Abstract
Background
Infective endocarditis due to Escherichia coli is a rare disease but is increasing in frequency, especially among older women. In addition, its mortality rate is higher than that of endocarditis due to the HACEK-group gram-negative bacteria (Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, and Kingela spp.).
Case presentation
A 58-year-old Japanese woman with a history of alcohol abuse was admitted to our hospital because of a fever. She was diagnosed with infective endocarditis due to E. coli based on repeated blood cultures and transthoracic echocardiography, which revealed vegetations attached to the anterior leaflet and chordae tendineae of the mitral valve. Despite administration of sulbactam/ampicillin and gentamycin, she developed purulent spondylitis during hospitalization and required treatment with meropenem administration for 6 weeks, leading to resolution of the endocarditis. She took oral levofloxacin for 2 months, and the spondylitis was completely cured 7 months after discharge.
Conclusion
Escherichia coli affects native valves without degenerative valvulopathy rather than prosthetic valves, especially in patients with risk factors such as an immunosuppressive status, excessive alcohol consumption, or treatment with hemodialysis. Peripheral embolization, congestive heart failure, and valve-ring abscesses are major complications of E. coli endocarditis; notably, infective myocarditis can also occur. The mortality and surgical intervention rates are 21% and 42%, respectively. Physicians should be cognizant of the necessity of surgical intervention when E. coli endocarditis is resistant to antibiotic therapy.
Similar content being viewed by others
Background
Infective endocarditis (IE) due to Escherichia coli is a rare disease. Escherichia coli is the causative microorganism in approximately 0.51% of cases of IE [1]. Thirty-six cases of E. coli native valve IE that met the Duke criteria were reported in the literature from 1909 to 2002, and urinary tract infection was the most common cause of endocarditis due to E. coli [2]. The low incidence of E. coli IE has been attributed to the inability of this bacterium to adhere to the endocardium as well as the existence of antibodies to E. coli in normal serum [3]. Notably, however, the number of > 70-year-old patients with E. coli IE has recently increased, and about 70% of affected patients are older women [2]. In addition, the mortality rate of E. coli IE (21%) is higher than that of IE due to HACEK-group gram-negative bacteria (4%), including Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, and Kingela spp. [1, 4].
We herein present a patient with native valve IE resulting from E. coli infection. This patient was admitted to our hospital for examination because of a fever of unknown origin. Repeated blood cultures revealed E. coli, but no E. coli grew in sputum or urine cultures. Transthoracic echocardiography demonstrated two vegetations adhered to the anterior leaflet of the mitral valve (MV) and its chordae tendineae. During hospitalization, the patient’s condition was complicated by purulent spondylitis. After 8 weeks of antibiotic administration, the patient was discharged without any sequelae.
Case presentation
A 58-year-old Japanese woman was admitted to our hospital by ambulance because of a 1-week history of malaise, lumbago, and fever of unknown origin. She had no relevant medical history and no family history. She was a nonsmoker, but she had drunk about 60 to 80 g of alcohol per day for 30 years. Liver dysfunction had been noted for the past 10 years.
On admission, her height was 158 cm, weight 56.2 kg, and body temperature 39.5 °C. Her blood pressure was 101/60 mmHg. Her heart rate was 106 beats per minute. A physical examination showed no major abnormalities. She was alert but short of breath on exertion, with an arterial blood oxygen saturation of 94%, partial oxygen pressure of 72.0 mmHg, and partial carbon dioxide pressure of 27.2 mmHg. A chest radiograph showed no obvious signs suggesting pneumonia or pulmonary congestion. Electrocardiography showed sinus tachycardia and no other abnormalities. Plain computed tomography of the neck, chest, and abdomen and ultrasound of the abdomen revealed no significant abnormalities except for fatty change of the liver. Laboratory tests showed an elevated white blood cell count; elevated levels of serum liver enzymes, blood urea nitrogen, creatinine, uric acid, C-reactive protein (CRP), and serum brain natriuretic peptide; hypoproteinemia; and hypokalemia (Table 1). Serum rheumatoid factor was negative. Urinalysis was positive for ketone bodies, but the urinary sediment showed no abnormalities. Cultures of blood, urine, and sputum were carried out on admission. Intravenous administration of sulbactam/ampicillin (SBT/ABPC) (9 g/day) was begun from the day of hospitalization.
