentation to hexitol fermentation to lactate, formate, ethanol and acetate (P461-PWY, \( \beta \)=-0.099, P = 0.022), and purine nucleotides de novo biosynthesis (PWY-841, \( \beta \)=-0.092, P = 0.022). Considering the pleiotropy of IVs, the causal effects of P164-PWY (P = 0.031) and PPGPPMET-PWY (P = 0.026) with anti-measles virus IgG were explained by the results of MR-Egger regression method.
Anti-rubella virus IgG
MR analyses showed that three microbial taxa and 17 functional pathways had the potential to influence the anti-rubella virus IgG level (Fig. 4, A-B; Supplementary Table S5). These taxa only involving phylum Firmicutes and Bacteroidetes. The species Bacteroides intestinalis (\( \beta \)=0.116, P = 0.016) and genus Ruminococcaceae noname (\( \beta \)=0.067, P = 0.045) perhaps had a positive impact on increasing anti-rubella virus IgG level while the species Eubacterium hallii (\( \beta \)=-0.104, P = 0.024) had a negative impact on this IgG level.
Causal effects of gut microbial features on anti-rubella virus IgG and anti-mumps virus IgG levels. The forest plots represent the MR estimates beta and 95%CI values of the OR of gut microbial features on anti-rubella virus IgG (A, B) and anti-mumps virus IgG levels (C, D), as estimated using the fixed effect (IVW) or MR-Egger method
The pathways positively correlated with anti-rubella virus IgG were almost biosynthesis pathways, such as L-threonine biosynthesis (THRESYN-PWY,\( \beta \)=0.142, P = 0.043), palmitate biosynthesis (PWY-5971,\( \beta \)=0.130, P = 0.042), and L-glutamate and L-glutamine biosynthesis (PWY-5505,\( \beta \)=0.111, P = 0.014). An increase functional capacity of other eight pathways may decrease anti-measles virus IgG level mainly about biosynthesis or degradation pathways, like purine nucleotides de novo biosynthesis (PWY-841,\( \beta \)=-0.213, P = 0.002), and glycogen degradation (GLYCOCAT-PWY,\( \beta \)=-0.130, P = 0.035). Given that there was pleiotropy of IVs (P = 0.048), we used MR-Egger regression method to show the causal effect between the capacity of a kind of heme biosynthesis (HEMESYN2-PWY) and the anti-rubella virus IgG level.
Anti-mumps virus IgG
Five taxa and 13 functional pathways may adjust anti-mumps virus IgG (Fig. 4, C-D; Supplementary Table S6). Similar to anti-rubella virus IgG, these taxa also belonged to phylum Firmicutes and Bacteroidetes. In which, the rise abundance of genus Subdoligranulum (\( \beta \)=0.410, P = 0.015), species Bacteroides ovatus (\( \beta \)=0.274, P = 0.025), family Lachnospiraceae (\( \beta \)=0.083, P = 0.017) and species Eubacterium rectale (\( \beta \)=0.077, P = 0.022) may contribute to a higher serum anti-mumps virus IgG. However, species Ruminococcus callidus (\( \beta \)=-0.063, P = 0.028) perhaps decrease it.
Moreover, several pathways including L-methionine biosynthesis (PWY-5345, \( \beta \)=0.464, P = 0.039), D-galactose degradation (PWY66-422, \( \beta \)=0.084, P = 0.042), purine nucleobases degradation (P164-PWY, \( \beta \)=0.073, P = 0.001), L-rhamnose degradation (RHAMCAT-PWY, \( \beta \)=0.072, P = 0.027), and pyrimidine ribonucleosides degradation (PWY-7209, \( \beta \)=0.065, P = 0.008) could up-regulate the concentrate of anti-mumps virus IgG in serum. On the contrary, a high capacity of several functional pathways like phospholipid biosynthesis in bacteria (PHOSLIPSYN-PWY, \( \beta \)=-0.315, P = 0.044), purine nucleotides de novo biosynthesis (PWY-841, \( \beta \)=-0.085, P = 0.008), L-arginine biosynthesis in archaebacteria (PWY-7400, \( \beta \)=-0.077, P = 0.025), and enterobacterial common antigen biosynthesis (ECASYN-PWY, \( \beta \)=-0.038, P = 0.020) played a role in lowering anti-mumps virus IgG. Since the pleiotropy of IVs presented, we selected MR-Egger regression method to access the causal relationships between Subdoligranulum (P = 0.022), Bacteroides ovatus (P = 0.029), L-methionine biosynthesis pathways (P = 0.046) and anti-mumps virus IgG.
