Abstract
Background
Systemic immune-inflammation index (SII, platelet × neutrophil/lymphocyte ratio), a new marker of inflammation, is associated with adverse cardiovascular events, but its relationship with coronary slow flow phenomenon (CSFP) is unclear. Therefore, we aimed to investigate the relationship between SII and CSFP.
Methods
We enrolled consecutive patients who presented with chest pain, with normal/near-normal coronary angiography findings (n = 89 as CSFP group; n = 167 as control group). The baseline characteristics, laboratory parameters and angiographic characteristics of the two groups were compared.
Results
SII levels were significantly higher in the CSFP group than in the control group (409.7 ± 17.7 vs. 396.7 ± 12.7, p < 0.001). A significant positive correlation between SII and the mean thrombolysis in myocardial infarction frame count (mTFC) was found (r = 0.624, p < 0.001). SII increased with the number of coronary arteries involved in CSFP. In multivariate logistic regression analysis, SII/10 was an independent predictor of CSFP (odds ratio: 1.739, p < 0.001). In addition, the SII level > 404.29 was a predictor of CSFP with 67.4% sensitivity and 71.9% specificity.
Conclusions
SII can predict the occurrence of CSFP.
Similar content being viewed by others
Background
Coronary slow flow phenomenon (CSFP) is characterized by normal/near-normal epicardial coronary arteries (stenosis < 40%) but delayed vessel opacification in the coronary angiogram (CAG) [1, 2]. The prevalence of CSFP in patients undergoing CAG for suspected coronary artery disease (CAD) ranges from 1 to 7% [3].Although studies have shown that inflammation, oxidative stress, diffuse atherosclerosis, microvascular vasomotor and endothelial dysfunction are associated with CSFP [4,5,6,7,8], the exact pathogenesis of CSFP is unknown.
On the other hand, systemic immune-inflammation index (SII) is a novel marker of inflammation and is related to cardiovascular disease involving mechanisms such as atherosclerosis and inflammation [9,10,11,12], but the relationship with CSFP is unclear. Therefore, in view of the pathogenesis of CSFP and the significance of SII in cardiovascular disease, the aim of this study was to evaluate the relationship between increased SII and CSFP.
Methods
Study population
All patients who complained of chest pain, but with normal or nearly normal coronary angiography results (stenosis < 40%) from October 2020 to January 2022 at the People's Hospital of Liaoning Province were selected. Baseline clinical data, laboratory and angiographic data of all patients were retrospectively analysed. [13]. Patients with one of the three coronary arteries whose TFC values above this standard were considered to have CSFP while those with TFC values of all three coronary arteries below this criterion were considered controls [14]. Finally, 89 patients with CSFP and 167 patients with normal coronary blood flow were included in this study (Fig. 1). The exclusion criteria were as follows: recent acute coronary syndrome (< 3 months), previous myocardial infarction, previous history of revascularization, coronary artery dilation or spasm, dissection, severe cardiomyopathy, moderate to severe valvular heart disease, congenital heart disease, decompensated heart failure, non-sinus rhythm, malignancy, severe liver or renal failure, acute or chronic infection or lung disease, peripheral vascular disease, autoimmune disease, hematologic disorders, and anaemia (haemoglobin level < 12 g/dL for women or < 13 g/dL for men, according to the World Health Organization criteria) [15]. The study protocol was approved by the Institutional Ethics Committee and informed consent was obtained from all individuals involved in the study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Coronary angiography
All patients underwent coronary angiography via radial or femoral approach using the Judkins technique for typical chest pain. The coronary arteries were observed at 30 frames/second (fps). Two experienced cardiologists measured coronary blood flow velocity using the TFC, while blinded to the patients' clinical parameters [13]. The TFC was calculated based on the difference between the first and last frames, the initial frame referred to the contrast agent filling more than 70% of the arterial lumen in a prograde direction, while the last frame showing the contrast agent appearing at distal coronary landmarks, which were defined as follows: the distal landmark of the LAD was the distal bifurcation (i.e., the "moustache"), usually at the apex of the heart; that of the LCX was the distal bifurcation of the longest branch, and in the RCA, it was the first side branch of the posterolateral artery. As the LAD artery is usually longer than the other major coronary arteries, the TFC of LAD was divided by 1.7 to obtain the corrected TFC. The cut off values for normal epicardial coronary arteries filling were 36.2 ± 2.6 frames for the LAD (21.1 ± 1.5 frames for the corrected cutoff value for LAD), 22.2 ± 4.1 frames for the LCX, and 20.4 ± 3 frames for the RCA [13]. Patients with one of the three coronary arteries whose values above this threshold were defined as CSFP. The mean thrombolysis in myocardial infarction frame count (mTFC) was defined as the sum of the TFC of the LAD, LCX and RCA divided by 3 [13].