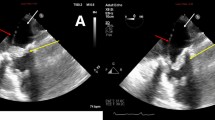
On day 2, the patient remained febrile. The sputum culture showed no significant growth of pathogenic bacteria, and the urine culture was sterile. However, gram-negative rods were detected in the blood culture. Subsequent echocardiography revealed two vegetations, one of which was attached to the anterior leaflet of the MV and was approximately 4 × 1 mm in size (Fig. 1a). The other was attached to the chorda tendinea adjacent to the posterior papillary muscle, and its size was larger (10 × 2 mm) than that of the MV vegetation (Fig. 1b, c). Based on these findings, combined antibiotics including SBT/ABPC (9 g/day) and gentamycin (GM) (80 mg/day) were administered on day 2. Repeated blood cultures were also performed on days 2 and 3, and all culture sets were positive for gram-negative rods, which were proven to be pansensitive E. coli on day 6. Based on these findings, the patient was diagnosed with IE due to E. coli in accordance with the modified Duke criteria [5]. An ophthalmologist and dermatologist also examined her on day 6, but they observed no remarkable findings associated with IE. Contrast-enhanced computed tomography of the chest and abdomen on day 7 showed no remarkable findings; magnetic resonance imaging (MRI) and magnetic resonance angiography of the brain on day 8 also showed no abnormalities.
Transthoracic echocardiography images on day 2. a: A parasternal long-axis tomogram demonstrated a vegetation attached to the anterior leaflet of the mitral valve (4 × 1 mm, white arrow). b, c: Parasternal long-axis tomograms at (b) presystole and (c) end-diastole (white arrows.) A mobile and elongated vegetation (10 × 2 mm) was attached to the chorda tendinea of the mitral valve
After antibiotic medication including SBT/ABPC and GM, the patient’s general condition improved. Her body temperature normalized on day 5, and her white blood cell count returned to a normal level (8600/mm3); her CRP level significantly decreased (5.09 mg/dL) on day 6. Blood culture analysis on day 7 and thereafter revealed no bacterial growth, but her CRP level remained mildly elevated (about 2.0 mg/dL). Echocardiography on day 14 showed a decrease in the size of the vegetations; those attached to the MV and chorda tendinea were 2 × 1 and 5 × 1 mm, respectively. However, the patient developed severe lumbago on day 20. Her white blood cell count was normal (5900/mm3), but her CRP level had increased to 3.1 mg/dL. MRI of the lumbar spine on day 21 revealed purulent spondylitis lesions in the L1 and L2 lumbar vertebrae. Intravenous meropenem (6 g/day) was substituted for SBT/ABPC and GM on day 21 despite the sterile blood culture. The patient’s clinical course was good thereafter; her lumbago improved. Transthoracic echocardiography on day 35 revealed complete resolution of the vegetations. On day 54, her CRP level decreased to 0.34 mg/dL. Administration of meropenem was terminated, and she was discharged from our hospital on day 56. After discharge, oral levofloxacin (500 mg/day) was administered for 2 months in accordance with the suggestions of orthopedic surgeons. Her CRP level normalized after 2 months. Both upper endoscopy and colonoscopy performed 3 months after discharge showed no abnormalities. MRI findings of the lumbar spine normalized after 7 months. She also stopped drinking alcohol for 12 months and remained well during that time.
