Abstract
Background
Recombinant fusion protein ESAT6-CFP10 (EC) is a newly developed skin test reagent for detecting Mycobacterium tuberculosis (M. tuberculosis) infection. In this study, we evaluated whether induration and erythema could be used as diagnostic indicators for EC skin test to detect M. tuberculosis infection.
Methods
A total of 743 tuberculosis patients and 1514 healthy volunteers underwent an EC skin test. The diameters of induration and erythema were measured with Vernier caliper, 24 h, 48 h, and 72 h after skin testing. Related indicators of EC reagent diagnostic test were tested, and the diagnostic effects of the four diagnostic indicators for EC skin test were compared.
Results
The sensitivity of induration / erythema measurement was lower at 24 h after EC skin test than at 48 h or 72 h (P<0.01). There was no difference in consistency (P = 0.16) between induration with clinical diagnosis, and erythema with clinical diagnosis at 48 h (88.88 and 90.16%, Kappa value was 0.75 and 0.78, respectively). In patients, the sensitivity of erythema measurement was higher than induration measurement (P<0.01). In healthy volunteers, the specificity of erythema measurement was lower than induration at 24 h after skin test, but there was no difference at 48 h after skin test (P = 0.22). In BCG vaccination volunteers, the specificity of induration and erythema were higher than 90%. In addition, there was a high consistency of induration and erythema. When induration or erythema was used as a positive diagnostic indicator, the sensitivity of the EC skin test was improved, and was no different from the other three indicators in terms of specificity and consistency with clinical diagnosis.
Conclusions
Induration or erythema diameter not less than 5 mm could be used as a diagnostic indicator for detecting M. tuberculosis infection.
Trial registration
Phase III clinical trial of recombinant Mycobacterium tuberculosis ESAT6-CFP10 allergen; CTR20150695; registered in December 16, 2015.
Similar content being viewed by others
Background
Tuberculosis (TB) remains a leading cause of morbidity and mortality, and a major public health problem worldwide [1]. World Health Organization (WHO) has estimated that 10.4 million new cases and 1.8 million deaths from TB occur every year [2, 3]. Approximately, one-third of the world’s population is infected with Mycobacterium tuberculosis (M. tuberculosis), and about 1.7 billion people have latent tuberculosis infection (LTBI) [4, 5], especially in developing countries [1]. China is one of the countries with a high burden of TB and LTBI. Although the prevalence of TB is gradually decreasing [6], it still remains an important threat due to its high infectiousness. The Chinese government is making strenuous efforts to prevent further spread of infection and provide rapid detection and treatment facilities through national support and project grants [7].
Currently, the most common method used to detect LTBI implies testing the hosts’ immune response to M. tuberculosis derived antigens [8, 9]. Tuberculin pure protein derivative (PPD) skin test and Interferon-γ (IFN-γ) release assays (IGRAs) are commonly used for detecting M. tuberculosis infection [10,11,12], but the tuberculin skin test (TST) has a high false positive rate [13, 14]. IGRAs is an immunodiagnostic test that measures effector T cell-mediated IFN-γ response to M. tuberculosis-specific antigens. Both 6 kDa early secretory antigenic target (ESAT6) and 10 kDa culture filtrate protein (CFP10) are co-transcribed and specifically secreted by M. tuberculosis [15, 16], which is absent in most non-tuberculous mycobacteria (NTM) and Bacillus Calmette-Guérin (BCG) [17,18,19,20]. IGRAs can limit the false positive reaction [21, 22] and overcome the operational drawbacks of TST [23]. However, the detection reagents used for IGRAs are expensive, and the operation process is difficult to be automated [24]; thus, IGRAs are not suitable for use in remote areas and wide-range screening.
According to the characteristics of M. tuberculosis-specific antigens, China has recently developed a recombinant fusion protein ESAT6-CFP10 (EC) as a new skin test reagent, which was expected to have high sensitivity and specificity. In this study, we conducted large sample research to clarify whether induration and erythema diameter could be used as diagnostic indicators for EC skin test to detect M. tuberculosis infection.