Overlapping gut microbial features in the MR analyses
If we identify some common microbial traits that modulate different IgG levels, altering the abundance of specific microbial taxa or pathways was an excellent way to increase antiviral IgG levels simultaneously. Interestingly, ten certain microbial taxa or functional pathways were causally associated with multiple serum-specific antiviral IgG levels (Table 1). Both genus Collinsella and species Bifidobacterium longum were positively correlated with anti-IAV IgG and anti-measles virus IgG. The genus Ruminococcaceae noname was causally correlated with anti-measles virus IgG and anti-rubella virus IgG. In addition, seven pathways were positively or negatively associated with multiple anti-virus IgG levels, in which, a higher function capacity of purine nucleotides de novo biosynthesis (PWY-841) could decrease anti-measles virus IgG, anti-rubella virus IgG, and anti-mumps virus IgG levels at the same time. Moreover, the improvements in the L-alanine biosynthesis and nicotinamide adenine dinucleotide (NAD) salvage (PWY0-1061 and PYRIDNUCSAL-PWY) also could increase in these IgG levels.
As can be seen, the directions of associations of any microbial features with different IgG levels were consistent, contributing to the rise or fall of IgG levels, except a pathway of purine nucleobases degradation (P164-PWY). We suspected that the discordance may be caused by fewer IVs of this pathway and different choices of MR methods.
Reverse MR analysis
Using anti-IAV IgG, anti-measles virus IgG, anti-rubella virus IgG, and anti-mumps virus IgG as exposure, we extracted 12, 11, 10, and six SNPs as IVs. The sum of values of PVE was 10.6%, 6.6%, 7.7%, and 5.1%. The F statistics of the IVs screened for the four IgG levels were all above 10, indicating that these IVs are strong instruments (Supplementary Table S7).
After reverse MR analyses, we found 24, 16, 20, and 10 gut microbial features affected by these four IgG levels respectively (Supplementary File: Figure S1 and S2) In addition, we identified five taxa (phylum Verrucomicrobia, species Parabacteroides merdae, Alistipes finegoldii, Eubacterium eligens, and Bacteroides plebeius) without any pathway that could be affected by more than one IgG levels (Table 2).
Discussion
This study performed comprehensive two-sample MR analyses to reveal the causal relationships between gut microbial features and antiviral IgG levels. Importantly, these inferred relationships were proved to be robust through various sensitivity analyses.
Previous studies have showed that some taxa influenced human immune system such as Bacteroidetes which could stimulate the innate immune system and Ruminococcus which contributed to upregulate immunity by producing short-chain fatty acid (SCFA), supporting the results of these taxa had positive effect on anti-IAV IgG in this study [37, 38]. In addition, we found that species Desulfovibrio piger may increase serum anti-measles virus IgG level. In fact, Desulfovibrio piger was the most common sulfate-reducing bacteria in healthy adults, and positively associated with beneficial genera like Bacteroides and Ruminococcus [39, 40]. For another, species Coprococcus catus had a negative impact on anti-measles virus IgG level, and it has proved to generate propanoic acid (a SCFAs) which had immunomodulatory properties [41, 42]. Subdoligranulum has been proved to simulate TH17 cell expansion and serum specific IgG [43]. Bacteroides ovatus was not only previously reported to modulate intestinal immunity, but also correlated with the host genetic variant [44, 45]. Lachnospiraceae of gut bacteria are abundant in healthy humans, and influence the hosts by producing SCFAs, converting bile acids, and facilitating colonization resistance against specific pathogens [46].
Interestingly, we also found ten microbial features could simultaneously regulate multiple antiviral IgG levels, such as genus Collinsella, Ruminococcaceae noname, and species Bifidobacterium longum, which will provide a reference for preventing different respiratory infectious diseases by adjusting same gut microbial traits. Previous studies have shown that Collinsela was one of the core microbiotas in healthy people, and a lower abundance of Collinsela predicted a higher respiratory infectious disease mortality [47]. Bifidobacterium longum has also attracted considerable attention among gut bacteria. In a double-blind study, Bifidobacterium longum stimulated immune function in 45 elderly hospitalized patients who had received influenza vaccines [48]. These surveys supported our findings of a high abundance of Collinsela and Bifidobacterium longum could enhance human immunity by elevating antibody concentrations. Moreover, this study confirmed that the functional capacity of microbial pathways, especially those involved in nucleotide and amino acid synthesis, may also affect the long-term immunity levels against respiratory infectious diseases.
The reverse MR analyses illuminated that human immune capacity also had an impact on the abundance of gut microbial features. However, the specific mechanism under antiviral antibodies regulate microbes was still unclear.
