Abstract
Some individuals who go to fitness centers for various purposes perform resistance exercise (RE) alone, while others engage in combined exercise (CE) by including cardio exercises along with RE. Studying the effects of these two different training methods on left ventricular (LV) systolic and diastolic parameters and left atrial mechanical function is an important step toward understanding the effects of different types of exercise on cardiac function. This knowledge has significant implications for public health, as it can inform the development of targeted and effective exercise programs that prioritize cardiovascular health and reduce the risk of adverse outcomes. Therefore, the primary aim of this study is to comprehensively investigate the LV systolic and diastolic parameters of athletes who engage in RE and CE using ECHO, to contribute to the growing body of literature on the cardiovascular effects of different types of exercise. Forty-two amateur athletes aged between 17 and 52 were included in our study. The participants consisted of the RE (n = 26) group who did only resistance exercise during the weekly exercise period, and the CE group (n = 16) who also did cardio exercise with resistance exercises. After determining sports age (year), weekly exercise frequency (day), and training volume (min) in addition to demographic information of RE and CE groups, left ventricular systolic and diastolic parameters and left atrial functions were determined by ECHO. Findings from our study revealed that parameters including the left ventricular end-diastolic diameter (LVEDD) (p = .008), left ventricular end-diastolic volume (LVEDV) (p = .020), stroke volume index (SV-I) (p = .048), conduit volume (CV-I) (p = .001), and aortic strain (AS) (p = .017) were notably higher in the RE group compared to the CE group. Also left atrial active emptying volüme (LAAEV) of CE was higher than the RE group (p = .031). In conclusion, the cardiac parameters of the RE group showed more athlete’s heart characteristics than the CE group. These results may help to optimize the cardiovascular benefits of exercise routines while minimizing the potential risks associated with improper training.
Similar content being viewed by others
Background
Cardiovascular diseases are the most common cause of mortality and morbidity today [1]. Sport is an important phenomenon in the rehabilitation and prevention of cardiovascular diseases. Chronic exercise causes some improvements and some morphological changes in the heart [2]. Athlete’s heart refers to electrophysiological, structural, and functional myocardial adaptations associated with continuous training stimuli without pathological significance [3]. Regular physical training leads to an increase in left ventricular (LV) cavity size and mass (LVM) due to increased wall thickness, with adaptations occurring in endurance athletes with the greatest volume and exercise intensity [4]. In general, in the athlete’s heart, an enlargement of the left and right ventricles by 10–15% and of the left ventricular wall thickness by 10–20% is observed [5]. Echocardiographic findings can help to identify adaptive and exercise-induced cardiac changes associated with underlying pathology. Researchers have been studying these changes in cardiac structure and function for many years, as they are important for understanding the effects of exercise on the heart [6].
The wide range of cardiovascular hemodynamic changes requires classification into two main categories: static and dynamic, with several differences depending on the sport. Resistance exercise is generally characterized by static, short, and strong muscle contractions. Static sports lead to an increase in systemic vascular resistance, systolic blood pressure (SBP), and heart rate (HR) to maintain cardiac output, which eventually manifests as concentric LV hypertrophy [7]. On the other hand, dynamic and endurance activities such as running are characterized by repeated contractions and relaxations of the large skeletal muscles. Dynamic exercise results in an increase in HR, an increase in stroke volume (SV), and an increase in SBP while decreasing systemic vascular resistance and diastolic blood pressure (DBP) to maintain adequate oxygen delivery [8].
Resistance exercise (RE), also known as weight or strength training, is a specialized form of conditioning to increase muscular strength, muscular endurance, and muscular power. In response to this type of training, both skeletal and cardiac muscles adapt to this condition, resulting in hypertrophy [9]. However, RE and endurance exercises lead to different changes in the heart. These changes in RE are characterized by an increase in LV wall thickness and no change in the diastole LV cavity [10]. A study by Longhurst et al. comparing elite endurance athletes and weightlifters reported that LVM increased in both groups compared with the control groups (amateur endurance athletes and weightlifters). However, he concluded that LVM increased more in elite endurance athletes than in elite weightlifters [11]. Therefore, research has shown that chronic exercise generally increases the aerobic ability of muscles and reduces CO2 at rest. These changes are associated with a slow heartbeat and increased myocardial contractility. The increased muscle oxygenation and hypertrophy with RE leads to higher oxygen perfusion [12]. Therefore, the increased amount of venous blood returning to the heart increases cardiac contractility via the Starling effect, which also increases the amount of blood leaving the heart. This resulting volume overload causes concentric cardiac remodeling in the heart muscle of resistance exercisers [13].