Methods
Clinical trial units
The study on TB patients was completed by Shanghai Public Health Clinical Center, Tianjin Haihe Hospital, Wuhan Tuberculosis Prevention and Treatment Center, Beijing Chest Hospital, First Affiliated Hospital of Chongqing Medical University, Wuxi Infectious Disease Hospital, Fuzhou Pulmonary Hospital, Zhenjiang Third People’s Hospital, Anhui Provincial hospitals and Shenzhen Third People’s Hospital; Shanghai Public Health Clinical Center was the main responsible unit. The research was performed from December 17, 2015 to March 02, 2018. Jiangsu Provincial Center for Disease Control and Prevention completed the research on healthy volunteers from February 27, 2016 to June 18, 2016.
The Human Research Ethics Committee granted the research protocol. All participants read and signed the informed consent.
Inclusion and exclusion criteria
Healthy volunteers aged 18 to 65 were recruited and included in this study if they met the following criteria: (a) no history or family history of TB; (b) no X-ray chest abnormality; (c) normal physical condition and temperature. Healthy volunteers were excluded if they (a) had serious diseases such as advanced cancer, acute exacerbation of the chronic obstructive pulmonary disease, acute or progressive liver disease, acute or progressive kidney disease or congestive heart failure; (b) had a mental illness; (c) had known or suspicious immune function impairment or abnormality; (d) had an acute febrile illness and infectious disease; (e) were currently participating in other drug clinical trials or have participated in any other new drug clinical trials within 3 months before the testing; (f) had a history of drug allergy; (g) were pregnant or lactating; (h) had a clear history of hypertension; (i) had other reasons that investigators believed might affect the evaluation of the test.
TB patients’ inclusion criteria were: patients 18 to 65 years old who were definitively diagnosed according to the criteria for pulmonary TB of the Ministry of Health of China (WS 288–2008, supplementary). TB patients were excluded if they met the (a) (b) (e) (f) (g) (i) six items in the health volunteers’ exclusion criteria.
Finally, 743 TB patients and 1514 healthy volunteers were enrolled in this study.
Skin test reagent
ESAT6-CFP10 included the following: a recombinant fusion protein of ESAT6 and CFP10, which was manufactured by Anhui Longcom Biologic Pharmacy Co. Ltd. This reagent was supplied as a liquid formulation (0.3 ml/bottle), consisting of ESAT6-CFP10 antigen (50 U/10μg/ml), phosphate-buffered saline (PBS, 1.0 mmol/liter), phenol (3%) and Tween 80 (0.0005%).
Skin test operation
Before starting the skin test, skin test reagent, rescue medicine and rescue equipment were prepared. Each numbered skin test reagent was only be used by one subject and could not be mixed with others. The authorized medical staff checked the drug number/subject number of each subject, and then checked the expiration date, bottle wall, and drug contents.
The skin was sterilized with ethanol in the first 1/3 of the subjects’ forearm volar side without scars or lesions. Then, 0.1 ml skin test reagent was inhaled into a 1 ml disposable syringe, the scale and the tip hole slope were consistently upward. The medical staff’s left thumb tightened the skin of the subject where the injection was required, and the right hand hold the syringe, which penetrated into the skin at 5° ~ 10°from the skin. The reagent was injected slowly; the white rounded ridge was visible, the pores were revealed, and the boundary was clear after the injection. After the injection was completed, the needle was pulled out while rotating 90 degrees. Subjects were instructed not to get wet or rub the injection site for 72 h. Medical staff was required to change gloves for each patient to avoid contamination.
