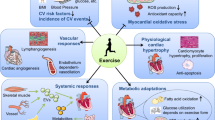
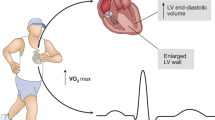
Abstract
The heart is the primary pump that circulates blood through the entire cardiovascular system, serving many important functions in the body. Exercise training provides favorable anatomical and physiological changes that reduce the risk of heart disease and failure. Compared with pathological cardiac hypertrophy, exercise-induced physiological cardiac hypertrophy leads to an improvement in heart function. Exercise-induced cardiac remodeling is associated with gene regulatory mechanisms and cellular signaling pathways underlying cellular, molecular, and metabolic adaptations. Exercise training also promotes mitochondrial biogenesis and oxidative capacity leading to a decrease in cardiovascular disease. In this review, we summarized the exercise-induced adaptation in cardiac structure and function to understand cellular and molecular signaling pathways and mechanisms in preclinical and clinical trials.


Similar content being viewed by others
References
Abrahams A (1946) Exercise and cardiac hypertrophy. Lancet 2:565
Aengevaeren VL, Hopman MTE, Thijssen DHJ, van Kimmenade RR, de Boer MJ, Eijsvogels TMH (2017) Endurance exercise-induced changes in BNP concentrations in cardiovascular patients versus healthy controls. Int J Cardiol 227:430–435. https://doi.org/10.1016/j.ijcard.2016.11.016
Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA (2001) Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985) 90:1900–1908. https://doi.org/10.1152/jappl.2001.90.5.1900
Almeida PW, Gomes-Filho A, Ferreira AJ, Rodrigues CE, Dias-Peixoto MF, Russo RC, Teixeira MM, Cassali GD, Ferreira E, Santos IC, Garcia AM, Silami-Garcia E, Wisloff U, Pussieldi GA (2009) Swim training suppresses tumor growth in mice. J Appl Physiol (1985) 107:261–265. https://doi.org/10.1152/japplphysiol.00249.2009
Arad M, Seidman CE, Seidman JG (2007) AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100:474–488. https://doi.org/10.1161/01.RES.0000258446.23525.37
Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE (2015) Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 175:959–967. https://doi.org/10.1001/jamainternmed.2015.0533
Aubert G, Vega RB, Kelly DP (2013) Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta 1833:840–847. https://doi.org/10.1016/j.bbamcr.2012.08.015
Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE (2016) Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133:706–716. https://doi.org/10.1161/CIRCULATIONAHA.115.017545
Beisvag V, Kemi OJ, Arbo I, Loennechen JP, Wisloff U, Langaas M, Sandvik AK, Ellingsen O (2009) Pathological and physiological hypertrophies are regulated by distinct gene programs. Eur J Cardiovasc Prev Rehabil 16:690–697. https://doi.org/10.1097/HJR.0b013e32833158a2
Bersell K, Arab S, Haring B, Kuhn B (2009) Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270. https://doi.org/10.1016/j.cell.2009.04.060
Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, Cao Y, Mann N, Spiegelman BM, Rosenzweig A (2016) CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight 1. doi:https://doi.org/10.1172/jci.insight.85904
Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH (2013) The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24:1250–1261. https://doi.org/10.1681/ASN.2012121216
Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH (2014) Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171:2017–2028. https://doi.org/10.1111/bph.12468
Blomqvist CG, Saltin B (1983) Cardiovascular adaptations to physical training. Annu Rev Physiol 45:169–189. https://doi.org/10.1146/annurev.ph.45.030183.001125
Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM (2010) C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143:1072–1083. https://doi.org/10.1016/j.cell.2010.11.036
Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED (2009) Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119:1272–1283. https://doi.org/10.1161/CIRCULATIONAHA.108.792101
Budiono BP, See Hoe LE, Peart JN, Sabapathy S, Ashton KJ, Haseler LJ, Headrick JP (2012) Voluntary running in mice beneficially modulates myocardial ischemic tolerance, signaling kinases, and gene expression patterns. Am J Physiol Regul Integr Comp Physiol 302:R1091–R1100. https://doi.org/10.1152/ajpregu.00406.2011
Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, Hochachka PW, Allard MF (2004) Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol 287:H1055–H1063. https://doi.org/10.1152/ajpheart.00925.2003
