Abstract
Background
Although there is increasing understanding of the changes in the laboratory parameters of Coronavirus disease 2019 (COVID-19), the correlation between circulating Mid-regional Proadrenomedullin (MR-proADM) and mortality of patients with COVID-19 is not fully understood. In this study, we conducted a systematic review and meta-analysis to evaluate the prognostic value of MR-proADM in patients with COVID-19.
Methods
The PubMed, Embase, Web of Science, Cochrane Library, Wanfang, SinoMed and Chinese National Knowledge Infrastructure (CNKI) databases were searched from 1 January 2020 to 20 March 2022 for relevant literature. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to assess quality bias, STATA was employed to pool the effect size by a random effects model, and potential publication bias and sensitivity analyses were performed.
Results
14 studies comprising 1822 patients with COVID-19 met the inclusion criteria, there were 1145 (62.8%) males and 677 (31.2%) females, and the mean age was 63.8 ± 16.1 years. The concentration of MR-proADM was compared between the survivors and non-survivors in 9 studies and the difference was significant (P < 0.01), I2 = 46%. The combined sensitivity was 0.86 [0.73–0.92], and the combined specificity was 0.78 [0.68–0.86]. We drew the summary receiver operating characteristic (SROC) curve and calculated the area under curve (AUC) = 0.90 [0.87–0.92]. An increase of 1 nmol/L of MR-proADM was independently associated with a more than threefold increase in mortality (odds ratio (OR) 3.03, 95% confidence interval (CI) 2.26–4.06, I2 = 0.0%, P = 0.633). The predictive value of MR-proADM for mortality was better than many other biomarkers.
Conclusion
MR-proADM had a very good predictive value for the poor prognosis of COVID-19 patients. Increased levels of MR-proADM were independently associated with mortality in COVID-19 patients and may allow a better risk stratification.
Similar content being viewed by others
Introduction
Coronavirus disease-19 (COVID-19) is a clinical syndrome caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The COVID-19 pandemic spread rapidly with more than 535 million cases and 6.3 million deaths. COVID-19 is a global problem and a significant cause of death. Therefore, accurate evaluation of patients’ prognosis is very important. Many inflammatory markers [1,2,3,4,5] and critical illness scores [6,7,8,9,10] are used to predict the prognosis and mortality of patients with COVID-19 infection.
The emergence of an increasing number of biomarkers may provide a new way to improve the accuracy of prognosis simply and quickly. Mid-regional Proadrenomedullin (MR-proADM) is a peptide produced by multiple tissues that is used to stabilize microcirculation and protect endothelial function [11,12,13,14]. Previous studies have shown that with the deterioration of microcirculation integrity and capillary leakage, the concentration of MR-proADM increases, reflecting the early development stage of organ dysfunction [14, 15]. Therefore, early evaluation of microcirculation function may provide a valuable tool to evaluate disease progression and treatment effect [15].
Some studies were devoted to investigate the relationship between MR- proADM and the outcome of patients with COVID-19 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. However, their sample sizes were small, and the conclusions were inconsistent. Using a meta-analytic method, the aim of the present study was to provide an overview of the concentration of the MR-proADM between the survivors and non-survivors and to explore the predictive value of the MR-proADM for the mortality of the hospitalized patients with COVID-19.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34]. The review protocol was registered on PROSPERO (CRD42022308638).
Search strategy
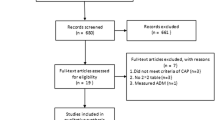
The systematic literature search was conducted in PubMed, Embase, Web of Science, the Cochrane Library, Wanfang, SinoMed and Chinese National Knowledge Infrastructure (CNKI) for studies published from 1 January 2020 to 20 March 2022. The following search terms were used: “MR-proADM” OR “MR-pro-adrenomedullin” OR “midregional pro-adrenomedullin” OR “MR-pro-ADM” and “COVID-19” OR “SARS-CoV-2 Infection” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease”. There was no restriction on the language of the records. See Fig. 1 for details.
Selection and eligibility criteria
All prospective and retrospective studies of MR-proADM and outcomes in patients with COVID-19 were included, and there were no restrictions on age, sex, setting of treatment, severity of disease, sample size, etc. Death was the primary endpoint, and studies that did not include death outcomes were excluded.
Data extraction
Two authors independently extracted data into a standardized data collection form. Discrepancies were resolved by a third party. The following data were extracted from each study: first author, publication year, country, study period, study design, setting, population, number, age, male ratio, clinical outcomes, levels of MR-proADM, mortality rate, AUC for death, cut-off value, sensitivity and specificity. See additional files for detail.
Risk of bias assessment
The quality of the included studies was assessed by two independent assessors using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) which consists of four key domains: patient selection, index test, reference standard and flow and timing (Fig. 2). Each was assessed in terms of risk of bias and the first three in terms of concerns regarding applicability. Signaling questions were included to assist in judgements about the risk of bias.