Outcome and measurement
Previous literature reports and small sample clinical trial suggested that erythema can be used as a specific response to the skin test. In this study, outcome measures were induration and erythema diameters in every subject. All patients were observed at 24 h, 48 h, and 72 h after a skin test, and healthy volunteers were observed at 24 h and 48 h after skin test. Firstly, the medical staff analyzed the induration (touching the skin with fingers) and observed the erythema outer ring to determine edges of induration and erythema. The upper and lower edges (longitudinal diameter), left and right edges (transverse diameter) of the induration were marked by a short line with a black pen. The boundary of the erythema was marked by a short line with a red pen. The longitudinal and transverse diameters of induration and erythema were measured with Vernier caliper. Two medical staff measured and read the size of the skin test response to ensure the reading error between the two people controlled within ±0.2 mm. The averages of the longitudinal and transverse diameters was the sizes of induration and erythema diameter. A previous clinical trial showed that the area under the ROC curve was the largest when the cutoff value of the diameter of induration and erythema was 5 mm. Therefore, in this study, the diameter of induration and erythema of 5 mm was the positive criterion for the EC skin test response. This clinical trial expanded the sample size for further verification. So, the results of the EC skin test were considered positive when the diameter of induration or erythema was ≥5 mm, and strongly positive if a local blister, necrosis, or lymphangitis were observed.
Statistical analysis
Statistical analysis was conducted using SAS 9.3 software. The diameters of induration and erythema after EC skin test in all subjects were described with mean ± SD, number, median, minimum and maximum. Sensitivity, Specificity, Positive predict value (PV+), Negative predict value (PV−), Youden index (YI), consistency, and Kappa value (95% confidence interval) were used to compare the consistency of induration / erythema and clinical diagnostic results. Positive coincidence rate (CR+), Negative coincidence rate (CR−), consistency, and Kappa value (95% CI) were carried out to compare the consistency of induration and erythema results. A Chi-square test was applied to compare the rates in different groups. P < 0.05 was considered to be statistically significant.
Results
Characteristics of participants
In total, 743 TB patients were enrolled in the study; they were 38.77 ± 14.11 years old, 513 subjects (69.04%) were male, and 118 subjects had a history or family history of TB. There were 1514 healthy volunteers, who were 45.43 ± 9.57 years old, and 398 subjects (26.29%) were male. Among them, 777 volunteers had BCG vaccination scars (were vaccinated during infancy, which was a basic health policy in China). BCG is very effective in preventing severe tuberculosis in childhood, but is less effective in adults [25,26,27]. Therefore, healthy adults vaccinated with BCG during infancy were considered as a generally healthy population without BCG protection. In order to evaluate the specificity of the EC skin test in the BCG vaccination population, 315 healthy volunteers were selected for BCG vaccination according to the purpose of the clinical trial. The other characteristics are shown in Table 1.
The diameters of induration and erythema after EC skin test
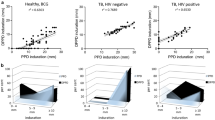
In TB patients, the average diameter of induration was 14.23 mm, 22.68 mm, and 21.00 mm at 24 h, 48 h and 72 h after EC skin test, while the maximum diameter was 82.50 mm, 96.00 mm and 112.50 mm, respectively. Moreover, the average diameter of erythema was 24.72 mm, 37.55 mm, and 37.64 mm at 24 h, 48 h, and 72 h after EC skin test, while the maximum diameter was 108.00 mm, 153.50 mm and 158.50 mm, respectively (Fig. 1a).
In healthy volunteers, the average diameter of induration was 0.36 mm and 1.81 mm at 24 h and 48 h after EC skin test, while the maximum diameter was 30.50 mm and 60.50 mm respectively; the average diameter of erythema was 1.23 mm and 3.86 mm at 24 h and 48 h after EC skin test, and the maximum diameter was 73.00 mm and 124.00 mm, respectively (Fig. 1b).
