Abstract
Background
ESAT6-CFP10 (EC) skin test has been reported accurate and safe in identifying tuberculosis infection. We aimed to demonstrate the safety of EC skin test compared with tuberculin skin test (TST) in university freshmen.
Methods
We conducted a double-blind, randomized, controlled clinical study in a university freshmen population with 16,680 participates in China, and finally 14,579 completed the study. About a half received an EC skin test and the others received TST. Adverse reactions were evaluated.
Results
Out of the 14,579 participants, 48.2% (7029/14,579) were males. The average age was 18.1 ± 0.8 years and the average BMI was 20.9 ± 3.1 kg/m2. 50.4% (7351/14,579) participants received EC skin test and 49.6% (7228/14,579) received TST. The EC group had significantly less adverse reactions compared with the TST group (21.3%, 1565/7351 vs. 34.6%, 2499/7228, P = 0.000). The most common adverse reactions for EC were bleeding (5.63%, 414), dermatodyschroia (4.27%, 314), induration (3.90%, 287), swelling (2.49%, 183), pain (1.59%, 117) and pruritus (1.48%, 109). Bleeding, dermatodyschroia, swelling and erythema were significantly less in EC group (P < 0.05), while others were similar to those of TST.
Conclusion
the EC skin test was safe in our cohort. And its incidence of total adverse drug reactions (ADRs) is less than that of TST. Most adverse reactions were mild or moderate, lasting less than 48 h and self-limiting. Considering the satisfactory diagnostic accuracy in identifying tuberculosis infection, the cost and safety, the EC skin test might be a potential candidate for replacing TST in high burden countries or those with routine BCG vaccination. Clinical Trials Registration. ChiCTR2000038622, Safety of the EC skin test to screen tuberculosis infection in two universities, compared with the tuberculin skin test: a double-blind, randomized, controlled trial. registered on 26/09/2020 at http://www.chictr.org.cn.
Similar content being viewed by others
Background
Tuberculosis is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent (ranking above HIV/AIDS) [1]. Approximately 10 million people fell in with TB and it was responsible for over 1 million death each year globally in recent ten years. About a quarter of the world’s population is infected with Mycobacterium tuberculosis, and it is estimated their lifetime risk of developing tuberculosis is 5-10% [2]. In order to effectively control the epidemic of TB, scanning out people with tuberculosis infection is important. Two currently available diagnostic tool for tuberculosis infection are interferon-gamma release assays (IGRAs), including T-SPOT.TB test and QuantiFERON-TB Gold In-Tube, and the tuberculin skin test (TST) [3, 4]. Compares with TST, IGRAs is more specific because it is less likely to be affected by Calmette Guerin (BCG) vaccination or exposure to nontuberculous mycobacteria[5,6,7]. Since over 95% of tuberculosis cases are in developing countries, IGRAs is not an affordable choice. Thus, TST is widely used in high-burden countries for its high sensitivity and low cost. While its poor specificity caused by prior BCG vaccination and exposure to nontuberculous mycobacteria results many false positive cases. ESAT6-CFP10 (EC) skin test is based on tuberculosis specific antigens and comparable to the T-SPOT.TB test on diagnostic accuracy [8]. In addition, considering the low cost of EC skin test, it is a promising tool to replace TST in high burden countries or those with routine BCG vaccination. It has been reported EC skin test had a good safety profile [8, 9]. In our study, we further explored the safety of EC skin test compared with that of TST.
Methods
Study Design and participants
This was a double-blind, randomized, controlled clinical study conducted in college freshmen in Xiangtan University and Hunan University of Science and Technology in Xiangtan City, Hunan Province, China. The inclusion criteria were: 1) willing to receive EC or TST testing and provide information on past medical history. The exclusion criteria were: (1) had been vaccinated with any live vaccine within the preceding 6 months. (2) had received TST in the previous 12 months (to eliminate the potential risk for boosting). (3) had received Immunosuppressive therapy treatment. (4) refusing to participate in this study. 4) could not provide written informed consent. (5) could not complete all surveys and testing items. (6) current skin conditions that could interfere with the measurement of induration after the EC test or TST. (7) were currently participating in other drug clinical trials. (8) other disorders that investigators deemed unsuitable.