Data synthesis and statistical analysis
The main analysis was to determine the predictive ability of MR-proADM on COVID-19 mortality. The mean and standard deviation (SD) of the MR-proADM values were included in the pooled analysis. A random model was used to calculate and separately pool the concentration of MR-proADM in the survivors and non-survivors, and the weighted mean difference (WMD) and 95% confidence interval (95% CI) of survivors and non-survivors were estimated. When the mean and standard deviation could not be obtained, conversion calculations were carried out according to the sample size, median and interquartile range (IQR) as suggested by Hozo et al. [35].
The heterogeneity between the different studies was presented as I2 and P values for the I2 statistic were derived from the chi-square distribution of Cochran’s Q test. The standard for the category of heterogeneity was defined as I2 > 50%. We used STATA 16.0 to calculate the sensitivity and specificity of MR-proADM and performed the summary receiver operating characteristic (SROC) curve of MR-proADM for mortality. Public bias was examined by Egger’s test.
All statistical analyses were performed using RevMan version 5.4 (the Cochrane Collaboration) and STATA 16.0 software (StataCorp, College Station, Texas, USA). P values less than 0.05 were considered statistically significant.
Results
Literature search
We found 83 relevant studies from seven databases, Medline (21), Embase (37), Web of Science (18), Wanfang (7) and the other three databases contain zero records. Endnote software was used to delete duplications, and 37 articles remained. By checking the titles and abstracts, another 20 articles were excluded. Three of the remaining 17 articles did not meet the inclusion criteria, and finally 14 articles were ultimately included. The literature search and selection process are depicted in the PRISMA flow diagram (Fig. 1).
The included studies were published between 2021 and 2022. We looked for an association between MR-proADM and the mortality of patients with COVID-19. A total of 1822 patients met the inclusion criteria, including 1145 (62.8%) males and 677 (31.2%) females, aged 63.8 ± 16.1 years. Eight of the studies were from Italy, three were from Spain and others were from the Netherlands, Switzerland and United Kingdom. Three studies were conducted in hospital settings, three in the intensive care unit (ICU), three in the emergency department, one in a COVID center, one in an intermediate medical care unit (IMCU), one in a medium intensity-of-care COVID-19 department, one in an acute national health service (NHS) setting and the remaining study site was not determined. Nine studies used a prospective study design, and five studies were retrospective studies. Of these studies, 12 were single-center studies and two were multicenter studies. Three studies reported 28-day mortality, six reported in-hospital mortality, four reported 30-day mortality and one reported 90-day mortality. We summarized the characteristics of the included studies in Supplementary Tables 1, 2, 3.
Meta-analysis of the effects of MR-proADM on mortality
Of 14 studies, 10 studies described the specific concentration of MR-proADM in the survivors and non-survivors. Therefore, the concentration of MR-proADM was compared between the survivors and non-survivors in 10 studies and the difference was significant (P < 0.01), I2 = 79%. Due to the obvious heterogeneity, we looked for the source of heterogeneity and found that after removing the first study [24], the heterogeneity of the results decreased significantly. Finally, nine studies were included, and there was a significant difference in the concentration of MR- proADM between the survivors and the non-survivors (P < 0.01), I2 = 46% (Fig. 3). STATA was used to calculate the combined sensitivity and specificity, the combined sensitivity was 0.86 [0.73–0.92], I2 = 51.47%, and the combined specificity was 0.78 [0.68–0.86], I2 = 92.95% (Fig. 4).
To evaluate the predictive ability of MR- proADM for the mortality of patients with COVID-19, we drew the SROC curve, and calculated the AUC = 0.90 [0.87–0.92] (Fig. 5).
Odds ratios (OR) and hazard ratios (HR)
We calculated an overall mortality rate of 19.0% (347/1822) for all studies, ranging from 7.2 to 54.4%. Seven studies used OR to indicate that MR-proADM was associated with mortality, and the pooled OR was 3.03 (2.26–4.06), which means that an increase of 1 nmol/L of MR-proADM was independently associated with a more than threefold increase in mortality. There was no significant heterogeneity between these studies (I2 = 0.0%, P = 0.633) (Fig. 6). Four studies used HR as an effect measure, and the pooled HR was 5.40 (1.82–16.06). Significant heterogeneity was observed (I2 = 84.0%, P = 0.000) (Fig. 6). Both of them indicated that MR-proADM can be an independent predictor for mortality among patients with COVID-19.
Comparation with other biomarkers and critical illness scores
Some studies compared the area under the curve (AUC) of MR-proADM with other biomarkers and critical illness scores. The predictive value of MR-proADM for mortality was better than the Sequential Organ Failure Assessment (SOFA) score, Acute Physiological and Chronic Health Evaluation IV (APACHE IV), Confusion blood Urea nitrogen Respiratory rate Blood pressure age 65 or older (CURB-65) score, D-dimer, cardiac troponin T (cTNT), C-reactive protein (CRP), procalcitonin (PCT), ferritin, lactate and C-terminal proendothelin-1 (CT-proET-1) [33]. Another study found that the AUC of MR-proADM was greater than those of PCT, creatinine, albumin, platelet count, interleukin-6 (IL-6) and lymphocyte count [19]. The study of Benedetti showed that its predictive power was stronger than SOFA and Simplified Acute Physiology Score II (SAPS II), but weaker than the Acute Physiology and Chronic Health Evaluation II (APACHE II) score [17]. MR-proADM was superior to CRP, PCT, neutrophils, lymphocytes [27] and many other biomarkers [18]. However, only a few studies indicated the sensitivity and specificity, and we could not calculate the combined effect of each item.