Laboratory measurements
The baseline characteristics of all patients were reviewed and blood samples were collected from the cubital vein in the forearm after 12 h of fasting prior to the coronary angiography. Laboratory parameters (including complete blood count, biochemical parameters and lipid parameters collected before coronary angiography) were recorded for all patients. And the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) were calculated. Total preoperative peripheral platelets count (P) × NLR were used to obtain SII (SII = P × N/L) [9].
Statistical analysis
Statistical analysis was performed using SPSS software version 26.0. Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as numbers and percentages. The differences between groups were compared using Student t test for continuous variables and the Chi-square (χ2) test was used for comparison of categorical variables. Spearman correlation analysis was used to identify the correlation between SII and mTFC. Univariate and multivariate logistic regression analyses were performed to determine the relationship between clinical data and laboratory parameters and CSFP. The factors showing statistical significance at the p < 0.05 level between groups were included in the univariate regression model, and those with p < 0.05 in the univariate regression analysis were finally analyzed by multivariate logistic regression. The receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity of the SII and the optimal cut off for predicting CSFP. A P value of < 0.05 was considered statistically significant.
Results
A total of 256 patients (89 in the CSFP group and 167 in the control group) were enrolled in this study. Baseline characteristics of the two groups are demonstrated in Table 1. There were no statistically significant intergroup differences in terms of age, gender, BMI, and smoking (p > 0.05), but the incidence rates of hypertension (36.0% vs. 24.0%, p = 0.042) and diabetes mellitus (29.2% vs. 18.0%, P = 0.038) in the CSFP group were significantly higher than those in the control group. No statistically significant differences were observed in medication between the two groups (P > 0.05). In this study, patients were also divided into two groups respectively according to gender and whether smoking or not to compare the incidence of CSFP and SII (Figs. 2, 3). The results showed that SII was significantly higher in male (403.9 ± 16.5 vs. 397.8 ± 14.2, p = 0.002); and smokers (405.7 ± 17.4 vs. 399.5 ± 14.9, p = 0.005). Although there were no significant differences in the incidence of CSFP in the gender and smoking group, the prevalence of CSFP was also higher in male and smokers in the study.
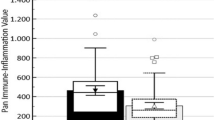
The laboratory parameters of the two groups are shown in Table 2. There were no statistically significant differences between the two groups in the following laboratory values: neutrophil count, lymphocyte count, monocyte count and eosinophil count, haemoglobin and total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol and urea, creatinine, uric acid, mean platelet volume, and PLR (P > 0.05). The white blood cell count (6.8 ± 1.2 vs. 6.4 ± 1.0*103/mm3, p = 0.006), platelet count (235.7 ± 9.7 vs. 232.5 ± 13.2*103/mm3, p = 0.031) in the CSFP group as well as fasting plasma glucose (5.1 ± 0.5 vs. 5.0 ± 0.6 mmol/l, p = 0.034), NLR (1.74 ± 0.09 vs. 1.71 ± 0.12, p = 0.034) and SII (409.7 ± 17.7 vs. 396.7 ± 12.7, p < 0.001, Fig. 4) were significantly higher than in the control group, and SII increased with the number of vessels in which CSFP occurred (Fig. 5). However, high-density lipoprotein (HDL) cholesterol (1.0 ± 0.1 vs. 1.1 ± 0.2 mmol/L, P = 0.030) was significantly lower than in the control group.