Randomization and masking
Participants were enrolled and randomly assigned into two groups at 1:1 (EC group and TST group). The randomization schedule was generated centrally by an independent statistician not involved in the study, by random permutation programmed in SPSS Statistics version 25. Investigators and participants were masked to skin test allocations. To mask which test was being injected in forearm, physicians were provided with test kits that contained two kinds of identical vials, one containing EC and one containing TST, and instructions about in which participant each test should be administered. The vials and the cardboard box of each kit were all labelled with the same randomization number.
Procedures
The EC antigen is a recombinant reagent of the ESAT-6 and CFP-10 tests, developed by Zhifei Longcom Biologic Pharmacy Company, China. The TST (TB-PPD) was manufactured by Beijing Gaoke Life and Technology, China, and administrated by trained nurses, following the national standard guideline [10]. Both the EC and TST agent were clear, colorless solutions. Participants received the EC skin test (1.0 µg/0.1 mL) [8] or TST on the volar surface of one forearm. Injection site ADRs such as bleeding, swelling, pain, pruritus, erythema, blistering and ulceration, systemic ADRs (all non-injection-site reactions) such as fatigue, headache, nausea, vomiting and palpitation were assessed by investigators at 30 min, 24 h, 48 h, 72 h after skin test, respectively.
Follow-up and data collection
Participants attended follow-up visits on 24 h, 48 h, 72 h and day 28 after skin test, respectively for evaluation of skin test ADRs. The evaluation of the results was done by trained doctors, and they were all blinded about the groups of intervention. General information such as age, gender, height and weight, as well as medical history were obtained and recorded by investigators after the assignment of written informed consent.
Statistical analysis
Numerical data are presented as number (%) of participants, mean (SD). Categorical data are presented as number (%) of participants. Differences in number of participants, gender, and adverse reactions between EC group and TST group were evaluated with χ2 test, and Continuity Correction or Fisher’s Exact test would be used when necessary. Differences in age and body-mass index (BMI) between EC group and TST group were evaluated with t test. Statistical analyses were done with SPSS Statistics version 25. This study is registered with http://www.chictr.org.cn (ChiCTR2000038622).
Results
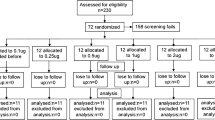
From October 15, 2020, to October 30, 2020, 16,680 healthy freshmen were screened and all participants or their legal guardians signed written informed consent. They were randomly assigned to the EC group or TST group. 973 and 1098 participants in the EC and TST group were excluded and 7363 and 7242 were enrolled, respectively. Finally, 14,579 participants completed the study. 7351/14,579(50.4%)and 7228/14,579 (49.6%) were analyzed in the EC and TST group, respectively (Fig. 1). In all the 14,579 participants, males accounted for 7029/14,579 (48.2%). The average age was 18.1 ± 0.8 years. The average BMI was 20.9 ± 3.1 kg/m2. The EC group had significantly less adverse reactions compared with the TST group (21.3%, 1565/7351 vs. 34.6%, 2499/7228, P = 0.000). Proportions of ADRs of EC and TST are listed in Table 1. In the EC group, common ADRs (≥ 1.0% and < 10.0%) were bleeding, dermatodyschroia, induration, swelling, pain and pruritus. Uncommon ADRs (≥ 0.1% and < 1.0%) were erythema, blistering, fatigue, headache and injection site rash. Rare ADRs (≥ 0.01% and < 0.1%) were nausea, dyspnea, vomiting, body ache, palpitation and ulceration. In the TST group, common ADRs (≥ 1.0% and < 10.0%) were induration, bleeding, dermatodyschroia, swelling, pain, pruritus and erythema. Uncommon ADRs (≥ 0.1% and < 1.0%) were blistering, injection site rash, fatigue, headache and nausea. Rare ADRs (≥ 0.01% and < 0.1%) were dyspnea, vomiting, palpitation, ulceration and body ache. Compared with the ADRs of TST group, bleeding, dermatodyschroia, induration, swelling and erythema were significantly less in the EC group (P < 0.05, see Table 1). All of the ADRs arose during the first 24 h after injection and were mild to moderate and lasted less than 48 h and were and self-limiting. In addition, no related serious ADRs were found during follow-up.