Burgos JI, Yeves AM, Barrena JP, Portiansky EL, Vila-Petroff MG, Ennis IL (2017) Nitric oxide and CaMKII: critical steps in the cardiac contractile response to IGF-1 and swim training. J Mol Cell Cardiol 112:16–26. https://doi.org/10.1016/j.yjmcc.2017.08.014
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP (2007) Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. https://doi.org/10.1172/JCI32578
Cai MX, Shi XC, Chen T, Tan ZN, Lin QQ, Du SJ, Tian ZJ (2016) Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci 149:1–9. https://doi.org/10.1016/j.lfs.2016.02.055
Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ (2011) Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res 108:1448–1458. https://doi.org/10.1161/CIRCRESAHA.111.241117
Chung E, Heimiller J, Leinwand LA (2012) Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One 7:e42297. https://doi.org/10.1371/journal.pone.0042297
Corrado D, Basso C, Schiavon M, Thiene G (1998) Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 339:364–369. https://doi.org/10.1056/NEJM199808063390602
D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E (2015) ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol 17:627–638. https://doi.org/10.1038/ncb3149
Dailey SM, Oberman A (1993) The athletic heart. Heart Dis Stroke 2:53–58
de Waard MC, van Haperen R, Soullie T, Tempel D, de Crom R, Duncker DJ (2010) Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol 48:1041–1049. https://doi.org/10.1016/j.yjmcc.2010.02.005
DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ (2006) Akt1 is required for physiological cardiac growth. Circulation 113:2097–2104. https://doi.org/10.1161/CIRCULATIONAHA.105.595231
Dehghani M, Kargarfard M, Rabiee F, Nasr-Esfahani MH, Ghaedi K (2018) A comparative study on the effects of acute and chronic downhill running vs uphill running exercise on the RNA levels of the skeletal muscles PGC1-alpha, FNDC5 and the adipose UCP1 in BALB/c mice. Gene 679:369–376. https://doi.org/10.1016/j.gene.2018.09.024
Diffee GM, Nagle DF (2003) Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes. J Appl Physiol (1985) 95:35–42. https://doi.org/10.1152/japplphysiol.00951.2002
Djouadi F, Brandt JM, Weinheimer CJ, Leone TC, Gonzalez FJ, Kelly DP (1999) The role of the peroxisome proliferator-activated receptor alpha (PPAR alpha) in the control of cardiac lipid metabolism. Prostaglandins Leukot Essent Fatty Acids 60:339–343
Duncker DJ, van Deel ED, de Waard MC, de Boer M, Merkus D, van der Velden J (2016) Erratum to: exercise training in adverse cardiac remodeling. Pflugers Arch 468:367. https://doi.org/10.1007/s00424-015-1761-x
Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL (2005) Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol 289:H2030–H2038. https://doi.org/10.1152/ajpheart.00526.2005
Esposito F, Mathieu-Costello O, Wagner PD, Richardson RS (2018) Acute and chronic exercise in patients with heart failure with reduced ejection fraction: evidence of structural and functional plasticity and intact angiogenic signalling in skeletal muscle. J Physiol 596:5149–5161. https://doi.org/10.1113/JP276678
Fagard RH (1996) Athlete’s heart: a meta-analysis of the echocardiographic experience. Int J Sports Med 17(Suppl 3):S140–S144. https://doi.org/10.1055/s-2007-972915
Fagard RH, Celis H, Thijs L, Wouters S (2009) Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension 54:1084–1091. https://doi.org/10.1161/HYPERTENSIONAHA.109.136655
Fernandes T, Barauna VG, Negrao CE, Phillips MI, Oliveira EM (2015) Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol 309:H543–H552. https://doi.org/10.1152/ajpheart.00899.2014
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP (2002) The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109:121–130. https://doi.org/10.1172/JCI14080
Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A (2018) Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 15:731–743. https://doi.org/10.1038/s41569-018-0065-1
Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP, Investigators H-A (2009) Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301:1451–1459. https://doi.org/10.1001/jama.2009.457
Foryst-Ludwig A, Kintscher U (2013) Sex differences in exercise-induced cardiac hypertrophy. Pflugers Arch 465:731–737. https://doi.org/10.1007/s00424-013-1225-0
Frey N, Katus HA, Olson EN, Hill JA (2004) Hypertrophy of the heart: a new therapeutic target? Circulation 109:1580–1589. https://doi.org/10.1161/01.CIR.0000120390.68287.BB
Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB (2003) Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35:1473–1479