The angiographic characteristics of patients in the two groups are shown in Table 3. In the CSFP group, the corrected TFC for LAD (43.2 ± 24.8 vs. 19.7 ± 1.0, p < 0.001), and the TFC for LCX (29.8 ± 10.1 vs. 18.8 ± 2.2, p < 0.001), for RCA (27.9 ± 8.8 vs. 17.2 ± 2.2, p < 0.001) and the mean TFC (33.6 ± 9.4 vs. 18.6 ± 1.6, p < 0.001) were significantly higher than those in the control group. In the CSFP group, a total of 47 (52.8%) patients developed CSFP in the LAD, 54 (60.7%) patients showed CSFP in the LCX, and 61 (68.5%) in the RCA. Moreover, 37 (41.6%) patients had CSFP in one major coronary artery, 31 (34.8%) in two major coronary arteries, and 21 (23.6%) in three coronary arteries. The mean number of coronary arteries with CSFP was 1.82 ± 0.79.
In the correlation analysis, a significant positive correlation was found between SII and mTFC (r = 0.624, p < 0.001; Fig. 6). Multivariate logistic regression analysis revealed that SII/10 was an independent predictor of CSFP (odds ratio: 1.739, 95% confidence interval [CI] 1.408–2.148, p < 0.001), i.e., each 10-unit increase in SII was associated with a 73.9% increase in CSFP prevalence in the study population (Table 4).
The ROC curve was used to evaluate the discriminatory capability of SII for the occurrence of CSFP. The ROC curve showed an area under the curve of 0.715 (95% CI 0.655–0.769, p < 0.001; Fig. 7), and using a cut off level of 404.29, SII predicted the presence of CSFP with an 67.4% sensitivity and 71.9% specificity.
Discussion
In this study, we found that higher SII before coronary angiography was significantly and independently associated with the presence of CSFP. The SII level in patients with CSFP was significantly elevated and increased with the number of vessels involved and was also positively correlated with mTFC. Multivariate regression analysis indicated that SII/10 was an independent predictor of CSFP. To the best of our knowledge, this is the first study in the literature reporting the relationship between SII and CSFP.
CSFP is an angiographic phenomenon with specific pathogenesis and diagnostic criteria [2], which usually occurs in young men, smokers and those with comorbid metabolic syndrome [16]. Similarly, in our study, CSFP was more common in male, smokers, and patients with diabetes mellitus and hypertension. The main clinical symptom of CSFP is unstable angina pectoris, although CSFP is usually a benign phenomenon, it has also been reported to be associated with life-threatening adverse cardiovascular events such as acute coronary syndrome, ventricular fibrillation, and sudden cardiac death [17]. The exact pathophysiological mechanisms of CSFP are still unclear, but inflammation [4, 18], diffuse atherosclerosis [8, 19], microvascular [20] and endothelial dysfunction [7], and oxidative stress [21] have been thought to be involved. In addition, cardiovascular risk factors such as diabetes mellitus and hypertension [22, 23], lipid index such as HDL cholesterol and triglyceride, and conventional clinical parameters including fasting glucose, uric acid, etc. were also considered to be associated with CSFP [16, 24, 25]. As in line with previous studies, we also found the prevalence of diabetes mellitus and hypertension and fasting glucose levels were significantly higher in the CSFP group, whereas HDL cholesterol was significantly lower in the CSFP group as compared with the control group.