The diagnostic value of induration and erythema after EC skin test
The sensitivity of induration measurement was 72.14, 84.25, and 83.78% at 24 h, 48 h, and 72 h after EC skin test, respectively; the sensitivity was the lowest at 24 h (χ2 = 43.80, P < 0.01), while there was no statistical difference between the sensitivity of 48 h and 72 h (χ2 = 0.06, P = 0.81). The specificity of induration measurement was 97.49 and 91.15% at 24 h and 48 h after EC skin test, respectively; the specificity was lower at 48 h (χ2 = 56.81, P < 0.01). The Kappa value was 0.75 at 48 h. The consistency of 24 h and 48 h after the skin test between induration and clinical diagnosis was close to 90% (χ2 = 0.08, P = 0.78). The remaining results are shown in Table 2.
The sensitivity of erythema measurement was 85.33, 90.85, and 91.08% at 24 h, 48 h, and 72 h after EC skin test, respectively; the sensitivity was the lowest at 24 h (χ2 = 16.18, P < 0.01), and there was no statistical difference between the sensitivity at 48 h and 72 h (χ2 = 0.03, P = 0.88). The specificity of erythema measurement was 95.38 and 89.83% at 24 h and 48 h after EC skin test, respectively; the specificity was lower at 48 h (χ2 = 34.02, P < 0.01). Kappa values were 0.82 at 24 h and 0.78 at 48 h. The consistency of 24 h and 48 h after the skin test between erythema and clinical diagnosis were more than 90%; at 48 h after a skin test, the consistency was slightly lower than that at 24 h after skin test (χ2 = 5.06, P = 0.02). The remaining results are shown in Table 2.
When comparing the consistency between induration with a clinical diagnosis and erythema with clinical diagnosis, there was a significant difference at 24 h after EC skin test (χ2 = 11.34, P < 0.01), and no difference at 48 h after skin test (χ2 = 1.99, P = 0.16). The consistency of erythema with the clinical diagnosis was higher than that of induration with clinical diagnosis at 24 h after a skin test, and there was no difference in the consistency between two of them at 48 h after the skin test.
The comparison of sensitivity and specificity between induration and erythema
In TB patients, there were more patients with positive erythema response than those with positive induration response. The sensitivity in judging induration and erythema were compared at three time points, P < 0.01 (χ224 h = 38.60, χ248 h = 14.82, χ272 h = 17.93) (Table 3). These results demonstrated that the sensitivity of erythema measurement was significantly higher than that of induration. In bacteriologically positive TB patients, the sensitivity of erythema measurement was higher than that of induration, P < 0.01 (χ224 h = 20.72, χ248 h = 11.16, χ272 h = 16.58) (Table 3). In bacteriologically negative TB patients and extrapulmonary TB patients, there was no differences in sensitivity between induration and erythema measurement at 48 h and 72 h after a skin test, P ≥ 0.05 (Table 3).
Among healthy volunteers, there were more subjects with negative induration response than those with negative erythema response. The specificity for analyzing induration and erythema were compared. There was a significant difference at 24 h after EC skin test (χ2 = 9.83, P < 0.01), and no difference at 48 h after EC skin test (χ2 = 1.54, P = 0.22) (Table 3), which suggested that the specificity of erythema measurement was lower than induration at 24 h after skin test, but not at 48 h after skin test.
In BCG vaccination volunteers, the specificity of induration and erythema measurement were > 90%, and the specificity were compared at two time points, P > 0.05 (χ224 h = 1.79, χ248 h = 1.01). These proved there was no difference in the specificity between induration and erythema (Table 3).
Comparison the consistency of induration and erythema
In TB patients, the CR+ (χ2 = 31.99, P <0.01), Kappa value, and consistency (χ2 = 18.36, P < 0.01) between induration and erythema were lower at 24 h after the skin test than that at 48 h and 72 h. In bacteriologically positive TB patients, the CR+ (χ2 = 8.62, P = 0.01) and consistency (χ2 = 8.19, P = 0.02) between induration and erythema were lower at 24 h after the skin test, while Kappa value was higher at 48 h after skin test. In bacteriologically nagative TB patients, CR+ (χ2 = 27.02, P <0.01), Kappa value and consistency (χ2 = 27.27, P < 0.01) were lower at 24 h after the skin test. In extrapulmonary TB patients, Kappa value was higher at 48 h after skin test. In healthy volunteers, CR+ (χ2 = 30.84, P < 0.01) and Kappa value at 24 h after skin test were lower than that at 48 h after skin test. The comparisons results are shown in Table 4.