Sensitivity analysis and potential publication bias
Begg’s test was used to assess for publication bias, and the results showed no potential bias (P = 0.965) (Fig. 7). We also conducted a sensitivity analysis, and the results indicated that our study was stable (Fig. 8).
Discussion
Using a meta-analytic approach, we systematically reviewed and analyzed the contribution of MR-proADM to mortality in COVID-19 patients. In this study, we found that MR-proADM had a very good predictive value for the poor prognosis of COVID-19 patients. Increased levels of MR-proADM were independently associated with mortality in COVID-19 patients. These findings also confirm the feasibility of risk stratification by MR-proADM in COVID-19 patients.
COVID-19 patients have a high mortality rate. The mortality rate of in-hospital patients was 12.44% [36], that of ICU patients was 26% [37], and that of patients with acute respiratory distress syndrome (ARDS) was 50% [38]. The study population we included had different severities of illness, and the overall mortality rate was 19%. Many studies have used various biomarkers, such as CRP [39,40,41,42,43,44], PCT [39, 43, 45, 46], IL-6 [39, 43, 46], WBC [40, 47,48,49], D-dimer [42, 44, 46, 50, 51], lactate dehydrogenase (LDH) [39, 42,43,44, 46, 47], N-terminal pro-B-type natriuretic peptide (NT-proBNP) [39, 52, 53], and Troponin T [39, 54], and critical illness scores, such as APACHE II [55,56,57], SOFA [55, 56, 58,59,60], SAPS [61,62,63,64], and CURB65 [59, 61, 65], to evaluate the prognosis of patients with COVID-19. The APACHE II and SOFA scoring systems require the worst values of the clinical and biological parameters to be recorded within 24 h of admission [66]. In contrast, biomarkers can be measured rapidly within hours of the admission. WBC, CRP and IL-6 have a lack of specificity, and PCT is mainly used to determine if a bacterial infection is present and to guide the use of antibiotics [67].
Adrenomedullin (ADM) is a 52 amino acid peptide hormone that is associated with cardiovascular, endocrine and renal mechanisms regulating water and electrolyte balance [68]. ADM can reduce the permeability of capillaries during septic shock [11], and plays an important role in the regulation of inflammatory mediators, vascular endothelial barrier and microcirculation stability [11, 69]. Because of the rapid degradation and clearance of ADM in the cycle, the measurement of ADM in the cycle becomes complex [70]. Meanwhile, MR-proADM has a longer half-life and is relatively stable in the circulation, which can directly reflect the level of rapidly degraded active ADM peptide. It has been shown that measuring MR-proADM is more suitable for clinical practice [71].
In fact, patients with decreasing PCT concentrations but continuously high MR-proADM concentrations had a significantly increased mortality risk [72], and MR-proADM had an important role in predicting the development of organ failure over 24 h [73], suggesting that MR-proADM may have important clinical value in the early risk stratification of patients with sepsis. Recent studies have proposed that virus-induced endothelial dysfunction and damage, resulting in impaired vascular blood flow, coagulation and leakage, may partially explain the development of organ dysfunction [74, 75]. Another study showed that myocardial injury induced by SARS-CoV-2 is strongly associated with high MR-proADM values and mortality [76]. The assessment of MR-proADM may provide vital information to explain the pathophysiological mechanisms of vascular endothelial injury and subsequent organ dysfunction in patients with COVID-19.
Our study found that there was a significant difference in the concentration of MR-proADM between the survivors and the non-survivors (P < 0.01), the combined sensitivity was 0.86, and the combined specificity was 0.78. These findings are consistent with those of prior studies [72, 77, 78]. MR-proADM may be a good predictive marker for the prognosis of patients. Our data indicated that an increase of 1 nmol/L of MR-proADM was independently associated with a more than threefold increase in mortality (OR 3.03, 95% CI 2.26–4.06). In another meta-analysis, all of included studies showed that an elevated MR-proADM level was associated with higher risk of death from community-acquired pneumonia, the pooled RR was 5.83 (95% CI 4.53–7.52) [79]. In addition, PCT and MR-proADM test combination presents a high positive predictive value in pneumonia diagnosis and prognosis [80]. Furthermore, MR pro-ADM value provides additional information for better risk stratification especially for patients with high pneumonia severity score [81]. In a recent narrative review [82], directly testing for MR-proADM in the emergency department could contribute to improving the prognostic assessment of patients with sepsis. Spoto et al. [83] conducted a retrospective analysis of MR-proADM in 571 consecutive patients with sepsis and found that MR-proADM cut-off values > 3.39 nmol/L in sepsis and > 4.33 nmol/L in septic shock were associated with a significantly higher risk of 90-day mortality. These findings confirm that MR pro-ADM is a prognostic tool that can guide clinicians to develop more personalized treatment for patients.