Inflammation plays an important role in the development of CSFP, neutrophils can infiltrate endothelial tissue and release pro-oxidants and pro-inflammatory mediators, which in turn can form neutrophil extracellular traps (NETs) and promote the formation and development of atherosclerotic plaques [26, 27]. Doğan et al. [15] reported NLR as an inflammatory marker to be associated with the presence of CSFP. It has also been reported that high sensitive CRP (hs-CPR) may be an early indicator that could predict the occurrence of CSFP [18]. While SII as an inflammatory marker has been considered to predict the occurrence of some cardiovascular diseases. for example, one study showed that SII can act as a circulating immune inflammatory cell to predict major cardiovascular events after coronary intervention in patients with coronary heart disease [9]. Another study concluded that elevated SII may have a predictive value for coronary artery dilation [28]. Meanwhile, CSFP as a common cardiovascular disease, this study also confirmed that SII can be used as a predictor of CSFP. And the results show that the values of white blood cell count, NLR, and SII were all significantly higher in the CSFP group than in the control group. On the other hand, lymphocyte levels decrease in number during chronic inflammation due to stress response. In addition to increased apoptosis, downregulation of proliferation and redistribution of lymphocytes can lead to low lymphocyte counts [29, 30]. Furthermore, a decrease in lymphocyte counts also has an effect on cardiovascular disease, as found by Major et al. [31] in experimental studies in B-cell-deficient mice where a low lymphocyte count promoted atherosclerosis, and another study also showed that low lymphocyte counts were associated with poor prognosis in cardiovascular disease [32]. Similarly, our study showed that patients in the case group had lower lymphocyte counts and higher NLR, PLR and SII. These findings all suggest that SII, an indicator of inflammation, may be a causative factor for CSFP. Platelets play an important connecting role in inflammation, thrombosis, and atherosclerosis formation. Platelets can recruit leukocytes and monocytes to the site of inflammation and secrete inflammatory mediators such as chemokines and cytokines, which can lead to vascular inflammation [33]. At the same time, enhanced thrombosis is related to the development of CSFP as well, and it has been documented that the platelet activation ability is enhanced in CSFP patients, when compared with controls [34]. Akboga et al. [22] also found that PLR was not only significantly correlated with CSFP as an inflammatory indicator, but also could lead to the occurrence of CSFP through enhanced pro-systemic coagulant activity. The results of this study also confirmed that platelet, PLR, and SII levels were higher in the CSFP group. In addition, it has been reported that SII is superior to NLR and PLR in predicting the occurrence of certain cardiovascular diseases [10, 11, 30]. The present study also found no significant difference in PLR between the two groups and that NLR was not a predictor of CSFP in the regression model, suggesting that SII could better predict the occurrence of CSFP compared with NLR and PLR.
Diffuse atherosclerosis has also been shown to be an important causative factor in cardiovascular diseases such as CSFP. Avşar et al. [35] reported that carotid intima-media thickness (CIMT) was a marker of early atherosclerosis in blood vessels and that CIMT was significantly increased in patients with CSFP. It has also been demonstrated that NLR and SII are significantly associated with atherosclerosis, as in a study by Kaya et al. [36] who found NLR to be a predictor of severe coronary atherosclerosis and another study which showed that SII was one of the risk factors for atherosclerosis in predicting the severity of coronary artery lesions [10]. The present study also demonstrated that the NLR and SII levels were significantly higher in the CSFP group compared to the control group, and that SII was positively correlated with mTFC and increased with an increase in the number of coronary arteries involved. Therefore, SII may also assume an important role in the development of CSFP through atherosclerosis.
Limitations
Our study has some potential limitations. Firstly, this study was a single-centre study with a relatively small sample size and no long-term follow-up of the patients with CSFP. Secondly, we did not measure C-reactive protein, an inflammatory mediator. Further confirmation is needed in more rigorous large-scale, prospective, and randomized controlled studies.
Conclusions
In conclusion, the results of our study suggested that SII is an easily available and inexpensive new biomarker, and that higher levels of SII were significantly and independently related to the occurrence of CSFP. Moreover, its value increased as the number of involved vessels increased, Therefore, SII as a mediator of inflammation can predict the occurrence and severity of CSFP. we should pay attention to patients with high SII levels in clinical practice, and further studies are needed to confirm these results and the mechanism of action of SII in CSFP and further exploration is also needed in the treatment of CSFP.
Future perspective
Coronary slow flow phenomenon (CSFP) is a common cardiovascular disease, but the pathophysiological mechanism is still unclear. Systemic immune-inflammation index (SII) as a new inflammatory biomarker in the present study can predict the occurrence of CSFP. Therefore, for patients with CSFP, SII would be important for the early diagnosis and stratification of the disease, also it would provide more insights into treatment in future clinical practice.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to the need for further research in this area but are available from the corresponding author on reasonable request.