Fulton N, Rajiah P (2017) Utility of magnetic resonance imaging in the evaluation of left ventricular thickening. Insights Imaging 8:279–293. https://doi.org/10.1007/s13244-017-0549-2
Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, Tseng MT, Wang J, Jones SP, Bhatnagar A, Hill BG (2017) Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation 136:2144–2157. https://doi.org/10.1161/CIRCULATIONAHA.117.028274
Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M (2003) Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res 92:518–524. https://doi.org/10.1161/01.RES.0000060700.55247.7C
Gleim GW, Coplan NL, Nicholas JA (1986) Acute cardiovascular response to exercise. Bull N Y Acad Med 62:211–218
Haram PM, Kemi OJ, Lee SJ, Bendheim MO, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisloff U (2009) Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81:723–732. https://doi.org/10.1093/cvr/cvn332
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A, American College of Sports M, American Heart A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116:1081–1093. https://doi.org/10.1161/CIRCULATIONAHA.107.185649
Hirai DM, Musch TI, Poole DC (2015) Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309:H1419–H1439. https://doi.org/10.1152/ajpheart.00469.2015
Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6:25–37. https://doi.org/10.1016/j.cmet.2007.06.005
Hydock DS, Wonders KY, Schneider CM, Hayward R (2009) Voluntary wheel running in rats receiving doxorubicin: effects on running activity and cardiac myosin heavy chain. Anticancer Res 29:4401–4407
Isgaard J, Kujacic V, Jennische E, Holmang A, Sun XY, Hedner T, Hjalmarson A, Bengtsson BA (1997) Growth hormone improves cardiac function in rats with experimental myocardial infarction. Eur J Clin Investig 27:517–525
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022. https://doi.org/10.1073/pnas.0705070104
Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O (2005) Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67:161–172. https://doi.org/10.1016/j.cardiores.2005.03.010
Kim TT, Dyck JR (2015) Is AMPK the savior of the failing heart? Trends Endocrinol Metab 26:40–48. https://doi.org/10.1016/j.tem.2014.11.001
Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, Abel ED (2008) Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol 22:2531–2543. https://doi.org/10.1210/me.2008-0265
Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA (2004) Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol 287:H2768–H2776. https://doi.org/10.1152/ajpheart.00292.2004
Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA (2006) Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res 98:540–548. https://doi.org/10.1161/01.RES.0000205766.97556.00
Konhilas JP, Chen H, Luczak E, McKee LA, Regan J, Watson PA, Stauffer BL, Khalpey ZI, McKinsey TA, Horn T, LaFleur B, Leinwand LA (2015) Diet and sex modify exercise and cardiac adaptation in the mouse. Am J Physiol Heart Circ Physiol 308:H135–H145. https://doi.org/10.1152/ajpheart.00532.2014
Korkmaz A, Yildiz A, Turker Duyuler P, Duyuler S, Yilmaz S, Basyigit F, Elalmis OU, Guray U, Ileri M (2018) Combination of change in hematological parameters with exercise stress test to predict coronary artery disease. J Clin Lab Anal 32. https://doi.org/10.1002/jcla.22205
Kwak HB (2013) Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil 9:338–347. https://doi.org/10.12965/jer.130049
Kwak HB (2013) Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil 9:212–219. https://doi.org/10.12965/jer.130002
Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR, Vega RB, Attie AD, Muoio DM, Kelly DP (2014) Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 7:1022–1031. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001469
Lee YI, Cho JY, Kim MH, Kim KB, Lee DJ, Lee KS (2006) Effects of exercise training on pathological cardiac hypertrophy related gene expression and apoptosis. Eur J Appl Physiol 97:216–224. https://doi.org/10.1007/s00421-006-0161-5
Lin YY, Chen JS, Wu XB, Shyu WC, Chaunchaiyakul R, Zhao XL, Kuo CH, Cheng YJ, Yang AL, Lee SD (2018) Combined effects of 17beta-estradiol and exercise training on cardiac apoptosis in ovariectomized rats. PLoS One 13:e0208633. https://doi.org/10.1371/journal.pone.0208633
Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM, Rosenzweig A (2015) miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21:584–595. https://doi.org/10.1016/j.cmet.2015.02.014
MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR (1985) Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58:785–790. https://doi.org/10.1152/jappl.1985.58.3.785
Maillet M, van Berlo JH, Molkentin JD (2013) Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 14:38–48. https://doi.org/10.1038/nrm3495
Malik AB, Abe T, O’Kane H, Geha AS (1973) Cardiac function, coronary flow, and oxygen consumption in stable left ventricular hypertrophy. Am J Phys 225:186–191. https://doi.org/10.1152/ajplegacy.1973.225.1.186
Maron BJ (2009) Distinguishing hypertrophic cardiomyopathy from athlete’s heart physiological remodelling: clinical significance, diagnostic strategies and implications for preparticipation screening. Br J Sports Med 43:649–656. https://doi.org/10.1136/bjsm.2008.054726
McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S (2003) Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A 100:12355–12360. https://doi.org/10.1073/pnas.1934654100
McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S (2004) The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem 279:4782–4793. https://doi.org/10.1074/jbc.M310405200
McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL (2007) Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A 104:612–617. https://doi.org/10.1073/pnas.0606663104
Meijer JH, Robbers Y (2014) Wheel running in the wild. Proc Biol Sci 281:20140210. https://doi.org/10.1098/rspb.2014.0210
Meyer C, Rana OR, Saygili E, Gemein C, Becker M, Nolte KW, Weis J, Schimpf T, Knackstedt C, Mischke K, Hoffmann R, Kelm M, Pauza D, Schauerte P (2010) Augmentation of left ventricular contractility by cardiac sympathetic neural stimulation. Circulation 121:1286–1294. https://doi.org/10.1161/CIRCULATIONAHA.109.874263
Moore RL, Musch TI, Yelamarty RV, Scaduto RC Jr, Semanchick AM, Elensky M, Cheung JY (1993) Chronic exercise alters contractility and morphology of isolated rat cardiac myocytes. Am J Phys 264:C1180–C1189. https://doi.org/10.1152/ajpcell.1993.264.5.C1180
Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM (2012) Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 9:e1001335. https://doi.org/10.1371/journal.pmed.1001335
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407. https://doi.org/10.1038/s41569-018-0007-y
Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134:405–415. https://doi.org/10.1016/j.cell.2008.06.051
Natali AJ, Turner DL, Harrison SM, White E (2001) Regional effects of voluntary exercise on cell size and contraction-frequency responses in rat cardiac myocytes. J Exp Biol 204:1191–1199
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301:1439–1450. https://doi.org/10.1001/jama.2009.454
O’Neill BT, Abel ED (2005) Akt1 in the cardiovascular system: friend or foe? J Clin Invest 115:2059–2064. https://doi.org/10.1172/JCI25900
O’Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED (2007) A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab 6:294–306. https://doi.org/10.1016/j.cmet.2007.09.001
Olver TD, Ferguson BS, Laughlin MH (2015) Molecular mechanisms for exercise training-induced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci 135:227–257. https://doi.org/10.1016/bs.pmbts.2015.07.017
Otaka N, Shibata R, Ohashi K, Uemura Y, Kambara T, Enomoto T, Ogawa H, Ito M, Kawanishi H, Maruyama S, Joki Y, Fujikawa Y, Narita S, Unno K, Kawamoto Y, Murate T, Murohara T, Ouchi N (2018) Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury. Circ Res 123:1326–1338. https://doi.org/10.1161/CIRCRESAHA.118.313777
Pan SS (2008) Alterations of atrial natriuretic peptide in cardiomyocytes and plasma of rats after different intensity exercise. Scand J Med Sci Sports 18:346–353. https://doi.org/10.1111/j.1600-0838.2007.00684.x
Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8:33–40. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001615
Pattyn N, Coeckelberghs E, Buys R, Cornelissen VA, Vanhees L (2014) Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Med 44:687–700. https://doi.org/10.1007/s40279-014-0158-x
Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA (2006) Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest 116:1547–1560. https://doi.org/10.1172/JCI25397
Pilegaard H, Saltin B, Neufer PD (2003) Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546:851–858
Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE (2000) The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101:336–344
Poole DC, Hirai DM, Copp SW, Musch TI (2012) Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302:H1050–H1063. https://doi.org/10.1152/ajpheart.00943.2011