In the literature, cardiac morphology is affected by many exercise-related variables. Although there are many studies examining the effects of exercises such as RE on the heart [14,15,16], the number of studies examining the changes in LV diastolic and systolic parameters and left atrial (LA) mechanical function in individuals performing cardio exercises on different equipment in fitness centers is limited. Therefore, this study aimed to examine the effects of chronic RE and combined exercise (CE) on cardiac morphologies and to provide some suggestions for updating training content.
Method
Participants
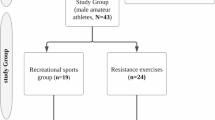
The research sample was selected by simple random sampling method from the participants attending fitness centers [17]. The differences between the left ventricular end-diastolic diameter (LVEDV) values (experimental group: 65 ± 5 mL/m2, control group: 60 ± 5.2 mL/m2) in the results of the study conducted by Hassanpour Dehkordi and Khaledi Far were taken to determine the minimum sample size in the study [2]. In the power analysis performed according to the results of this study (α = 0.05 1-β(power) = 0.80, actual power = 81.9) and the effect size was taken as 1.05, it was determined that there should be at least 16 subjects for each group in our study. The study included volunteers who had been attending a fitness center regularly (at least three days per week) for at least 12 months. Participants with hypertension, tachycardia or bradycardia, thyroid disease, heart valve disease, previous surgery for a heart problem, and any comorbidities were excluded, as well as athletes taking caffeine, ephedrine, anabolic steroids, beta-androgenic agonists for performance enhancement. Participants who were found to have a sleep disorder during the study, who did not comply with the researcher’s instructions, or who were found to have health problems during testing were excluded from the study. Accordingly, 42 male amateur participants doing resistance exercises who met the inclusion and exclusion criteria were included in the study. Participants were divided into two groups according to the type of training: the RE group (n = 26), which performed only resistance exercises, and the CE group (n = 16), which combined cardio and resistance exercises. Participants’ time spent in sport (sport age), weekly exercise frequency, and exercise volume were recorded. RE group (sports age: 5.34 ± 7.31 years, weekly exercise frequency: 4.46 ± 1.36 days, daily training volume: 84.03 ± 17.88 s) and CE group (sports age: 7.15 ± 9.47 years, weekly exercise frequency: 4.06 ± 1.12 days, daily training volume: 84.31 ± 28.34 s) sports age, weekly training frequency and training volumes were similar (Table 1).
Institutional Review Board Statement: This study was conducted at Bandırma Onyedi Eylul University Health Sciences Institute Non-Interventional Ethics Committee, according to the principles outlined Declaration of Helsinki. Ethics committee approval was obtained from Bandırma Onyedi Eylul University Health Sciences Institute Non-Interventional Ethics Committee Ethics Committee (number: 2022 − 172). All experimental protocols were approved by the Institute’s Clinical Research Ethics Committee. Informed consent was obtained from all subjects and/or their legal guardian(s) agreed to participate in this study.
Study design
In this study, the quantitative data collection technique known as the cross-sectional method was employed. This method involves measuring or observing specific behaviors of individuals from various age groups simultaneously, within the same time frame [18]. The RE group stated that they did not include cardio exercises (treadmill, elliptical trainer, vertical and horizontal cycling exercises, etc.) in their exercise program. The group CE stated that it includes at least 150 min of cardio exercises per week in addition to resistance exercises. They were asked to attend fully provided they slept at least 8 h before the test and ate at least 2 h before. Before the test, participants were informed that they should not engage in high-intensity sports and should not consume substances such as alcohol and caffeine. After the demographic information and training content of the participants were determined, they were taken to the cardiology department for analysis. After the necessary rest period was given, the DBP, SBP, and HR data of the participants were recorded. ECHO measurements were then performed by the cardiologist.
Training characteristics of RE and CE groups
The RE group worked with free weights and machines as part of resistance exercises. All participants set their own 1 repetition maximum (1-RM). Each participant performed 6–12 repetitions*4 sets with 60–80% of 1-RM. The CE group included cardio exercises for 15–30 min in addition to RE exercises. Within the scope of these exercises, they stated that they worked between 60 and 70% of their HR on the treadmill, horizontal and vertical bicycle. They stated that they performed resistance exercises at the same standards as the RE group (60–80% 1-RM, 6–12 repetitions*4 sets). The exercise programs of the participants were prepared and followed by the expert trainer in the fitness center.
Data Collection
Body surface area
After determining the demographic characteristics of the participants, such as age, height, and weight, body mass index (BMI) was calculated using the formula body weight (kg)/height squared (m²). Body surface area (BSA); Calculated according to the formula: Body weight (kg)0.425 ×Height (cm)0.725 × 0.007184 [19].