Abbreviations
- SII:
-
Systemic immune-inflammation index
- CSFP:
-
Coronary slow flow phenomenon
- mTFC:
-
The mean thrombolysis in myocardial infarction frame count
- CAG:
-
Coronary angiogram
- CAD:
-
Coronary artery disease
- TFC:
-
TIMI frame count
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- RCA:
-
Right coronary artery
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- ROC:
-
Receiver operating characteristic
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- NETs:
-
Neutrophil extracellular traps
- hs-CPR:
-
High sensitive CRP
- CIMT:
-
Carotid intima-media thickness
- TIMI:
-
Thrombolysis in myocardial infarction
References
Beltrame JF. Defining the coronary slow flow phenomenon. Circ J. 2012;76(4):818–20.
Tambe AA, Demany MA, Zimmerman HA, Mascarenhas E. Angina pectoris and slow flow velocity of dye in coronary arteries–a new angiographic finding. Am Heart J. 1972;84(1):66–71.
Saadat M, Masoudkabir F, Afarideh M, Ghodsi S, Vasheghani-Farahani A: Discrimination between obstructive coronary artery disease and cardiac syndrome X in women with typical angina and positive exercise test; Utility of Cardiovascular Risk Calculators. Medicina (Kaunas, Lithuania) 2019, 55(1).
Kalay N, Aytekin M, Kaya MG, Ozbek K, Karayakalı M, Söğüt E, Altunkas F, Oztürk A, Koç F. The relationship between inflammation and slow coronary flow: increased red cell distribution width and serum uric acid levels. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2011;39(6):463–8.
Enli Y, Turk M, Akbay R, Evrengul H, Tanriverdi H, Kuru O, Seleci D, Kaftan A, Ozer O, Enli H. Oxidative stress parameters in patients with slow coronary flow. Adv Ther. 2008;25(1):37–44.
Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon–a new coronary microvascular disorder. Cardiology. 2002;97(4):197–202.
Sezgin AT, Sigirci A, Barutcu I, Topal E, Sezgin N, Ozdemir R, Yetkin E, Tandogan I, Kosar F, Ermis N, et al. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis. 2003;14(2):155–61.
Cin VG, Pekdemir H, Camsar A, Ciçek D, Akkus MN, Parmaksýz T, Katýrcýbaý T, Döven O. Diffuse intimal thickening of coronary arteries in slow coronary flow. Jpn Heart J. 2003;44(6):907–19.
Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, Lin SJ, Chou CY, Chen JW, Pan JP, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5): e13230.
Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–81.
Kelesoglu S, Yilmaz Y, Elcık D, Kalay N: Systemic immune inflammation index: a novel predictor for coronary collateral circulation. Perfusion 2021:2676591211014822.
Adali MK, Buber I, Kilic O, Turkoz A, Yilmaz S: Ticagrelor improves systemic immune-inflammation index in acute coronary syndrome patients. Acta cardiologica 2021:1–7.
Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–88.
Wang Y, Liu MJ, Yang HM, Ma CY, Jia PY, Jia DL, Hou AJ. Association between increased serum alkaline phosphatase and the coronary slow flow phenomenon. BMC Cardiovasc Disord. 2018;18(1):138.
Doğan M, Akyel A, Çimen T, Bilgin M, Sunman H, Kasapkara HA, Arslantaş U, Yayla KG, Açıkel S, Yeter E. Relationship between neutrophil to lymphocyte ratio and slow coronary flow. Clin Appl Thrombosis/Hemostasis. 2015;21(3):251–4.
Yilmaz H, Demir I, Uyar Z. Clinical and coronary angiographic characteristics of patients with coronary slow flow. Acta Cardiol. 2008;63(5):579–84.
Chalikias G, Tziakas D. Slow coronary flow: pathophysiology, clinical implications, and therapeutic management. Angiology. 2021;72(9):808–18.
Barutcu I, Sezgin AT, Sezgin N, Gullu H, Esen AM, Topal E, Ozdemir R, Kosar F, Cehreli S. Increased high sensitive CRP level and its significance in pathogenesis of slow coronary flow. Angiology. 2007;58(4):401–7.