Above all, induration and erythema could be used for M. tuberculosis detection. These data suggested that the best sensitivity, specificity, and other diagnostic results are obtained 48 h after the skin test is performed.
Comparison of diagnostic indicators for the EC skin test
We selected induration, erythema, induration or erythema, induration and erythema as the diagnostic indicators of the EC skin test, and compared the diagnostic effects of these four indicators in different types of patients at 48 h after EC skin test .
In different types of patients, the sensitivity of induration or erythema measurement was the highest, the sensitivity of induration and erythema measurement was the lowest, however, in extrapulmonary TB patients, there were no differences in the sensitivity of the four indicators. The specificity of induration or erythema measurement was the lowest, the specificity of induration and erythema measurement was the highest, however, there was no difference in the specificity of the four indicators. In different subjects, there was no difference in the consistency of the four indicators with clinical diagnosis. Detailed data are shown in Table 5.
Discussion
Currently, there is no golden standard for LTBI diagnosis. Therefore, in order to detect M. tuberculosis infection, TB patients were used instead of LTBI [28,29,30]. All enrolled patients underwent a bacteriological test of M. tuberculosis. Based on the bacteriological results, imaging results, and clinical symptoms, patients were divided into bacteriologically positive and negative patients, and extrapulmonary tuberculosis patients. In previous clinical trials, we validate the consistency and safety of the recombinant fusion protein EC for detecting M. tuberculosis. In this phase III clinical trial, we expanded the number of subjects and found that induration or erythema diameter not less than 5 mm could be used as a diagnostic indicator for detecting M. tuberculosis infection.
Combined with the results of previous clinical trials [31, 32], these data suggested that the best sensitivity, specificity, and other diagnostic results are obtained 48 h after testing. Furthermore, we found that diagnostic results of induration and erythema had higher sensitivity, specificity, consistency, and Kappa values compared with clinical diagnosis. Therefore, we believed that induration and erythema could be used as diagnostic indicators for EC skin test to detect M. tuberculosis infection. On the one hand, the recombinant fusion protein is more stable than recombinant mixed protein [33] and could produce a stronger cellular immune response. Thus, the erythema response could not be ignored. On the other hand, EC skin test reagent contains only M. tuberculosis-specific antigens proteins; skin responses should be specific. Therefore, induration and erythema as skin response should be measured.
Previous small sample clinical trial suggested that erythema was a specific response to the EC skin test. After the test, the sensitivity of the erythema was higher then 80%, the specificity was higher then 90%, and the test results were consistent with clinical diagnosis results (Kappa value ≥0.78). In the BCG vaccination population, more than 90% of the subjects had a negative erythema response. A large prospective study of PPD skin test conducted in Japan confirmed that the specificity of erythema measurement was closely related to induration, and erythema was more likely to produce a specific response without being confused by induration [34]. Moreover, another study showed that erythema and induration had the same response trend. In this study, subjects were divided into M. tuberculosis infection negative and positive groups according to the erythema response [35]. We believed that erythema should be a specific response to the EC skin test according to the above results. Therefore, we included erythema as a judgement indicator and further verified its detection effect by expanding the sample size. In TB patients, the sensitivity of erythema measurement was higher than that of induration. If only the induration is used, many patients may be misdiagnosed, which could reduce the sensitivity of the test. In this study, erythema could improve the sensitivity of the test in TB patients. Although the erythema response had a certain false-positive rate in healthy volunteers, the false positive rate was low, and its specificity was still high. Especially in BCG vaccination volunteers, whether induration or erythema, its specificity was higher than 90%, which explained that EC skin test response would not be affected by BCG. In addition, the analysis found that the consistency of erythema and clinical diagnosis was high. Overall, the erythema response in different types of TB patients kept the sensitivity of about 90% after 48 h; its sensitivity was higher than that of induration. In healthy volunteers, the erythema had a specificity of about 90%. Above all, the sensitivity and specificity of the erythema response were both high. Erythema used as the judgment indicator can improve the positive detection rate in TB patients without affecting the specificity of healthy volunteers. Therefore, erythema was a specific response to the EC skin test and could be used for detecting M. tuberculosis infection. We believed that erythema could be used as a diagnostic indicator of the EC skin test.