While our data-driven approach highlighted the potential of MR to uncover associations between microbiota and immune indicators, we should be cautious about the causalities. There were some limitations of this study that should be considered. First, similar to other MR studies, we have assumed linear relationships between gut microbial features and antiviral IgG levels in the MR model [49]. Nonetheless, we could not rule out the possibility that the relationships between gut microbial features and IgG levels were actually non-linear. Second, gender and age are the most common confounding factors in epidemiology, but we were unable to use GWAS summary data for stratified analyses to estimate and validate specific causal effects by gender or age stage. Besides, although MR design used genetic variants as IVs to minimize the effects of environmental confounding factors, it still cannot completely remove the confounding bias. Third, part of the microbial features in this study utilized few SNPs as IVs, resulting in a limited ability to identify causal relationships. Fourth, we performed FDR correction for P values, and used a nominal significance level of 0.05. If there are larger sample GWAS data available in the future, the relationship between gut microbiome and IgG level may become more significant after correction.
Conclusions
This study has identified causal relationships between certain gut microbial features and serum-specific antiviral IgG levels. The results added new evidence for the influence of gut microbes on the human immune system, which provides a reference for enhancing population immunity to prevent respiratory infectious diseases after vaccination by adjusting gut microbes from a clinical and public health perspective.
Data availability
The summary statistics of 412 gut microbial features (Study accession numbers: GCST90027446-GCST90027857) and four anti-viral IgG level (Study accession numbers: GCST006350-GCST006353) used in this article could be downloaded from NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics ). The main data generated or analyzed during this study are included in this published article/ Supplementary File, and Supplementary Tables, and further inquiries can be directed to the corresponding author.
Abbreviations
- IAV:
-
Influenza A virus
- IgG:
-
Immunoglobulin G
- IVW:
-
Inverse variance weighted
- WM:
-
Weighted-median
- GWAS:
-
Genome-wide association study
- MR:
-
Mendelian randomization
- IVs:
-
Instrumental variables
- DMP:
-
Dutch Microbiome Project
- MI:
-
Milieu Interieur
- S/CO:
-
Signal/cut-off ratio
- IA:
-
Index Antibody
- H2 :
-
Heritability
- LD:
-
Linkage disequilibrium
- LDSC:
-
Linkage disequilibrium score regression
- MAF:
-
Minimum allele frequency
- CI:
-
Confidence interval
- MR-PRESSO:
-
Mendelian Randomization Pleiotropy RESidual Sum analysis
- PVE:
-
Variance in phenotype explain
- FDR:
-
False discovery rate
References
Bourouiba L. Fluid Dynamics of Respiratory Infectious diseases. Annu Rev Biomed Eng. 2021;23:547–77. https://doi.org/10.1146/annurev-bioeng-111820-025044.
Hübschen JM, Gouandjika-Vasilache I, Dina J, Measles. Lancet. 2022;399(10325):678–90. https://doi.org/10.1016/S0140-6736(21)02004-3.
Winter AK, Moss WJ, Rubella. Lancet. 2022;399(10332):1336–46. https://doi.org/10.1016/S0140-6736(21)02691-X.
Orenstein WA, Ahmed R. Simply put: Vaccination saves lives. Proc Natl Acad Sci U S A. 2017;114(16):4031–3. https://doi.org/10.1073/pnas.1704507114.
Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol. 2017;33:577–99. https://doi.org/10.1146/annurev-cellbio-100616-060718.
Zimmermann P, Curtis N. Factors that influence the Immune response to vaccination. Clin Microbiol Rev. 2019;32(2). https://doi.org/10.1128/CMR.00084-18.
Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202–14. https://doi.org/10.1016/j.chom.2016.07.001.
Valenzuela NM, Schaub S. The Biology of IgG subclasses and their clinical relevance to transplantation. Transplantation. 2018;102(1). https://doi.org/10.1097/TP.0000000000001816.
Wang TT, Ravetch JV. Functional diversification of IgGs through fc glycosylation. J Clin Invest. 2019;129(9):3492–8. https://doi.org/10.1172/JCI130029.
Montoya JG, Adams AE, Bonetti V, Deng S, Link NA, Pertsch S, Olson K, Li M, Dillon EC, Frosch DL. Differences in IgG antibody responses following BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines. Microbiol Spectr. 2021;9(3):e0116221. https://doi.org/10.1128/Spectrum.01162-21.
Miyazaki H, Yamanaka G, Furukawa K, Ichiki M. Effect of vaccine program on IgG antibody titers for measles, rubella, varicella, and mumps in young adults in Japan: survey between 2018 and 2021. J Infect Chemother. 2022;28(10):1410–4. https://doi.org/10.1016/j.jiac.2022.06.016.