Discussion
Our study further explored the safety of EC skin test in a large size population, with 14,579 participants. Out of the 14,579 participants, the EC group had less adverse reactions compared with the TST group (21.3%, 1565/7351 vs. 34.6%, 2499/7228, P = 0.000). EC skin test has satisfactory diagnostic accuracy and high consistency (96.3%, 95% CI, 92.0-100.0) with the T-SPOT.TB test in identifying tuberculosis infection, which means an increased specificity over traditional TST. At present, the cost of EC skin test is about 10% of T-SPOT.TB test, 14.3% of QuantiFERON-TB Gold test, 2 times of TB-pure protein derivative (PPD) and 9 times of BCG-PPD. The efficiency, effectiveness and cost of EC skin test refers it the potential replacing the extensive usage of TST in countries with high burden or with routine BCG vaccination, which are also low-to-moderate income countries[8]. Phase I and II clinical trials of EC skin test have demonstrated it is safe in both healthy people and TB patients aged between 18 and 65, without any serious adverse reaction [8, 9, 11]. In the phase I clinical trial of EC skin test [9], 24 healthy volunteers aged 18 to 40 were enrolled to evaluate its safety, and the incidence of ADRs was 4.2% (1/24). The volunteer developed a mild local reaction of red spots scattered at the injection site 15 min after the skin test and disappeared on the next day. In the phase IIa clinical trial of EC skin test [11], 56 healthy adults and 88 TB patients aged 18 to 65 were enrolled and all received EC skin test. Besides, all healthy participants and 56 out of 88 TB patients received TB-PPD skin test at the same time while the rest 32 patients received placebo. In this IIa trial, the main ADRs for EC and TB-PPD skin test were local pruritus and pain. The incidence of the two ADRs in EC and TB-PPD were17.4% (25/144) and 22.3% (25/112), respectively, but without statistically difference. When it comes to the IIb clinical trial [8], 777 healthy participants, 96 TB patients and 95 nontuberculosis patients with other pulmonary disease whose age were between 18 and 65 were analyzed. The rate of ADRs of EC skin test and TST were 27.8% and 16.5% (P = 0.000), respectively. For both the EC skin test and the TST, pruritus and pain at the injection site were the most common ADRs, which was consistent to that in the phase IIa trial. In summary of all previous trials and our study, all the EC-related ADRs were mild to moderate and self-limiting, arising during the first 24 h after injection and lasting less than 48 h. No serious ADRs related to EC skin test was observed. Besides, in our study, which has a much larger sample size of participants, the rate of total ADRs in EC skin test group is statistically lower than that in the TST group. Instead of pruritus and pain at injection site in the previous studies, bleeding and dermatodyschroia were the most common ADRs of EC group while induration and bleeding were the most common ADRs of TST group in our study. Since other ADRs except for injection site pruritus and pain of EC in previous clinical trials were not known, we are not able do the comparison. The different sample size (the number of participants) and the selection of participants (healthy or patients, common adults or university freshmen) might be an explanation. The strength of our study included: (1) large number of participants. (2) easy procedure with good repeatability. (3) minimal loss to follow up with 5 participants lost to follow up and 25 withdrew out of 14,609 enrolled participants in total. The limitation in our study is the population was constrained to university freshmen. In future studies, wider population should be included.
Conclusion
the EC skin test was safe in participants from our cohort. And its incidence of total ADRs is less than that of TST. Most adverse reactions were mild or moderate, lasting less than 48 h and self-limiting. Considering the satisfactory diagnostic accuracy in identifying tuberculosis infection, the cost and safety, the EC skin test might be a potential candidate for replacing TST in high burden countries or those with routine BCG vaccination.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to Confidentiality Agreement, but are available from the corresponding author on reasonable request.