Echocardiographic examinations
ECHO evaluations were made by a specialist cardiologist in the cardiology clinic. All tests were performed at the same time of day (morning). Participants were asked to take eight hours of sleep before the measurements. At least 3 h before the test, participants were warned not to use food, drink, performance-enhancing products, or products containing caffeine other than water. All ECHO examinations were performed with the Vivid T8 device and the 3ScRS transducer. All measurements were performed following the recommendations of the American Society of Echocardiography Guidelines [20]. Echocardiographic images and recordings were obtained in parasternal long axis, apical four-chamber, and apical two-chamber views in the left decubitus position and at rest [21]. The following 2-dimensional and M-mode echocardiographic parameters were measured: aortic diameters both in systole (ADs) and diastole (ADd), left ventricular end-diastolic diameter (LVDd, mm), left ventricular end-systolic diameter (LVDs), interventricular septal thickness (IVST), and posterior wall thickness (PWT).
Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), stroke volume (SV), and ejection fraction (EF) were measured in the apical four-chamber view by the modified Simpson method [22]. Pulsed wave (PW) and early diastolic flow velocity (E), late diastolic flow parameter (A), E/A ratio, ejection time (ET), isovolumic relaxation time (IVRT), isovolumic contraction time (IVCT), and transmissible flow parameters were measured. To determine the group with stage 2 diastolic dysfunction (pseudonormal), patients performed the Valsalva maneuver, and the measurement was repeated. Tissue Doppler imaging of annulus motion was measured from lateral mitral annulus and peak early systolic (Sm), peak early diastolic (Em), and peak late diastolic (Am) velocities.
Tissue doppler imaging (TDI)
TDI was performed from the LV lateral wall in apical 4-chamber views from the mitral lateral annulus. A clear image signal was obtained by adjusting the TDI filter and Nyquist cutoff to a value of 16–20 cm/s. The left ventricular mass index (LVM-I) was calculated according to the Devereux formula [23]. Aortic systolic and diastolic diameters were obtained from the recording made after the insertion of the M-mode rod through the area of the ascending aorta 3 cm distal to the aortic valve. Systolic and diastolic diameters were measured in the area corresponding to the R peak of the ECG from the point of maximum forward motion in the aortic curve. The measurements were repeated in three heartbeats and the average value was determined [24]. Aortic strain (AS) and aortic distensibility (AD) were used as aortic elasticity parameters. The following formulas were used to calculate these parameters [25]:
Left atrial volumes were calculated from apical four-chamber and two-chamber views using the biplane field length method. Maximum left atrial volume (LAVmax) was measured at the time when the mitral valve was fully open, minimum left atrial volume (LAVmin) was measured at the time when the mitral valve was fully closed, and left atrial volume before systole was measured at the onset of the p wave (LAVp) on the electrocardiogram. All measurements were repeated during three consecutive heartbeats and averaged. The LAVmax index was determined by dividing the maximum volume of the left atrium (LAVmax) by the body surface area. All volumes were then corrected by division by the LAVmax index. Left atrial functions were determined according to the following formula [26].
Intraobserver and interobserver variability
The absolute mean difference ± SD between measurements within a single observer and between two observers for ADs, 2D STI, and LS were 1.1 ± 1.2% and 1.4 ± 1.4%, respectively. The intraobserver and interobserver Intraclass Correlation Coefficients (ICCs) were 0.877 and 0.865, respectively.
Blood pressure
Participants’ blood pressure was measured by the cardiologist after 9 min of complete passive rest. Systolic and diastolic blood pressure were measured with a stethoscope and a sphygmomanometer (Erka Perfect Aneroid / Germany) [27].
Statistical analysis
Data were reported as mean ± SD. Data analysis was carried out by the SPSS (version 25.0) program. The Shapiro-Wilk test stated the data were normally distributed. Also, according to Mauchly’s test, variances were determined to be homogeneous for all parameters (p > .05). Therefore, sphericity assumed values were taken into account. In the study, an Independent Sample T-test was used for paired group comparisons. In the research, graphical analyses were made using the GraphPad 9 program. The Cohen’s D effect size (ES) was performed to determine the effect magnitude through the difference of two means divided by the standard deviation from the data, and the following criteria were used: ES: 0.2 was accepted as small, 0.5 as medium, and 0.8 as large [28]. The significance level was interpreted according to p < .05.
The statistical analyses of this study were conducted using the Benjamini-Hochberg procedure to control the false discovery rate (FDR) that may result from the results of multiple comparisons. This procedure is a correction method developed to keep the probability of falsely rejecting multiple hypothesis tests low [29]. In this context, p-values were ranked at the end of the tests. Starting from the lowest p-value, the formula:
was used for each p value. This procedure was used to limit possible false positives in the results and aimed to minimize the increased risk of type-1 error due to multiple comparisons.