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Rabinovich-Nikitin I, Kirshenbaum LA (2018) Exercise-induced myonectin protects against ischemia-reperfusion injury. Circ Res 123:1264–1266. https://doi.org/10.1161/CIRCRESAHA.118.314129
Ribeiro PA, Boidin M, Juneau M, Nigam A, Gayda M (2017) High-intensity interval training in patients with coronary heart disease: prescription models and perspectives. Ann Phys Rehabil Med 60:50–57. https://doi.org/10.1016/j.rehab.2016.04.004
Richter SH, Gass P, Fuss J (2014) Resting is rusting: a critical view on rodent wheel-running behavior. Neuroscientist 20:313–325. https://doi.org/10.1177/1073858413516798
Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, Bevins J, Valdez S, Noh J, Kim BJ, Moreira AB, Weatherford ET, Manivel R, Rawlings TA, Rech M, White MF, Abel ED (2014) Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol 34:3450–3460. https://doi.org/10.1128/MCB.00426-14
Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K (2016) Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail 9:e002206. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002206
Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG (2014) Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7:634–642. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001151
Santana MNS, Souza DS, Miguel-Dos-Santos R, Rabelo TK, Vasconcelos CML, Navia-Pelaez JM, Jesus ICG, Silva-Neto JAD, Lauton-Santos S, Capettini L, Guatimosim S, Rogers RG, Santos M, Santana-Filho VJ, Mesquita TRR (2018) Resistance exercise mediates remote ischemic preconditioning by limiting cardiac eNOS uncoupling. J Mol Cell Cardiol 125:61–72. https://doi.org/10.1016/j.yjmcc.2018.10.016
Santos MH, Higuchi Mde L, Tucci PJ, Garavelo SM, Reis MM, Antonio EL, Serra AJ, Maranhao RC (2016) Previous exercise training increases levels of PPAR-alpha in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response. Clinics (Sao Paulo) 71:163–168. https://doi.org/10.6061/clinics/2016(03)08
Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23:459–466. https://doi.org/10.1016/j.tem.2012.06.006
Scheinowitz M, Kessler-Icekson G, Freimann S, Zimmermann R, Schaper W, Golomb E, Savion N, Eldar M (2003) Short- and long-term swimming exercise training increases myocardial insulin-like growth factor-I gene expression. Growth Hormon IGF Res 13:19–25
Scheuer J, Tipton CM (1977) Cardiovascular adaptations to physical training. Annu Rev Physiol 39:221–251. https://doi.org/10.1146/annurev.ph.39.030177.001253
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2014) Humanized animal exercise model for clinical implication. Pflugers Arch 466:1673–1687. https://doi.org/10.1007/s00424-014-1496-0
Seo DY, Lee SR, Kwak HB, Seo KW, McGregor RA, Yeo JY, Ko TH, Bolorerdene S, Kim N, Ko KS, Rhee BD, Han J (2016) Voluntary stand-up physical activity enhances endurance exercise capacity in rats. Korean J Physiol Pharmacol 20:287–295. https://doi.org/10.4196/kjpp.2016.20.3.287
Serneri GG, Modesti PA, Boddi M, Cecioni I, Paniccia R, Coppo M, Galanti G, Simonetti I, Vanni S, Papa L, Bandinelli B, Migliorini A, Modesti A, Maccherini M, Sani G, Toscano M (1999) Cardiac growth factors in human hypertrophy. Relations with myocardial contractility and wall stress. Circ Res 85:57–67
Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T, Wang H, Xuan Q, Chen P, Xu J, Che L, Liu H, Zhong J, Sluijter JP, Li X, Rosenzweig A, Xiao J (2017) miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 7:664–676. https://doi.org/10.7150/thno.15162
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K (2005) Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115:2108–2118. https://doi.org/10.1172/JCI24682
Silva DA, ND J, Fernandes T, Soci UP, Monteiro AW, Phillips MI, EM DEO (2012) Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44:1453–1462. https://doi.org/10.1249/MSS.0b013e31824e8a36
Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM (2011) MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics 43:665–673. https://doi.org/10.1152/physiolgenomics.00145.2010
Soci UPR, Fernandes T, Barauna VG, Hashimoto NY, de Fatima Alves Mota G, Rosa KT, Irigoyen MC, Philips MI, de Oliveira EM (2016) Epigenetic control of exercise training-induced cardiac hypertrophy by miR-208. Clin Sci (Lond) 130:2005–2015. https://doi.org/10.1042/CS20160480
Solskov L, Magnusson NE, Kristiansen SB, Jessen N, Nielsen TT, Schmitz O, Botker HE, Lund S (2012) Microarray expression analysis in delayed cardioprotection: the effect of exercise, AICAR, or metformin and the possible role of AMP-activated protein kinase (AMPK). Mol Cell Biochem 360:353–362. https://doi.org/10.1007/s11010-011-1075-z