The skin test response produced in subjects showed that the diameter of erythema was larger, smaller, or equal to the diameter of induration. Therefore, it was one-sided and unreasonable if uniformly stipulated that it used induration alone as the diagnostic indicator for the EC skin test. The CR+ and the consistency of induration and erythema were higher than 90%, and the Kappa value was higher than 0.66 in different types of TB patients at 48 h after testing, which indicated the consistency of induration and erythema results was better in patients. The CR− of induration and erythema was more than 99% in healthy volunteers. Notably, the consistency was 98.28%, and Kappa value was 0.90 at 48 h after testing, indicating that the consistency of induration and erythema results was excellent in healthy volunteers. Since induration and erythema had a good consistency, both of them could be used as diagnostic indicators for EC skin test.
In terms of sensitivity, the use of induration and erythema in parallel was higher than that when the indicators used alone or in tandemly; In terms of specificity, although the two indicators used in parallel were lower than the two indicators used alone or in tandemly, there was no statistical difference. In terms of consistency with clinical diagnosis, the consistency of the four diagnostic indicators was about 90%. In summary, we believed that the parallel combination of induration and erythema should be used to evaluate the responses of the EC skin test.
In this study, only yellow-skinned people were included. People with darker skin will have a specific effect on the interpretation of erythema results. Therefore, in the darker-skinned population, we mainly take the diagnosis of induration, supplemented by the diagnosis of erythema.
The above content was only part of the phase III clinical trial for the EC skin test. In phase III clinical trials, we performed a PPD skin test, EC skin test, and T-SPOT test in TB patients and healthy volunteers, respectively. Our study indicated that an EC skin test can be used for screening M. tuberculosis infection and was an effective skin test reagent for clinical auxiliary diagnosis of tuberculosis. No adverse events and serious adverse events leading to withdrawal occurred in a clinical trial. In terms of sensitivity, the EC skin test was equivalent to the PPD skin test and T-SPOT test. In terms of specificity, the EC skin test was superior to the PPD skin test and non-inferior to the T-SPOT test. Compared the consistency of the three tests and clinical diagnosis, it was concluded that the consistency of PPD skin test and clinical diagnosis was lower than that of EC skin test or T-SPOT and clinical diagnosis.
Conclusion
Induration and erythema could be used as diagnostic indicators for EC skin test. Erythema as diagnostic indicators could improve the sensitivity of the EC skin test, without affecting the specificity. Induration or erythema diameter not less than 5 mm could be as a diagnostic indicator for positive response of the EC skin test. Although the determination of skin test results is subjective, the EC skin test is more specific than TST, and can be used to detect M. tuberculosis infection early, quickly, and accurately. Due to its convenient operation, it is more suitable to be used in countries with high TB burden to reduce the financial burden.
Availability of data and materials
The datasets used and/or analysed during the current study are de-identified and available from the corresponding author on reasonable request.