Results
Table 1 shows the descriptive information of the participants. Accordingly, there was no difference between RE’s age (27.03 ± 9.88 years), weight (81.53 ± 12.24 kg), height (178.61 ± 4.85 cm), BMI (25.50 ± 3.29 kg/m2), and BSA (2.00 ± 0.16) and CE’ age (28.00 ± 11.52 years), weight (78.12 ± 12.53 kg), height (178.56 ± 6.97 cm), BMI (24.38 ± 2.57 kg/m2), and BSA (1.92 ± 0.28) (p > .05).
Table 2 shows the statistical results of the participants’ SBP, DPB, HR, and routine ECHO parameters. Accordingly, LVEDD (p = .008, t = 2.805, ES: 0.84), SV-I (p = .048, t = 2.039, ES: 0.97), and LVEDV (p = .020, t = 2.415, ES: 0.75) in the RE group was significantly higher than the CE group. However, SBP, DBP, HR, ADs, ADd, LVESD, LVESV, IVST, and PWT values were similar between the two groups (p > .05).
Table 3 shows the ECHO results of the systolic and diastolic functions of the participants. According to this, there was no difference between groups in terms of E, A, ET, IVCT, IVRT, Em, Am, Sm, LVEF, E/A ratio, and E/Em among participants (p > .05).
Table 4 shows the participants’ atrial functions. Accordingly, CV -I (p = .008, t = 2.797, ES:0.87) was higher in the RE group than in the CE group. However, LAAEV-I scores were higher in the CE group (p = .031, t=-2.230, ES: 0.67). There were no differences between groups in LAPEV-I, LAPEF-I, LAAEF-I, LATEV-I, LATEF-I, LAmin-I, LAP -I, LAmax-I, LVM-I, and AD scores (p > .05).
Table 5 shows the results of the aortic stiffness and flexibility of the participants. Accordingly, the results of AS (p = .032, t = 2.217, ES:0.75) were higher in the RE group than in the CE group. AD was similar between the two groups (p > .05).
Figure 1 shows the parameters that differed in the ECHO results of the RE and CE groups. Accordingly, LVEDD (p = .008, t = 2.805, ES: 0.84), LVEDV (p = .020, t = 2.415, ES: 0.75), SV-I (p = .048, t = 2.039, ES: 0.97), CV -I (p = .008, t = 2.797, ES:0.87), and AS (p = .032, t = 2.217, ES:0.75) were significantly higher in the RE group than in the CE group. However, LAAEV-I scores were higher in the CE group (p = .031, t=-2.230, ES: 0.67).
Discussion
In our study, the results for LVDd, LVEDV, SV -I, CV -I, and AS were higher in the RE than in CE. Also, LAAEV was higher in CE. From a physiological point of view, long-term hemodynamic changes during exercise induce both LV internal size (LV dilation) and LV hypertrophy to normalize LV wall tension. LV dilation and LV hypertrophy may be marked enough to mimic a pathological condition, but LV systolic and diastolic functions are normal or even above normal, which is a very natural feature of the athlete’s heart [30].
Chronic exercise is known to affect systolic [2] and diastolic [31] parameters. The changes in these parameters vary depending on the intensity, duration, and type of exercise [32]. During heavy resistance exercise, there is a significant increase in arterial blood pressure [33]. Cardiac output also increases. As a result, a slight increase in LVDd is observed [34]. It was found that systolic and diastolic parameters and cardiac output increased in individuals who combined exercises such as cycling, rowing, and cross-country skiing with resistance exercise. This excessive pressure exercise increases LVDd even more [35]. In a study by Keul et al., it was found that LVDd was higher in strength athletes than in long and middle-distance runners [36]. Although combined exercise should increase diastolic function more, it was found in our study that LVDd was higher in the RE group. In this case, it can be assumed that the intensity of cardio exercises performed in the CE group did not impose a burden on the heart. In a meta-analysis that examined the effects of aerobic, resistance, and combined exercise on cardiovascular factors, a systematic review study by Liang et al., the effects of aerobic, resistance, and combined exercises on cardiovascular risk factors were examined. According to the results of this study, it was reported that combined exercises were the most effective method in minimizing cardiac risk factors, while aerobic exercises had minimal effect. It was concluded that future studies need more study results for resistance exercises [37]. Evaluating the results of this study and the results of our study, we think that the structural changes expected as a result of endurance training in the heart did not occur, because the exercises performed by the group CE in addition to the RE exercises were of low intensity.
A relationship between the athlete’s heart and systolic and diastolic functions has been confirmed. The diastolic functions of the athlete’s heart are manifested by a low A wave, a high Em wave, and a lower E/Em ratio [38]. Elevated LV and confirmed SV -I for body surface area reflect systolic recovery [39]. A meta-analysis by Pluim et al. concluded that there was no significant difference in LVEF between endurance athletes and bodybuilders [40]. In our study, SV-I results were higher in the RE group than in the CE group. Although there was no difference between the groups in terms of LVEF, a positive correlation was found between LV and EF. In this case, it may show that the systolic parameters of the RE group are also positively affected. In a study by Seals et al., it was concluded that high-intensity exercise contributes to the development of LV in older elite athletes who have undergone endurance training [41].