New JS, Dizon BLP, Fucile CF, Rosenberg AF, Kearney JF, King RG. Neonatal exposure to commensal-Bacteria-derived antigens directs polysaccharide-specific B-1 B cell Repertoire Development. Immunity. 2020;53(1). https://doi.org/10.1016/j.immuni.2020.06.006.
Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. https://doi.org/10.1038/s41575-018-0061-2.
Scepanovic P, Alanio C, Hammer C, Hodel F, Bergstedt J, Patin E, Thorball CW, Chaturvedi N, Charbit B, Abel L, et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10(1):59. https://doi.org/10.1186/s13073-018-0568-8.
Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18(1):312. https://doi.org/10.1186/s12916-020-01778-5.
Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600–5. https://doi.org/10.1038/s41588-019-0350-x.
Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sánchez S, Chen L, Vila AV, Gacesa R, Sinha T, Collij V, et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet. 2022;54(2):143–51. https://doi.org/10.1038/s41588-021-00992-y.
Scholtens S, Smidt N, Swertz MA, Bakker SJL, Dotinga A, Vonk JM, van Dijk F, van Zon SKR, Wijmenga C, Wolffenbuttel BHR, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44(4):1172–80. https://doi.org/10.1093/ije/dyu229.
Torbica A, Radosavljević M, Belović M, Djukić N, Marković S. Overview of nature, frequency and technological role of dietary fibre from cereals and pseudocereals from grain to bread. Carbohydr Polym. 2022;290:119470. https://doi.org/10.1016/j.carbpol.2022.119470.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. https://doi.org/10.1038/nature15393.
Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, Alanio C, Scepanovic P, Hammer C, Jönsson F, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19(3):302–14. https://doi.org/10.1038/s41590-018-0049-7.
Yang S, Zhou X. Accurate and scalable construction of polygenic scores in Large Biobank Data Sets. Am J Hum Genet. 2020;106(5):679–93. https://doi.org/10.1016/j.ajhg.2020.03.013.
Yang S, Zhou X. PGS-server: accuracy, robustness and transferability of polygenic score methods for biobank scale studies. Brief Bioinform. 2022;23(2). https://doi.org/10.1093/bib/bbac039.
Zeng P, Wang T, Zheng J, Zhou X. Causal association of type 2 diabetes with amyotrophic lateral sclerosis: new evidence from mendelian randomization using GWAS summary statistics. BMC Med. 2019;17(1):225. https://doi.org/10.1186/s12916-019-1448-9.
Chauquet S, Zhu Z, O’Donovan MC, Walters JTR, Wray NR, Shah S. Association of Antihypertensive Drug Target genes with Psychiatric disorders: a mendelian randomization study. JAMA Psychiatry. 2021;78(6):623–31. https://doi.org/10.1001/jamapsychiatry.2021.0005.
Huang P, Hou Y, Zou Y, Ye X, Yu R, Yang S. The Causal effects of primary biliary cholangitis on thyroid dysfunction: a two-sample mendelian randomization study. Front Genet. 2021;12:791778. https://doi.org/10.3389/fgene.2021.791778.
Huang P, Zou Y, Zhang X, Ye X, Wang Y, Yu R, Yang S. The Causal effects of Insomnia on Bipolar Disorder, Depression, and Schizophrenia: a two-sample mendelian randomization study. Front Genet. 2021;12:763259. https://doi.org/10.3389/fgene.2021.763259.
Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, Chen G, Zhao S, Zhang Z, Shen J, et al. Education and lung cancer: a mendelian randomization study. Int J Epidemiol. 2019;48(3):743–50. https://doi.org/10.1093/ije/dyz121.
Gu X, Dou M, Cao B, Jiang Z, Chen Y. Peripheral level of CD33 and Alzheimer’s disease: a bidirectional two-sample mendelian randomization study. Transl Psychiatry. 2022;12(1):427. https://doi.org/10.1038/s41398-022-02205-4.
Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11(1):376. https://doi.org/10.1038/s41467-019-14156-4.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. https://doi.org/10.1002/gepi.21758.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. https://doi.org/10.1093/ije/dyv080.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. https://doi.org/10.1002/gepi.21965.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7.
Wang K, Shi X, Zhu Z, Hao X, Chen L, Cheng S, Foo RSY, Wang C. Mendelian randomization analysis of 37 clinical factors and coronary artery disease in east Asian and European populations. Genome Med. 2022;14(1):63. https://doi.org/10.1186/s13073-022-01067-1.
Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16(8):457–70. https://doi.org/10.1038/s41579-018-0036-x.