Our study showed that MR-proADM was superior to most biomarkers and critical illness scores in predicting the prognosis of COVID-19 patients [17,18,19, 27, 33]. However, few of them indicated the sensitivity and specificity, and we could not calculate the combined effect of each item. A recent study [84] evaluated the usefulness of MR-proADM compared to CRP, PCT, and ferritin in the prognosis of influenza A (H1N1) pneumonia, and found that the initial MR-proADM, ferritin, CRP, and PCT levels effectively determined adverse outcomes and risk of ICU admission and mortality in patients with influenza virus pneumonia. MR-proADM has the highest potency for survival prediction (AUC = 0.853, P < 0.0001). The recently published TRIAGE study [85] is a multinational, prospective, observational cohort study that included consecutive medical patients presenting with a medical urgency at three tertiary-care hospitals. A total of 7,132 patients were included in the final analysis. The study found that MR-proADM was the best biomarker, especially for mortality prediction. Another study [72] indicated that the initial use of MR-proADM within the first 24 h after sepsis diagnosis resulted in the strongest association with short-term, mid-term and long-term mortality compared to all other biomarkers or clinical scores. Other studies also yielded similar results [86, 87]. Recently, a meta-analysis suggested that MR-proADM testing alone is poor at identifying invasive bacterial infections in young children [88], which may be related to the population under study, the measurement time and other factors. In addition, it may be more meaningful to determine the time trend of MR-proADM levels in septic patients with pulmonary infection [77].
There are some limitations in our study. First, selection bias and information bias are easily generated because of the observational design and different research locations. Second, there was considerable heterogeneity in some analyses, mainly due to the significant differences in the baseline patient characteristics. Third, converting the median to an average also affects our results. Due to the limited number of articles included, our analysis results may not be accurate, and more studies need to be included for verification in the future.
Conclusions
MR-proADM was associated with mortality in patients with COVID-19, and risk assessment using MR-proADM allows clinicians to identify patients with COVID-19 who are at higher risk of adverse clinical outcomes.
Data availability
The data that support the findings of this study are included in this published article and its supplementary material.
Abbreviations
- COVID-19:
-
coronavirus disease 2019
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- MR-proADM:
-
Mid-regional Proadrenomedullin
- PRISMA:
-
the Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CNKI:
-
Chinese National Knowledge Infrastructure
- QUADAS-2:
-
The Quality Assessment of Diagnostic Accuracy Studies
- SD:
-
standard deviation
- WMD:
-
weighted mean difference
- CI:
-
confidence interval
- IQR:
-
interquartile range
- ICU:
-
intensive care unit
- IMCU:
-
intermediate medical care unit
- NHS:
-
national health service
- AUC:
-
the area under the curve
- SOFA:
-
the Sequential Organ Failure Assessment
- APACHEIV:
-
Acute Physiological and Chronic Health Evaluation IV
- CURB-65:
-
Confusion blood Urea nitrogen Respiratory rate Blood pressure age 65 or older
- cTNT:
-
cardiac troponin T
- CRP:
-
C-reactive protein
- PCT:
-
procalcitonin
- CT-proET-1:
-
C-terminal proendothelin-1
- IL-6:
-
interleukin-6
- SAPS II:
-
Simplified Acute Physiology Score II
- APACHE II:
-
the Acute Physiology and Chronic Health Evaluation II score
- ARDS:
-
acute respiratory distress syndrome
- LDH:
-
lactate dehydrogenase
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- ADM:
-
Adrenomedullin
References
Regolo M, Vaccaro M, Sorce A, Stancanelli B, Colaci M, Natoli G, et al. Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to Intensive Care Unit of COVID-19 patients. J Clin Med. 2022;11(8):2235.
Abrishami A, Eslami V, Arab-Ahmadi M, Alahyari S, Azhideh A, Sanei-Taheri M. Prognostic value of inflammatory biomarkers for predicting the extent of lung involvement and final clinical outcome in patients with COVID-19. J Res Med Sci. 2021;26:115.
Menez S, Moledina DG, Thiessen-Philbrook H, Wilson FP, Obeid W, Simonov M, et al. Prognostic significance of urinary biomarkers in patients hospitalized with COVID-19. Am J Kidney Dis. 2022;79(2):257–267e1.
Donoso-Navarro E, Arribas Gómez I, Bernabeu-Andreu FA. IL-6 and other biomarkers associated with poor prognosis in a cohort of hospitalized patients with COVID-19 in Madrid. Biomark Insights. 2021;16:11772719211013363.
Twe CW, Khoo DKY, Law KB, Nordin NSBA, Sathasivan S, Lim KC, et al. The role of procalcitonin in predicting risk of mechanical ventilation and mortality among moderate to severe COVID-19 patients. BMC Infect Dis. 2022;22(1):378.
Beigmohammadi MT, Amoozadeh L, Motlagh FR, Rahimi M, Maghsoudloo M, Jafarnejad B, et al. Mortality predictive value of APACHE II and SOFA Scores in COVID-19 patients in the Intensive Care Unit. Can Respir J. 2022;2022:5129314.