In the study conducted by Andersen et al., systolic and diastolic indicators such as the E/Em ratio were analyzed as an estimate of Em, Sm, and left ventricular filling pressure [42], E increased significantly in both groups after sixteen weeks of football, and track and field training, noting that IVRT and Am scores decreased. However, it was observed that this change was greater in football players [43]. According to the results of our study, systolic and diastolic parameters (Em, Am, Sm, E/Em ratio) were similar to those reported in the literature and no significant difference was found between the two groups (RE, CE). Considering that resistance training increases aerobic capacity [1] and, accordingly, peak oxygen consumption and systolic parameters increase [44], the reason why the systolic and diastolic parameters of the RE group were better than those of the CE group is that the CE group could show that the groups training does not exert resistance on the heart.
With increasing age, significant changes occur in the cardiovascular system in apparently healthy people. There is thickening and hardening of the large arteries due to the loss of elastic fibers, collagen, and calcium deposits in the middle layer [45]. As a result of these arterial changes, SBP increases with age. Wall thickening occurs in the left ventricle due to cell hypertrophy [46]. LVD functions are preserved with age, the early diastolic filling rate decreases by 30–50% between the ages of 30–40 years [47]. These age-related cardiac changes can be ameliorated by physical activity. Acute and chronic exercise, on the other hand, elicit different responses in the cardiovascular system depending on age [48]. During acute exercise in healthy, sedentary individuals, SV increases by 20–30% [49]. As exercise intensity increases, LV develops in parallel [50]. In our study, it was found that SV -I values were significantly higher in the RE group. This indicates that in the RE group, due to muscle hypertrophy resulting from resistance exercise [51], the energy required by the muscles was provided by an increase in systolic parameters.
Aortic stiffness reflects the mechanical tension and elasticity of the aortic wall. Increased stiffness of the great arteries is an important determinant of adverse cardiovascular outcomes [25]. O’Rourke et al. reported that changes in vascular tone, smooth muscle cell hypertrophy and hyperplasia, and increased collagen synthesis lead to an increase in arterial stiffness [52]. In addition, in physically inactive individuals, large artery function in the cardiothoracic region may decrease with age [53]. However, aerobic exercise such as endurance sports is known to be an important method of preventing arterial stiffness [54]. Arterial stiffness did not change with less strenuous exercise [55]. The aorta is responsible for most of the total arterial stiffness [56]. Previous studies have cited AD and AS as the best predictors of subclinical arterial stiffness, and there is an inverse relationship between arterial stiffness and AS [57]. In our study, AS was significantly higher in the RE group. These results may resolve the uncertainties reported in previous studies [55] regarding arterial stiffness during resistance training.
In our study, there was no difference between the two groups in terms of left LVM and IVST. According to the Morganroth hypothesis, endurance training leads to eccentric LV hypertrophy (increase in LV cavity diameter at increased LVM) as a result of volume loading and increased diastolic wall stress [58]. Conversely, resistance training results in concentric LV hypertrophy (increased wall thickness with no change in cavity size) as a result of pressure loading and increased systolic wall stress [59]. Data from Italian screening programs reported LV dilatation and LV hypertrophy in a large cohort of young athletes studied by ECHO. Although LVDd varied widely, most athletes were above normal, whereas only a small percentage had an IVST > 12 mm [60]. Standard ECHO plays an important role in distinguishing physiological from pathological LV hypertrophy. In the 1309 athlete series in different disciplines, 55% of endurance athletes have increased LVDd and only 15% have a value > 60 mm, almost always with normal EF. Most of the 947 top athletes had an IVST ≤ 12 mm. Only 1.7% had an IVST > 13 mm (range = 13–16 mm). While an increase in LVM in athletes is usually associated with a normal EF at rest, HR may be normal or increased as a result of increased preload (LVDd) [61].