Du C-T, Gao W, Ma K, Yu S-X, Li N, Yan S-Q, Zhou F-H, Liu Z-Z, Chen W, Lei L-C, et al. MicroRNA-146a Deficiency protects against Listeria monocytogenes infection by modulating the gut microbiota. Int J Mol Sci. 2018;19(4). https://doi.org/10.3390/ijms19040993.
Xia T, Duan W, Zhang Z, Li S, Zhao Y, Geng B, Zheng Y, Yu J, Wang M. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res Int. 2021;140:110064. https://doi.org/10.1016/j.foodres.2020.110064.
Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci USA. 2013;110(33):13582–7. https://doi.org/10.1073/pnas.1312524110.
Chen Y-R, Jing Q-L, Chen F-L, Zheng H, Chen L-D. Yang Z-C. is not always associated with adverse health effects in the Guangdong gut Microbiome Project. PeerJ. 2021;9:e12033. https://doi.org/10.7717/peerj.12033.
El Hage R, Hernandez-Sanabria E, Calatayud Arroyo M, Props R, Van de Wiele T. Propionate-Producing Consortium restores antibiotic-Induced Dysbiosis in a dynamic model of the human intestinal microbial ecosystem. Front Microbiol. 2019;10:1206. https://doi.org/10.3389/fmicb.2019.01206.
Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, et al. Propionic Acid shapes the multiple sclerosis Disease Course by an Immunomodulatory mechanism. Cell. 2020;180(6). https://doi.org/10.1016/j.cell.2020.02.035.
Chriswell ME, Lefferts AR, Clay MR, Hsu AR, Seifert J, Feser ML, Rims C, Bloom MS, Bemis EA, Liu S, et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of. Sci Transl Med. 2022;14(668):eabn5166. https://doi.org/10.1126/scitranslmed.abn5166.
Yang C, Mogno I, Contijoch EJ, Borgerding JN, Aggarwala V, Li Z, Siu S, Grasset EK, Helmus DS, Dubinsky MC, et al. Fecal IgA levels are determined by strain-level differences in Bacteroides ovatus and are modifiable by gut microbiota manipulation. Cell Host Microbe. 2020;27(3). https://doi.org/10.1016/j.chom.2020.01.016.
Cheng M, Ge X, Zhong C, Fu R, Ning K, Xu S. Micro-coevolution of host genetics with gut microbiome in three Chinese ethnic groups. J Genet Genomics. 2021;48(11):972–83. https://doi.org/10.1016/j.jgg.2021.09.002.
Sorbara MT, Littmann ER, Fontana E, Moody TU, Kohout CE, Gjonbalaj M, Eaton V, Seok R, Leiner IM, Pamer EG. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals Inter- and intra-species diversity. Cell Host Microbe. 2020;28(1). https://doi.org/10.1016/j.chom.2020.05.005.
Hirayama M, Nishiwaki H, Hamaguchi T, Ito M, Ueyama J, Maeda T, Kashihara K, Tsuboi Y, Ohno K. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS ONE. 2021;16(11):e0260451. https://doi.org/10.1371/journal.pone.0260451.
Akatsu H, Iwabuchi N, Xiao J-Z, Matsuyama Z, Kurihara R, Okuda K, Yamamoto T, Maruyama M. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. JPEN J Parenter Enter Nutr. 2013;37(5):631–40. https://doi.org/10.1177/0148607112467819.
Zeng P, Zhou X. Causal effects of blood lipids on amyotrophic lateral sclerosis: a mendelian randomization study. Hum Mol Genet. 2019;28(4):688–97. https://doi.org/10.1093/hmg/ddy384.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China [grant number 82173585 and 82273741], the Natural Science Foundation of Jiangsu Higher Education Institutions of China [grant number 21KJB330005, 22KJB330007], the Nanjing Major Science and Technology Project [grant number 2021–11005], and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
R.Y. and P.H. designed the study. Y.Z., L.J. and Y.W. (Yifan Wang) performed datasets download and quality control. J.T., S.Y. and X.Y. carried out data analyzing and interpretation. J.T. and Y.W. (Yidi Wang) wrote the draft manuscript. Y.W. (Yidi Wang), R.Y., W.L. and P.H. reviewed and revised the article. All the authors accepted the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
This manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tu, J., Wang, Y., Ye, X. et al. Gut microbial features may influence antiviral IgG levels after vaccination against viral respiratory infectious diseases: the evidence from two-sample bidirectional mendelian randomization. BMC Infect Dis 24, 431 (2024). https://doi.org/10.1186/s12879-024-09189-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09189-0