Giamarellos-Bourboulis EJ, Poulakou G, de Nooijer A, Milionis H, Metallidis S, Ploumidis M, et al. Development and validation of SCOPE score: a clinical score to predict COVID-19 pneumonia progression to severe respiratory failure. Cell Rep Med. 2022;3(3):100560.
Peruzzo MB, Requião-Moura L, Nakamura MR, Viana L, Cristelli M, Silva HT et al. Predictive ability of severity scores and outcomes for mortality in kidney transplant recipients with coronavirus disease 2019 admitted to the intensive care unit: results from a Brazilian single-center cohort study. J Bras Nefrol. 2022; S0101–28002022005008401.
Surme S, Tuncer G, Bayramlar OF, Copur B, Zerdali E, Nakir IY, et al. Novel biomarker-based score (SAD-60) for predicting mortality in patients with COVID-19 pneumonia: a multicenter retrospective cohort of 1013 patients. Biomark Med. 2022;16(8):577–88.
Citu C, Gorun F, Motoc A, Ratiu A, Gorun OM, Burlea B, et al. Evaluation and comparison of the predictive value of 4 C mortality score, NEWS, and CURB-65 in poor outcomes in COVID-19 patients: a retrospective study from a single Center in Romania. Diagnostics (Basel). 2022;12(3):703.
Temmesfeld-Wollbruck B, Brell B, David I, Dorenberg M, Adolphs J, Schmeck B, et al. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. 2007;33(4):703–10.
Muller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, Pfeil U, et al. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit Care. 2014;18(2):R73.
Carrizo GJ, Wu R, Cui X, Dwivedi AJ, Simms HH, Wang P. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery. 2007;141(2):245–53.
Vigue B, Leblanc PE, Moati F, Pussard E, Foufa H, Rodrigues A, et al. Mid-regional pro-adrenomedullin (MR- proADM), a marker of positive fluid balance in critically ill patients: results of the ENVOL study. Crit Care. 2016;20(1):363.
Xie Z, Chen WS, Yin Y, Chan EC, Terai K, Long LM, et al. Adrenomedullin surges are linked to acute episodes of the systemic capillary leak syndrome (Clarkson disease). J Leukoc Biol. 2018;103(4):749–59.
Zaninotto M, Mion MM, Marchioro L, Padoan A, Plebani. Endothelial dysfunction and mid-regional proAdrenomedullin: what role in SARS-CoV-2 infected patients? Clin Chim Acta. 2021;523:185–90.
Benedetti I, Spinelli D, Callegari T, Bonometti R, Molinaro E, Novara E, et al. High levels of mid-regional proadrenomedullin in ARDS COVID-19 patients: the experience of a single, italian Center. Eur Rev Med Pharmacol Sci. 2021;25(3):1743–51.
García de Guadiana-Romualdo L, Calvo Nieves MD, Rodríguez Mulero MD, Calcerrada Alises I, Hernández Olivo M, Trapiello Fernández W, et al. MR-proADM as marker of endotheliitis predicts COVID-19 severity. Eur J Clin Invest. 2021;51(5):e13511.
García de Guadiana-Romualdo L, Martínez Martínez M, Rodríguez Mulero MD, Esteban-Torrella P, Hernández Olivo M, Alcaraz García MJ, et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int J Infect Dis. 2021;111:211–8.
Gregoriano C, Koch D, Kutz A, Haubitz S, Conen A, Bernasconi L, et al. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: an observational study. Clin Chem Lab Med. 2021;59(5):995–1004.
Honore PM, Redant S, Moorthamers S, Preseau T, Kaefer K, Barreto Gutierrez L, et al. MR-proADM has a good ability to predict 28-day mortality in critically ill patients with SARS-CoV-2 pneumonia: beware of some potential confounders! J Crit Care. 2022;67:212–3.
Indirli R, Bandera A, Valenti L, Ceriotti F, Di Modugno A, Tettamanti M, et al. Prognostic value of copeptin and mid-regional proadrenomedullin in COVID-19-hospitalized patients. Eur J Clin Invest. 2022;52(5):e13753.
Lippi G, Henry BM. Pooled analysis of mid-regional pro-adrenomedullin values in COVID-19 patients with critical illness. Intern Emerg Med. 2021;16(6):1723–5.
Lo Sasso B, Gambino CM, Scichilone N, Giglio RV, Bivona G, Scazzone C, et al. Clinical utility of Midregional Proadrenomedullin in patients with COVID-19. Lab Med. 2021;52(5):493–8.
Minieri M, Di Lecce VN, Lia MS, Maurici M, Bernardini S, Legramante JM. Role of MR-proADM in the risk stratification of COVID-19 patients assessed at the triage of the Emergency Department. Crit Care. 2021;25(1):407.
Montrucchio G, Sales G, Rumbolo F, Palmesino F, Fanelli V, Urbino R, et al. Effectiveness of mid-regional proadrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: an observational prospective study. PLoS ONE. 2021;16(2):e0246771.
Moore N, Williams R, Mori M, Bertolusso B, Vernet G, Lynch J et al. Mid-regional proadrenomedullin (MR-proADM), C-reactive protein (CRP) and other biomarkers in the early identification of disease progression in patients with COVID-19 in the acute NHS setting.“J Clin Pathol. 2022; jclinpath-2021-207750.