Strom CC, Aplin M, Ploug T, Christoffersen TE, Langfort J, Viese M, Galbo H, Haunso S, Sheikh SP (2005) Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J 272:2684–2695. https://doi.org/10.1111/j.1742-4658.2005.04684.x
Tatsuguchi M, Hiratsuka E, Machida S, Nishikawa T, Imamura S, Shimizu S, Nishimura M, Komuro I, Furutani Y, Furutani M, Nagao H, Komatsu K, Kasanuki H, Matsuoka R (2004) Swimming exercise in infancy has beneficial effect on the hearts in cardiomyopathic Syrian hamsters. J Muscle Res Cell Motil 25:69–76
Tikkanen E, Gustafsson S, Ingelsson E (2018) Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank study. Circulation 137:2583–2591. https://doi.org/10.1161/CIRCULATIONAHA.117.032432
van Rooij E, Olson EN (2012) MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11:860–872. https://doi.org/10.1038/nrd3864
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105:13027–13032. https://doi.org/10.1073/pnas.0805038105
Vanzelli AS, Medeiros A, Rolim N, Bartholomeu JB, Cunha TF, Bechara LR, Gomes ER, Mattos KC, Sirvente R, Salemi VM, Mady C, Negrao CE, Guatimosim S, Brum PC (2013) Integrative effect of carvedilol and aerobic exercise training therapies on improving cardiac contractility and remodeling in heart failure mice. PLoS One 8:e62452. https://doi.org/10.1371/journal.pone.0062452
Vega RB, Horton JL, Kelly DP (2015) Maintaining ancient organelles: mitochondrial biogenesis and maturation. Circ Res 116:1820–1834. https://doi.org/10.1161/CIRCRESAHA.116.305420
Vega RB, Konhilas JP, Kelly DP, Leinwand LA (2017) Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 25:1012–1026. https://doi.org/10.1016/j.cmet.2017.04.025
Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, Tedesco L, Ruocco C, Fossati A, Fabris R, Serra R, Carruba MO, Nisoli E (2014) Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab 306:E519–E528. https://doi.org/10.1152/ajpendo.00617.2013
Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, Cao Y, Chen P, Xiao J, Kong X (2015) Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem 35:2159–2168. https://doi.org/10.1159/000374021
Wang B, Xu M, Li W, Li X, Zheng Q, Niu X (2017) Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via beta3-AR-nNOS-NO activation. PLoS One 12:e0179648. https://doi.org/10.1371/journal.pone.0179648
Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Humen DP (2002) Myocardial response to incremental exercise in endurance-trained athletes: influence of heart rate, contractility and the Frank-Starling effect. Exp Physiol 87:613–622
Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM (2014) The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35:2722–2731. https://doi.org/10.1093/eurheartj/ehs338
Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, Franke TF, Qian H, Bogoyevitch MA, Woodcock EA, Febbraio MA, Gregorevic P, McMullen JR (2012) Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail 5:523–534. https://doi.org/10.1161/CIRCHEARTFAILURE.112.966622
Weiner RB, Baggish AL (2012) Exercise-induced cardiac remodeling. Prog Cardiovasc Dis 54:380–386. https://doi.org/10.1016/j.pcad.2012.01.006
Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP (2007) A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem 282:36642–36651. https://doi.org/10.1074/jbc.M707006200
Yang R, Bunting S, Gillett N, Clark R, Jin H (1995) Growth hormone improves cardiac performance in experimental heart failure. Circulation 92:262–267
Yang L, Jia Z, Yang L, Zhu M, Zhang J, Liu J, Wu P, Tian W, Li J, Qi Z, Tang X (2014) Exercise protects against chronic beta-adrenergic remodeling of the heart by activation of endothelial nitric oxide synthase. PLoS One 9:e96892. https://doi.org/10.1371/journal.pone.0096892
Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, Kelly DP (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 12:633–642. https://doi.org/10.1016/j.cmet.2010.11.008
Funding
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018S1A5A8027802), by the Ministry of Education of Korea (2010-0020224), and by the Korea government Ministry of Science and ICT (2018R1A2A3074998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Seo, D.Y., Kwak, HB., Kim, A.H. et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch - Eur J Physiol 472, 155–168 (2020). https://doi.org/10.1007/s00424-019-02266-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02266-3