Şatıroğlu Ö, Durakoğlugil ME, Çetin M, Çiçek Y, Erdoğan T, Duman H. The role of urotensin II and atherosclerotic risk factors in patients with slow coronary flow. Intervent Med Appl Sci. 2016;8(4):158–63.
Erdogan D, Caliskan M, Gullu H, Sezgin AT, Yildirir A, Muderrisoglu H. Coronary flow reserve is impaired in patients with slow coronary flow. Atherosclerosis. 2007;191(1):168–74.
Tanriverdi H, Evrengul H, Enli Y, Kuru O, Seleci D, Tanriverdi S, Tuzun N, Kaftan HA, Karabulut N. Effect of homocysteine-induced oxidative stress on endothelial function in coronary slow-flow. Cardiology. 2007;107(4):313–20.
Akboga MK, Canpolat U, Balci KG, Akyel A, Sen F, Yayla C, Cay S, Aras D, Aydogdu S. Increased platelet to lymphocyte ratio is related to slow coronary flow. Angiology. 2016;67(1):21–6.
Sanati H, Kiani R, Shakerian F, Firouzi A, Zahedmehr A, Peighambari M, Shokrian L, Ashrafi P. Coronary slow flow phenomenon clinical findings and predictors. Res Cardiovasc Med. 2016;5(1): e30296.
Aciksari G, Cetinkal G, Kocak M, Atici A, Celik FB, Caliskan M. The relationship between triglyceride/high-density lipoprotein cholesterol ratio and coronary slow-flow phenomenon. Int J Cardiovasc Imaging. 2022;38(1):5–13.
Xia S, Deng SB, Wang Y, Xiao J, Du JL, Zhang Y, Wang XC, Li YQ, Zhao R, He L, et al. Clinical analysis of the risk factors of slow coronary flow. Heart Vessels. 2011;26(5):480–6.
Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, Iyisoy A. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl thrombosis/hemostasis. 2016;22(5):405–11.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43.
Tosu AR, Biter H. Association of systemic immune-inflammation index (SII) with presence of isolated coronary artery ectasia. Arch Med Sci Atherosclerot Dis. 2021;6:e152–7.
Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17(4):506–14.
Erdoğan M, Erdöl MA, Öztürk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14(16):1553–61.
Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22(11):1892–8.
Núñez J, Miñana G, Bodí V, Núñez E, Sanchis J, Husser O, Llàcer A. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–33.
Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Investig. 2005;115(12):3378–84.
Celik T, Yuksel UC, Bugan B, Iyisoy A, Celik M, Demirkol S, Yaman H, Kursaklioglu H, Kilic S, Isik E. Increased platelet activation in patients with slow coronary flow. J Thromb Thrombolysis. 2010;29(3):310–5.
Avşar O, Demir I, Ekiz O, Altekin RE, Yalçinkaya S. Relationship between the slow coronary flow and carotid artery intima-media thickness. Anadolu kardiyoloji dergisi AKD Anatol J Cardiol. 2007;7(1):19–23.
Kaya H, Ertaş F, İslamoğlu Y, Kaya Z, Atılgan ZA, Çil H, Çalışkan A, Aydın M, Oylumlu M, Soydinç MS. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thrombosis/Hemostasis. 2014;20(1):50–4.
Acknowledgements
Not applicable.
Funding
Funding for this study was received from the Science and Technology Projects of Liaoning Province (2020JH1/10300002).
Author information
Authors and Affiliations
Contributions
Dr. XTD acquired the data, performed statistical analyses, and wrote the initial draft. Dr. TZK and Dr. XJZ conceived the study, participated in its design and coordination, helped to draft the manuscript. Professor BL revised the manuscript critically for important intellectual content. Professor AJH, Professor YW provided the experimental design, revised the manuscript and provided final approval. All authors contributed to the study design and data interpretation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study had already been approved by Ethics Committee of the People’s Hospital of China Medical University and all subjects provided their informed, written consent before participation. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Xt., Kong, Tz., Zhang, Xj. et al. Relationship between increased systemic immune-inflammation index and coronary slow flow phenomenon. BMC Cardiovasc Disord 22, 362 (2022). https://doi.org/10.1186/s12872-022-02798-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02798-0