Previous studies examined left atrial (LA) size and athletic activity in athletes and found that left atrial enlargement depended on the type of exercise (the greatest change occurred with combined exercise: Endurance and strength exercises) [62]. Although the LA is known as a transport space that carries blood from the pulmonary veins to the LV during active and passive diastolic filling, it also has many physiological functions and acts as a volume sensor. It is well known that LA size is an important indicator of mortality and morbidity in cardiomyopathy, LV dysfunction, aortic stenosis, mitral regurgitation, and arrhythmias [63]. Like the LV in LA also remodels after endurance exercise [64]. However, an important aspect is that LA reserve helps modulate LV filling pressure both at rest and during exercise [65]. Although the mechanisms of atrial development are not fully known [66], some sources indicate that the LA develops anterior to the LV in response to exercise [67]. Pellicia et al. found a prevalence of LA enlargement in athletes, a slight increase in LA anteroposterior diameter in 18% of athletes, significant dilation in 2%, and a close relationship between LA diameter and LA cavity [68]. In a study by Lakatos et al. comparing left atrial morphology in elite athletes with sedentary subjects, it was found that LATEF and LAAEF were lower in athletes [69]. A recent meta-analysis also found that both LA reservoir and contractile functions were lower in athletes [70]. In our study, LAAEF was significantly higher in the CE group than in the RE group. When participants’ left atrial functions are assessed according to the results of the study by Lakatos et al’, the LAAEV results of the CE group show that they do not have the characteristics of an athlete’s heart compared with the literature. Although no significant difference was found, the LAP -I and LATEV-I results had a moderate effect size and were higher in the CE group.
Our research is a study in which training loads and exercise intensities were analyzed according to the participants’ statements. This is one of the important limitations of our study. Therefore, research can be conducted on how the cardiac remodeling of the participants is shaped after chronic exercise. Another important limitation of this study is that it is cross-sectional in nature using a single time point. Therefore, in future randomised controlled trials, the changes in resistance exercises and aerobic exercise on cardiac morphology can be analysed in detail.
Conclusion
In conclusion, in our study, it was found that systolic and diastolic parameters were higher in the RE group than in the CE group. In addition, a significant increase in LAAEF in the CE group is indicative of an increase in active LA functions. Other findings on LA functions supported the increase in LAAEF in the CE group, although there was no significant difference. In addition, the higher systolic and diastolic parameters such as LVDd, LVEDV, SV -I, and CV -I in the RE group confirmed that the RE group had shown more athletes’ heart [71]. In the literature, combined exercise with resistance exercise is more effective on cardiac functions [33, 37, 72], but in our study, indices of cardiac functions were lower in the CE group than in the RE group. It is believed that this is because the CE group kept the exercise intensity of cardio training equipment such as treadmills, elliptical bikes, and vertical and horizontal bikes lower in the exercises they performed. For this reason, athletes who visit a fitness center can update their programs in the presence of a sports professional. In addition, the level of physical fitness of these people can be monitored at regular intervals and their development can be analyzed. Individuals who attend the fitness centre for different purposes can update their training programs and intensities according to the results of our research.
Data availability
Data are available for research purposes upon reasonable request to the corresponding author.
References
Giuliano C, Karahalios A, Neil C, Allen J, Levinger I. The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure — a meta-analysis. Int J Cardiol. 2017;227:413–23.
Hassanpour Dehkordi A, Khaledi Far A. Effect of exercise training on the quality of life and echocardiography parameter of systolic function in patients with chronic heart failure: a randomized trial. Asian J Sports Med. 2015;6.
Baumgartner L, Schulz T, Oberhoffer R, Weberruß H. Influence of vigorous physical activity on structure and function of the cardiovascular system in Young athletes—the MuCAYA-Study. Front Cardiovasc Med. 2019;6.
Kooreman Z, Giraldeau G, Finocchiaro G, Kobayashi Y, Wheeler M, Perez M, et al. Athletic remodeling in female college athletes: the morganroth hypothesis revisited. Clin J Sport Med. 2019;29:224–31.
Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J. 2015;36:1445–53.
White PD, The pulse after a, marathon race. JAMA: J Am Med Association. 1918;71:1047.
Baggish AL, Battle RW, Beckerman JG, Bove AA, Lampert RJ, Levine BD, et al. Sports cardiol. J Am Coll Cardiol. 2017;70:1902–18.
Levine BD, Baggish AL, Kovacs RJ, Link MS, Maron MS, Mitchell JH. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 1: classification of sports: dynamic, static, and impact. J Am Coll Cardiol. 2015;66:2350–5.
Leuchtmann AB, Mueller SM, Aguayo D, Petersen JA, Ligon-Auer M, Flück M, et al. Resistance training preserves high-intensity interval training induced improvements in skeletal muscle capillarization of healthy old men: a randomized controlled trial. Sci Rep. 2020;10:6578.
Parry-Williams G, Sharma S. The effects of endurance exercise on the heart: panacea or poison? Nat Rev Cardiol. 2020;17:402–12.
Longhurst JC, Kelly AR, Gonyea WJ, Mitchell JH. Echocardiographic left ventricular masses in distance runners and weight lifters. J Appl Physiol. 1980;48:154–62.
Seo DY, Kwak H-B, Kim AH, Park SH, Heo JW, Kim HK, et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch. 2020;472:155–68.
Foryst-Ludwig A, Kintscher U. Sex differences in exercise-induced cardiac hypertrophy. Pflugers Arch. 2013;465:731–7.