References
Organization WHO. Global tuberculosis report 2020. Geneva, Switzerland 2020, WHO, 2019.
Gao L, Li X, Liu J, Wang X, Lu W, Bai L, Xin H, Zhang H, Li H, Zhang Z, et al. Incidence of active tuberculosis in individuals with latent tuberculosis infection in rural China: follow-up results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2017;17(10):1053–61.
Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin–past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273–80.
Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20.
Jung YJ, Lyu J, Yoo B, Lee CK, Kim YG, Yang SK, Byeon JS, Kim KJ, Ye BD, Lee KH, et al. Combined use of a TST and the T-SPOT(R).TB assay for latent tuberculosis infection diagnosis before anti-TNF-alpha treatment. Int J Tuberc Lung Dis. 2012;16(10):1300–6.
Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl 4):1120–9.
King TC, Upfal M, Gottlieb A, Adamo P, Bernacki E, Kadlecek CP, Jones JG, Humphrey-Carothers F, Rielly AF, Drewry P, et al. T-SPOT.TB Interferon-gamma Release Assay Performance in Healthcare Worker Screening at Nineteen U.S. Hospitals. Am J Respir Crit Care Med. 2015;192(3):367–73.
Xu M, Lu W, Li T, Li J, Du W, Wu Q, Liu Q, Yuan B, Lu J, Ding X, et al: Sensitivity, specificity, and safety of a novel ESAT6-CFP10 skin test for tuberculosis infection in China: two randomized, self-controlled, parallel-group phase 2b trials. Clin Infect Dis 2021.
Li F, Xu M, Zhou L, Xiong Y, Xia L, Fan X, Gu J, Pu J, Lu S, Wang G. Safety of Recombinant Fusion Protein ESAT6-CFP10 as a Skin Test Reagent for Tuberculosis Diagnosis: an Open-Label, Randomized, Single-Center Phase I Clinical Trial. Clin Vaccine Immunol. 2016;23(9):767–73.
Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968–75.
Li F, Xu M, Qin C, Xia L, Xiong Y, Xi X, Fan X, Gu J, Pu J, Wu Q, et al. Recombinant fusion ESAT6-CFP10 immunogen as a skin test reagent for tuberculosis diagnosis: an open-label, randomized, two-centre phase 2a clinical trial. Clin Microbiol Infect. 2016;22(10):889. e889-889 e816.
Acknowledgements
Much gratitude to Pro. Zhixiong Fang for integrating the resources, and together with Pro. Shaojie Li and Pro. Shuihua Lu designing the study. We also thank all the staff and participants for their contribution to the revealing of the scientific truth.
Funding
This study was supported by grants from the Shanghai Clinical key specialist Construction Project (Tuberculosis) (shslczdzk03002), the National Science Foundation of China (81770014), Grants from Hunan Provincial Health Commission (20200855) and Grants from Shanghai Public Health Clinical Center (KY-GW-2021-21).
Author information
Authors and Affiliations
Contributions
Shuihua Lu, Shaojie Li and Zhixiong Fang designed the study. Zhixiong Fang generated the random allocation sequence. Yang Yang, Zhixiong Fang, Wei Huang, Haiming Zhang, Si Luo and Sha Lin conducted the study and collected the data, Yang Yang and Wei Huang analyzed the data, and Yang Yang wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from local ethics committees and the Center Hospital of Xiangtan City institutional review board. All participants aged 18 or over gave written informed consent before enrollment. For those aged under 18, their written informed consent was obtained from their parents or other legal guardians. Study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
#Yang Yang, Zhixiong Fang these authors contributed equally to this work
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Fang, Z., Huang, W. et al. Safety of a Novel ESAT6-CFP10 skin test compared with tuberculin skin test in a double-blind, randomized, controlled study. BMC Infect Dis 22, 780 (2022). https://doi.org/10.1186/s12879-022-07765-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07765-w