Oblitas CM, Galeano-Valle F, Ramírez-Navarro J, López-Cano J, Monterrubio-Manrique Á, García-Gámiz M, et al. Mid-regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as prognosis biomarkers in critically ill patients with COVID-19: an observational prospective study. Intensive Care Medicine Experimental. 2021;13(12):2445.
Roedl K, Jarczak D, Fischer M, Haddad M, Boenisch O, de Heer G, et al. MR-proAdrenomedullin as a predictor of renal replacement therapy in a cohort of critically ill patients with COVID-19. Biomarkers. 2021;26(5):417–24.
Saeed K, Legramante JM, Angeletti S, Curcio F, Miguens I, Poole S, et al. Mid-regional pro-adrenomedullin as a supplementary tool to clinical parameters in cases of suspicion of infection in the emergency department. Expert Rev Mol Diagn. 2021;21(4):397–404.
Sozio E, Tascini C, Fabris M, D’Aurizio F, De Carlo C, Graziano E, et al. MR-proADM as prognostic factor of outcome in COVID-19 patients. Sci Rep. 2021;11(1):5121.
Spoto S, Agrò FE, Sambuco F, Travaglino F, Valeriani E, Fogolari M, et al. High value of mid-regional proadrenomedullin in COVID-19: a marker of widespread endothelial damage, disease severity, and mortality. J Med Viro. 2021;93(5):2820–7.
van Oers JAH, Kluiters Y, Bons JAP, de Jongh M, Pouwels S, Ramnarain D, et al. Endothelium-associated biomarkers mid-regional proadrenomedullin and C-terminal proendothelin-1 have good ability to predict 28-day mortality in critically ill patients with SARS-CoV-2 pneumonia: a prospective cohort study. J Crit Care. 2021;66:173–80.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Asaduzzaman MD, Romel Bhuia M, Nazmul Alam Z, Zabed Jillul Bari M, Ferdousi T. Significance of hemogram-derived ratios for predicting in-hospital mortality in COVID-19: a multicenter study. Health Sci Rep. 2022;5(4):e663.
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–81.
Hartmann B, Verket M, Balfanz P, Hartmann NU, Jacobsen M, Brandts J, et al. Glycaemic variability is associated with all-cause mortality in COVID-19 patients with ARDS, a retrospective subcohort study. Sci Rep. 2022;12(1):9862.
Aziz F, Stöcher H, Bräuer A, Ciardi C, Clodi M, Fasching P, et al. Biomarkers predictive for In-Hospital mortality in patients with diabetes Mellitus and Prediabetes hospitalized for COVID-19 in Austria: an analysis of COVID-19 in Diabetes Registry. Viruses. 2022;14(6):1285.
Giorgino R, Soroush E, Soroush S, Malakouti S, Salari H, Vismara V, et al. COVID-19 Elderly Patients treated for proximal femoral fractures during the Second Wave of Pandemic in Italy and Iran: a comparison between two countries. Med (Kaunas). 2022;58(6):781.
Giner-Galvañ V, Pomares-Gómez FJ, Quesada JA, Rubio-Rivas M, Tejada-Montes J, Baltasar-Corral J, et al. C-Reactive protein and serum albumin ratio: a feasible prognostic marker in hospitalized patients with COVID-19. Biomedicines. 2022;10(6):1393.
Jawad Zaidi SM, Awan MH, Bhatti HW, Sabir S, Ahmed S, Arshad I, et al. Investigating the role of inflammatory markers at admission in defining the severity of moderate-to-critical COVID-19: a cross-sectional analysis. J Community Hosp Intern Med Perspect. 2022;12(2):1–5.
Cao B, Jing X, Liu Y, Wen R, Wang C. Comparison of laboratory parameters in mild vs. severe cases and died vs. survived patients with COVID-19: systematic review and meta-analysis. J Thorac Dis. 2022;14(5):1478–87.
Romero-Gameros CA, Vargas-Ortega G, Rendón-Macias ME, Cuevas-García CF, Colín-Martínez T, Sánchez-Hurtado LA, et al. Risk factors Associated with Mortality among patients with COVID-19: analysis of a cohort of 1213 patients in a Tertiary Healthcare Center. J Clin Med. 2022;11(10):2780.
Patel N, Adams C, Brunetti L, Bargoud C, Teichman AL, Choron RL. Evaluation of Procalcitonin’s utility to predict concomitant bacterial pneumonia in critically ill COVID-19 patients. J Intensive Care Med. 2022;16:8850666221108636.
Lee TA, Wang SH, Kuo CT, Li CW, McCullough LD, Bello D, et al. Prognostic serum biomarkers in cancer patients with COVID-19: a systematic review. Transl Oncol. 2022;21:101443.
Armin S, Mirkarimi M, Pourmoghaddas Z, Tariverdi M, Shamsizadeh A, Alisamir M, et al. Evidence-based prediction of COVID-19 severity in hospitalized children. Int J Clin Pract. 2022;2022:1918177.