Abbreviations
- TB:
-
Tuberculosis
- M. tuberculosis:
-
Mycobacterium tuberculosis
- LTBI:
-
Latent tuberculosis infection
- PPD:
-
Pure protein derivative
- IFN-γ:
-
Interferon-γ
- TST:
-
Tuberculin skin test
- IGRAs:
-
Interferon-γ release assays
- NTM:
-
Non-tuberculous mycobacteria
- BCG:
-
Bacillus Calmette-Guérin
- ESAT6:
-
6 kDa early secretory antigenic target
- CFP10:
-
10 kDa culture filtrate protein
- EC:
-
ESAT6-CFP10
- PV+ :
-
Positive predict value
- PV− :
-
Negative predict value
- YI:
-
Youden index
- CR+ :
-
Positive coincidence rate
- CR− :
-
Negative coincidence rate
References
Mandal N, Anand PK, Gautam S, Das S, Hussain T. Diagnosis and treatment of paediatric tuberculosis: an insight review. Crit Rev Microbiol. 2017;43(4):466–80.
Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the end TB era. Lancet Respir Med. 2018;6(4):299–314.
Sood S, Yadav A, Shrivastava R. Mycobacterium aurum is unable to survive Mycobacterium tuberculosis latency associated stress conditions: implications as non-suitable model organism. Indian J Microbiol. 2016;56(2):198–204.
Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152.
Azadi D, Motallebirad T, Ghaffari K, Shojaei H. Mycobacteriosis and tuberculosis: laboratory diagnosis. Open Microbiol J. 2018;12:41–58.
Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–64.
Yan L, Tang S, Yang Y, Shi X, Ge Y, Sun W, et al. A large cohort study on the clinical value of simultaneous amplification and testing for the diagnosis of pulmonary tuberculosis. Medicine (Baltimore). 2016;95(4):e2597.
Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33(5):956–73.
Dheda K, Van Zyl Smit R, Badri M, Pai M. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med. 2009;15(3):188–200.
Arend SM, Franken WP, Aggerbeck H, Prins C, Van Dissel JT, Thierry-Carstensen B, et al. Double-blind randomized phase I study comparing rdESAT-6 to tuberculin as skin test reagent in the diagnosis of tuberculosis infection. Tuberculosis (Edinb). 2008;88(3):249–61.
Nikitina IY, Karpina NL, Kasimceva OV, Gergert VY, Ergeshov A, Lyadova IV. Comparative performance of QuantiFERON-TB Gold versus skin test with tuberculosis recombinant allergen (Diaskintest) among patients with suspected pulmonary tuberculosis in Russia. Int J Infect Dis. 2019;86:18–24.
Gupta RK, Srivastava R. Resuscitation promoting factors: a family of microbial proteins in survival and resuscitation of dormant mycobacteria. Indian J Microbiol. 2012;52(2):114–21.
Greveson K. Can ELISpot replace the tuberculin skin test for latent tuberculosis? Br J Nurs. 2009;18(20):1248–54.
Fogan L. PPD antigens and the diagnosis of mycobacterial diseases. A study of atypical mycobacterial disease in Oklahoma. Arch Intern Med. 1969;124(1):49–54.
Holton SJ, Weiss MS, Tucker PA, Wilmanns M. Structure-based approaches to drug discovery against tuberculosis. Curr Protein Pept Sci. 2007;8(4):365–75.
Rachman H, Kaufmann SH. Exploring functional genomics for the development of novel intervention strategies against tuberculosis. Int J Med Microbiol. 2007;297(7–8):559–67.
Kaku T, Kawamura I, Uchiyama R, Kurenuma T, Mitsuyama M. RD1 region in mycobacterial genome is involved in the induction of necrosis in infected RAW264 cells via mitochondrial membrane damage and ATP depletion. FEMS Microbiol Lett. 2007;274(2):189–95.
Trajkovic V, Natarajan K, Sharma P. Immunomodulatory action of mycobacterial secretory proteins. Microbes Infect. 2004;6(5):513–9.
Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51(2):359–70.
Aagaard C, Govaerts M, Meikle V, Vallecillo AJ, Gutierrez-Pabello JA, Suarez-Guemes F, et al. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J Clin Microbiol. 2006;44(12):4326–35.
Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356(9235):1099–104.
Brock I, Weldingh K, Leyten EM, Arend SM, Ravn P, Andersen P. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J Clin Microbiol. 2004;42(6):2379–87.
Latorre I, De Souza-Galvao M, Ruiz-Manzano J, Lacoma A, Prat C, Fuenzalida L, et al. Quantitative evaluation of T-cell response after specific antigen stimulation in active and latent tuberculosis infection in adults and children. Diagn Microbiol Infect Dis. 2009;65(3):236–46.
Bergstedt W, Tingskov PN, Thierry-Carstensen B, Hoff ST, Aggerbeck H, Thomsen VO, et al. First-in-man open clinical trial of a combined rdESAT-6 and rCFP-10 tuberculosis specific skin test reagent. PLoS One. 2010;5(6):e11277.
Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012.
Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80.
Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):1339–45.
Kasprowicz VO, Churchyard G, Lawn SD, Squire SB, Lalvani A. Diagnosing latent tuberculosis in high-risk individuals: rising to the challenge in high-burden areas. J Infect Dis. 2011;204(Suppl 4):S1168–78.
Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20.
Hoff ST, Peter JG, Theron G, Pascoe M, Tingskov PN, Aggerbeck H, et al. Sensitivity of C-Tb: a novel RD-1-specific skin test for the diagnosis of tuberculosis infection. Eur Respir J. 2016;47(3):919–28.
Li F, Xu M, Qin C, Xia L, Xiong Y, Xi X, et al. Recombinant fusion ESAT6-CFP10 immunogen as a skin test reagent for tuberculosis diagnosis: an open-label, randomized, two-centre phase 2a clinical trial. Clin Microbiol Infect. 2016;22(10):889.e9–889.e16.
Li F, Xu M, Zhou L, Xiong Y, Xia L, Fan X, et al. Safety of recombinant fusion protein ESAT6-CFP10 as a skin test reagent for tuberculosis diagnosis: an open-label, randomized, single-center phase I clinical trial. Clin Vaccine Immunol. 2016;23(9):767–73.
Clark ED. Protein refolding for industrial processes. Curr Opin Biotechnol. 2001;12(2):202–7.
Kimura M, Comstock GW, Mori T. Comparison of erythema and induration as results of tuberculin tests. Int J Tuberc Lung Dis. 2005;9(8):853–7.
TUBERCULIN reaction size on five consecutive days. Bull World Health Organ. 1955;12(1–2):189–96..
Acknowledgements
Thanks to the professionals of Shanghai MedSci Medical Technology Co., Ltd. for revising the manuscript to improve the English.
Funding
This research was supported by the National major scientific and technological special project for significant new drugs development of China (2015 ZX09501008–004) to JX, and the National Natural Science Foundation of China (81773553) to LW. We acknowledge the contributions of the patients and volunteers enrolled into our study.
Author information
Authors and Affiliations
Contributions
FL, SL and JX contributed to the study design. FL and SL performed the clinical trial; JX contributed to design the analysis plan. HZ and LW performed the statistical analyses. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Shanghai Public Health Clinical Center was the main responsible unit for TB patients’ research; Jiangsu Provincial Center for Disease Control and Prevention was in charge of the research on healthy volunteers. Our research protocol was granted by Shanghai Public Health Clinical Center Ethics Committee (NO. 2015-E018–02) and Ethics Committee of Jiangsu Provincial Center for Disease Prevention and Control (NO. JSJK2015-C018–02). All participants read and signed the informed consent.
Consent for publication
Not applicable.
Competing interests
Authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Diagnostic criteria for pulmonary tuberculosis (WS288-2008).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Wang, L., Li, F. et al. Induration or erythema diameter not less than 5 mm as results of recombinant fusion protein ESAT6-CFP10 skin test for detecting M. tuberculosis infection. BMC Infect Dis 20, 685 (2020). https://doi.org/10.1186/s12879-020-05413-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05413-9