Ferreira FC, Bertucci DR, Barbosa MR, Nunes JE, Botero JP, Rodrigues MF et al. Circuit resistance training in women with normal weight obesity syndrome: body composition, cardiometabolic and echocardiographic parameters, and cardiovascular and skeletal muscle fitness. J Sports Med Phys Fit. 2017;57.
Haykowsky MJ, Dressendorfer R, Taylor D, Mandic S, Humen D. Resistance training and cardiac hypertrophy. Sports Med. 2002;32:837–49.
Dantas PS, Sakata MM, Perez JD, Watanabe RLH, Bizerra FC, Neves VJ das, et al. Unraveling the role of high-intensity resistance training on left ventricle proteome: is there a shift towards maladaptation? Life Sci. 2016;152:156–64.
Pathak PK. 4 Simple random sampling. 1988. 97–109.
Kesmodel US. Cross-sectional studies - what are they good for? Acta Obstet Gynecol Scand. 2018;97:388–93.
Zafrir B, Salman N, Crespo-Leiro MG, Anker SD, Coats AJ, Ferrari R, et al. Body surface area as a prognostic marker in chronic heart failure patients: results from the heart failure registry of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2016;18:859–68.
Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka P, et al. Recommendations for chamber quantification☆. Eur J Echocardiography. 2006;7:79–108.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713.
Ujino K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, et al. Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006;98:1185–8.
Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8.
Stefanadis C, Stratos C, Boudoulas H, Kourouklis C. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–6.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
Yilmaz M, Arican Ozluk FO, Akgumus A, Peker T, Karaagac K, Vatansever F, et al. Left atrial mechanical functions in patients with the metabolic syndrome. Acta Cardiol. 2013;68:133–7.
Johnson KA, Partsch DJ, Gleason P, Makay K. Comparison of two home blood pressure monitors with a Mercury Sphygmomanometer in an Ambulatory Population. Pharmacotherapy. 1999;19:333–9.
Hopkins WG, Marshall SW, Batterham AM, Hanın J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–12.
Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educational Behav Stat. 2000;25:60–83.
Lovic D, Narayan P, Pittaras A, Faselis C, Doumas M, Kokkinos P. Left ventricular hypertrophy in athletes and hypertensive patients. J Clin Hypertens. 2017;19:413–7.
Amundsen BH, Rognmo Ø, Hatlen-Rebhan G, Slørdahl SA. High-intensity aerobic exercise improves diastolic function in coronary artery disease. Scandinavian Cardiovasc J. 2008;42:110–7.
Lele SS, Macfarlane D, Morrison S, Thomson H, Khafagi F, Frenneaux M. Determinants of exercise capacity in patients with coronary artery disease and mild to moderate systolic dysfunction: role of heart rate and diastolic filling abnormalities. Eur Heart J. 1996;17:204–12.
Park W, Jung W-S, Hong K, Kim Y-Y, Kim S-W, Park H-Y. Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. 2020;17:7233.
Menapace FJ, Hammer WJ, Ritzer TF, Kessler KM, Warner HF, Spann JF, et al. Left ventricular size in competitive weight lifters. Med Sci Sports Exerc. 1982;14:72–5.
Abergel E, Chatellier G, Hagege AA, Oblak A, Linhart A, Ducardonnet A, et al. Serial left ventricular adaptations in world-class professional cyclists. J Am Coll Cardiol. 2004;44:144–9.
Keul J, Dickhuth HH, Simon G, Lehmann M. Effect of static and dynamic exercise on heart volume, contractility, and left ventricular dimensions. Circ Res. 1981;48(6 Pt 2):I162–70.
Liang M, Pan Y, Zhong T, Zeng Y, Cheng A. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: a systematic review and network meta-analysis. Rev Cardiovasc Med. 2021;22:1523.
Forteza-Albertí JF, Sanchis-Gomar F, Lippi G, Cervellin G, Lucia A, Calderón-Montero FJ. Limits of ventricular function: from athlete’s heart to a failing heart. Clin Physiol Funct Imaging. 2017;37:549–57.
Parry-Williams G, Gati S, Sharma S. The heart of the ageing endurance athlete: the role of chronic coronary stress. Eur Heart J. 2021;42:2737–44.
Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. Athlete’s Heart Circulation. 2000;101:336–44.
Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation. 1994;89:198–205.
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures. Circulation. 2000;102:1788–94.
Andersen LJ, Hansen PR, Søgaard P, Madsen JK, Bech J, Krustrup P. Improvement of systolic and diastolic heart function after physical training in sedentary women. Scand J Med Sci Sports. 2010;20:50–7.
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36.
Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12:293–7.
Lucas RAI, Sarma S, Schlader ZJ, Pearson J, Crandall CG. Age-related changes to cardiac systolic and diastolic function during whole-body passive hyperthermia. Exp Physiol. 2015;100:422–34.
Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17:545–54.
Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. J Appl Physiol. 2017;122:925–32.
Rowland TW. Circulatory responses to exercise. Chest. 2005;127:1023–30.
Rowland T, Heffernan K, Jae SY, Echols G. Tissue doppler assessment of ventricular function during cycling in 7- to 12-yr-old boys. Med Sci Sports Exerc. 2006;38:1216–22.
Ritter O, Isacco L, Rakobowchuk M, Tordi N, Laroche D, Bouhaddi M, et al. Cardiorespiratory and autonomic nervous system responses to prolonged eccentric Cycling. Int J Sports Med. 2019;40:453–61.
O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante G. e. E. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–44.
Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–82.
Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in Central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–32.
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, Habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–5.
Willum Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70.
Redheuil A, Yu W-C, Wu CO, Mousseaux E, de Cesare A, Yan R, et al. Reduced ascending aortic strain and distensibility. Hypertension. 2010;55:319–26.
Morganroth J. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521.
Horner K, Barinas-Mitchell E, DeGroff C, Kuk JL, Drant S, Lee S. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci. 2015;27:494–502.
Biffi A, Maron BJ, Di Giacinto B, Porcacchia P, Verdile L, Fernando F, et al. Relation between training-induced left ventricular hypertrophy and risk for ventricular tachyarrhythmias in Elite athletes. Am J Cardiol. 2008;101:1792–5.
Pelliccia A. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130:23.
Sørensen E, Myrstad M, Solberg MG, Øie E, Tveit A, Aarønæs M. Left atrial function in male veteran endurance athletes with paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2021;23:137–46.
Rossi A, Tomaino M, Golia G, Santini F, Pentiricci S, Marino P, et al. Usefulness of left atrial size in predicting postoperative symptomatic improvement in patients with aortic stenosis. Am J Cardiol. 2000;86:567–70.
George K, Whyte GP, Green DJ, Oxborough D, Shave RE, Gaze D, et al. The endurance athletes heart: acute stress and chronic adaptation. Br J Sports Med. 2012;46(Suppl 1):i29–36.
Maffeis C, Morris DA, Belyavskiy E, Kropf M, Radhakrishnan AK, Zach V, et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Heart Fail. 2021;8:116–28.
Iskandar A, Mujtaba MT, Thompson PD. Left atrium size in elite athletes. JACC Cardiovasc Imaging. 2015;8:753–62.
Liu M, Sun M, Li L, Li P, Hou S, Li Z, et al. Left atrial function in young strength athletes: four-dimensional automatic quantitation study. Int J Cardiovasc Imaging. 2022;38:1929–37.
Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–6.
Lakatos BK, Molnár AÁ, Kiss O, Sydó N, Tokodi M, Solymossi B, et al. Relationship between cardiac remodeling and exercise capacity in elite athletes: incremental value of left atrial morphology and function assessed by three-dimensional echocardiography. J Am Soc Echocardiogr. 2020;33:101–e1091.
Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Left atrial function in elite athletes: a meta-analysis of two‐dimensional speckle tracking echocardiographic studies. Clin Cardiol. 2019;42:579–87.
Albaeni A, Davis JW, Ahmad M. Echocardiographic evaluation of the Athlete’s heart. Echocardiography. 2021;38:1002–16.
Pedralli ML, Marschner RA, Kollet DP, Neto SG, Eibel B, Tanaka H, et al. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial. Sci Rep. 2020;10:7628.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.K; methodology, A.K., A.A., and A.B.; formal analysis, A.K., E.A., and E.K.; data curation, A.K., A.A., B.Ç., N.K., Ö.E.; writing—original draft preparation, A.K., A.A., A.B., E.A., E.K., B.Ç., N.K., Ö.E.; writing—review and editing, A.K., A.A., A.B., E.A., E.K., B.Ç., N.K., Ö.E., H.N.; Funding; H.N.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board Statement: This study was conducted at Bandırma Onyedi Eylul University Health Sciences Institute Non-Interventional Ethics Committee, according to the principles outlined Declaration of Helsinki. Ethics committee approval was obtained from Bandırma Onyedi Eylul University Health Sciences Institute Non-Interventional Ethics Committee Ethics Committee (number: 2022 − 172). All experimental protocols were approved by the Institute’s Clinical Research Ethics Committee. Informed consent was obtained from all subjects and/or their legal guardian(s) agreed to participate in this study.
Consent for publication
No individual or indemnifiable data is being published as part of this manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kurtoğlu, A., Akgümüş, A., Balun, A. et al. Investigation of left atrial mechanical function and left ventricular systolic and diastolic parameters in athletes performing resistance exercise and combined exercise. BMC Cardiovasc Disord 24, 237 (2024). https://doi.org/10.1186/s12872-024-03908-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03908-w