Feigin E, Levinson T, Wasserman A, Shenhar-Tsarfaty S, Berliner S, Ziv-Baran T. Age-dependent biomarkers for prediction of In-Hospital mortality in COVID-19 patients. J Clin Med. 2022;11(10):2682.
Bao PL, Deng KL, Yuan AL, Yan YM, Feng AQ, Li T, et al. Early renal impairment is associated with in-hospital death of patients with COVID-19. Clin Respir J. 2022;16(6):441–9.
Folkman R, Kamal H, Ahl M, Szum A, Magnusson M, Aleman S. Postacute elevation of D-dimer levels in severe acute respiratory syndrome coronavirus 2-positive nonhospitalized patients with mild symptoms. Blood Coagul Fibrinolysis. 2022;33(5):285–7.
Stavileci B, Ereren E, Özdemir E, Özdemir B, Cengiz M, Enar R. The impact of daily troponin I and D-dimer serum levels on mortality in COVID-19 pneumonia patients. Cardiovasc J Afr. 2022;33:1–7.
Qiang Z, Wang B, Garrett BC, Rainey RP, Superko HR. Coronavirus disease 2019: a comprehensive review and meta-analysis on cardiovascular biomarkers. Curr Opin Cardiol. 2021;36(3):367–73.
Calvo-Fernández A, Izquierdo A, Subirana I, Farré N, Vila J, Durán X, et al. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev Esp Cardiol. 2021;74(7):576–83.
Almeida Junior GLG, Braga F, Jorge JK, Nobre GF, Kalichsztein M, Faria PMP, et al. Prognostic value of Troponin-T and B-Type natriuretic peptide in patients hospitalized for COVID-19. Arq Bras Cardiol. 2020;115(4):660–6.
Wilfong EM, Lovly CM, Gillaspie EA, Huang LC, Shyr Y, Casey JD, et al. Severity of illness scores at presentation predict ICU admission and mortality in COVID-19. J Emerg Crit Care Med. 2021;5:7.
Asmarawati TP, Suryantoro SD, Rosyid AN, Marfiani E, Windradi C, Mahdi BA, et al. Predictive value of sequential organ failure Assessment, Quick Sequential Organ failure Assessment, Acute Physiology and Chronic Health evaluation II, and new early warning Signs Scores Estimate Mortality of COVID-19 patients requiring Intensive Care Unit. Indian J Crit Care Med. 2022;26(4):464–71.
Burrell AJ, Pellegrini B, Salimi F, Begum H, Broadley T, Campbell LT, et al. Outcomes for patients with COVID-19 admitted to australian intensive care units during the first four months of the pandemic. Med J Aust. 2021;214(1):23–30.
Citu C, Citu IM, Motoc A, Forga M, Gorun OM, Gorun F. Predictive value of SOFA and qSOFA for In-Hospital mortality in COVID-19 patients: a single-center study in Romania. J Pers Med. 2022;12(6):878.
Innocenti F, De Paris A, Lagomarsini A, Pelagatti L, Casalini L, Gianno A, et al. Stratification of patients admitted for SARS-CoV2 infection: prognostic scores in the first and second wave of the pandemic. Intern Emerg Med. 2022;22:1–9.
Torres A, Motos A, Cillóniz C, Ceccato A, Fernández-Barat L, Gabarrús A, et al. Major candidate variables to guide personalised treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study. Intensive Care Med. 2022;48(7):850–64.
Kim S, Choi H, Sim JK, Jung WJ, Lee YS, Kim JH. Comparison of clinical characteristics and hospital mortality in critically ill patients without COVID-19 before and during the COVID-19 pandemic: a multicenter, retrospective, propensity score-matched study. Ann Intensive Care. 2022;12(1):57.
Kokoszka-Bargieł I, Cyprys P, Madeja P, Rutkowska K, Wajda-Pokrontka M, Madowicz J, et al. Factors influencing death in COVID-19 patients treated in the ICU: a single-centre, cross-sectional study. Anaesthesiol Intensive Ther. 2022;54(2):132–40.
Lázaro APP, Albuquerque PLMM, Meneses GC, Zaranza MS, Batista AB, Aragão NLP, et al. Critically ill COVID-19 patients in northeast Brazil: mortality predictors during the first and second waves including SAPS 3. Trans R Soc Trop Med Hyg. 2022;22:trac046.
Aziz F, Reisinger AC, Aberer F, Sourij C, Tripolt N, Siller-Matula JM, et al. Simplified Acute Physiology score 3 performance in austrian COVID-19 patients admitted to Intensive Care units with and without diabetes. Viruses. 2022;14(4):777.
Olry de Labry-Lima A, Saez-de la Fuente J, Abdel-Kader Martin L, Alegre-Del Rey EJ, García-Cabrera E, Sierra-Sánchez JF. Factors associated with mortality in patients hospitalized for COVID-19 in Spain. Data from the RERFAR registry. Farm Hosp. 2022;46(2):57–71.
Minne L, Abu-Hanna A, De Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care. 2008;12(6):R161.
Méndez R, Aldás I, Menéndez R. Biomarkers in Community-Acquired Pneumonia (Cardiac and Non-Cardiac). J Clin Med. 2020;9(2):549.
Samson WK, Resch ZT, Murphy TC, Vargas TT, Schell DA. Adrenomedullin: is there physiological relevance in the Pathology and Pharmacology? News Physiol Sci. 1999;14:255–9.
Gonzalez-Rey E, Chorny A, Varela N, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol. 2006;168(6):1921–30.
Lewis LK, Smith MW, Yandle TG, Richards AM, Nicholls MG. Adrenomedullin (1–52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem. 1998;44:571–7.
Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–9.
Elke G, Bloos F, Wilson DC, Brunkhorst FM, Briegel J, Reinhart K, et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial. Crit Care. 2018;22(1):79.
Spoto S, Nobile E, Carnà EPR, Fogolari M, Caputo D, De Florio L, et al. Best diagnostic accuracy of sepsis combining SIRS criteria or qSOFA score with procalcitonin and mid-regional pro-adrenomedullin outside ICU. Sci Rep. 2020;10(1):16605.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8.
Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes. J Clin Med. 2020;9(6):1862.
Spoto S, Mangiacapra F, D’Avanzo G, Lemme D, Bustos Guillén C, Abbate A, et al. Synergistic effect of myocardial injury and mid-regional proAdrenomedullin elevation in determining clinical outcomes of SARS-CoV-2 patients. Front Med (Lausanne). 2022;9:929408.
Bima P, Montrucchio G, Caramello V, Rumbolo F, Dutto S, Boasso S, et al. Prognostic Value of Mid-Regional Proadrenomedullin sampled at Presentation and after 72 hours in septic patients presenting to the Emergency Department: an observational Two-Center Study. Biomedicines. 2022;10(3):719.
Li P, Wang C, Pang S. The diagnostic accuracy of mid-regional pro-adrenomedullin for sepsis: a systematic review and meta-analysis. Minerva Anestesiol. 2021;87(10):1117–27.
Liu D, Xie L, Zhao H, Liu X, Cao J. Prognostic value of mid-regional pro-adrenomedullin (MR-proADM) in patients with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect Dis. 2016;26:16:232.
Spoto S, Legramante JM, Minieri M, Fogolari M, Terrinoni A, Valeriani E, et al. How biomarkers can improve pneumonia diagnosis and prognosis: procalcitonin and mid-regional-pro-adrenomedullin. Biomark Med. 2020;14(7):549–62.
Legramante JM, Mastropasqua M, Susi B, Porzio O, Mazza M, Miranda Agrippino G, et al. Prognostic performance of MR-pro-adrenomedullin in patients with community acquired pneumonia in the Emergency Department compared to clinical severity scores PSI and CURB. PLoS ONE. 2017;12(11):e0187702.
Piccioni A, Saviano A, Cicchinelli S, Valletta F, Santoro MC, de Cunzo T, et al. Proadrenomedullin in Sepsis and septic shock: a role in the Emergency Department. Med (Kaunas). 2021;57(9):920.
Spoto S, Fogolari M, De Florio L, Minieri M, Vicino G, Legramante J, et al. Procalcitonin and MR-proAdrenomedullin combination in the etiological diagnosis and prognosis of sepsis and septic shock. Microb Pathog. 2019;137:103763.
Valenzuela-Méndez B, Valenzuela-Sánchez F, Rodríguez-Gutiérrez JF, Bohollo-de-Austria R, Estella Á, Martínez-García P, et al. Plasma levels of Mid-Regional Proadrenomedullin accurately identify H1N1pdm09 influenza virus patients with risk of Intensive Care Admission and Mortality in the Emergency Department. J Pers Med. 2022;12(1):84.
Schuetz P, Hausfater P, Amin D, Amin A, Haubitz S, Faessler L, et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care. 2015;19:377.
Enguix-Armada A, Escobar-Conesa R, La Torre AG, De La Torre-Prados MV. Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin Chem Lab Med. 2016;54(1):163–8.
Andaluz-Ojeda D, Cicuéndez R, Calvo D, Largo E, Nogales L, Muñoz MF, et al. Sustained value of proadrenomedullin as mortality predictor in severe sepsis. J Inf Secur. 2015;71(1):136–9.
Corr MP, Fairley D, McKenna JP, Shields MD, Waterfield T. Diagnostic value of mid-regional pro-adrenomedullin as a biomarker of invasive bacterial infection in children: a systematic review. BMC Pediatr. 2022;22(1):176.
Acknowledgements
The authors thank all staff of the emergency department of China rehabilitation research center, Capital Medical University.
Funding
This work was not supported by any funding.
Author information
Authors and Affiliations
Contributions
NW and LSL performed the data analysis, and drafted the manuscript. WH and NS were responsible for literature research. JYL and ZQ conducted data acquisition and extracted all of the raw date. XXD designed the study and helped to revise manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, N., Liu, L., He, W. et al. Circulating mid-regional proadrenomedullin is a predictor of mortality in patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis 23, 305 (2023). https://doi.org/10.1186/s12879-023-08275-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08275-z