Discussion
IE due to E. coli is rare and most commonly observed in older women, especially those with diabetes mellitus [1]. However, IE due to E. coli can also be seen in younger patients. Fayyaz et al. [6] reported that E. coli was detected from 8.4% of 20- to 40-year-old patients with IE. They also reported that no more than 60% of detected E. coli showed susceptibility to amikacin and amoxicillin/clavulanate. Other studies have shown that E. coli accounts for one-third of non-HACEK, gram-negative bacilli-induced IE and is the most common gram-negative bacillus that can cause IE [7, 8]. These findings suggest that the frequency of IE due to E. coli may be higher than previously believed. Because of the high in-hospital mortality and high rates of cardiac surgery in patients with IE due to non-HACEK gram-negative bacilli [1], physicians should pay close attention to the clinical course of patients with E. coli IE during hospitalization. In addition, although the E. coli detected from the blood culture samples in the present case showed susceptibility to both ABPC and GM, administration of both antibiotics did not prevent the onset of purulent spondylitis. Notably, the purulent spondylitis developed 3 weeks after hospitalization. This may reflect insufficient transferability of antibiotics because it takes 3 to 4 weeks for the formation of new blood vessels in infected bone to accomplish appropriate antibiotic migration [9].
Patients’ backgrounds
To the best of our knowledge, 32 cases of E. coli IE have been reported in the PubMed database in the past 30 years [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. In total, 33 cases (the 32 previously reported cases and the present case) were reviewed in this study (Table 2). These cases included 13 male and 16 female patients; the sex of 4 patients was unknown. The mean age of the patients was 59.6 ± 19.8 years (male, 52.4 ± 21.2 years; female, 64.3 ± 17.2 years). The MV was the most frequently affected structure (15 cases, including 5 cases of prosthetic valve endocarditis) [11, 13, 16, 17, 20, 22, 25, 27, 30, 32], and the aortic valve (AV) was the second most frequently affected structure (11 cases, including 4 cases of prosthetic valve endocarditis) [12, 14,15,16, 23, 24, 30, 31]. Both the MV and AV were affected in one patient [28]. Non-valvular IE was reported in two patients [26, 29]. The tricuspid or pulmonary valves were also affected; vegetations on the tricuspid valve and pulmonary valve were reported in three and one patient, respectively [1, 18, 19, 21].
Background comorbidities of E. coli IE
With respect to the comorbidities of E. coli IE, seven patients were diagnosed with diabetes mellitus [11, 16, 25, 30, 32], three had a history of malignancy [13, 16], and, notably, three had a history of excessive alcohol consumption [14, 19]. Moreover, three patients had renal disease [27, 29, 31]. Two patients required renal replacement therapy [27, 29], including one patient taking prednisolone and cyclophosphamide [27], and one patient had undergone renal transplantation [31]. Conditions such as diabetes, hemodialysis, and malignancy may reflect an underlying immunocompromised state. A past study showed an increasing incidence of non-HACEK gram-negative endocarditis in patients with cirrhosis [33]; interestingly, however, our review included three patients with a history of excessive alcohol consumption with or without cirrhosis. Chronic alcohol consumption causes disintegrity of the gut mucosa, which may lead to an increased risk of transmural migration of E. coli into the circulation and non-oral gastrointestinal tract. Commonly, the urinary tract is presumed to be a source of pathogen acquisition in patients with non-HACEK gram-negative endocarditis [33, 34]. In addition to this, excessive alcohol consumption may be an independent risk factor for E. coli IE. Patients undergoing renal replacement therapy also have a high incidence of endocarditis [35]; therefore, it should be noted that these comorbidities (diabetes, malignancy, excessive alcohol consumption, and hemodialysis) may be risk factors for E. coli IE. Preceding urinary tract infection was reported in 52% (17/33) of the patients [12,13,14,15, 20, 21, 25, 28, 30,31,32]. Additionally, 36% (12/33) of the patients were positive for E. coli in urine culture samples [12,13,14,15, 20, 21, 25, 31, 32].
Prosthetic valve endocarditis and native valve endocarditis
Endocarditis due to non-HACEK gram-negative bacteria was considered a disease of intravenous drug users in the past, but the reported frequency of this type of endocarditis in intravenous drug users is no more than 4% of all affected patients [33]. Our review included no intravenous drug users. Instead, E. coli IE is more common in patients with prosthetic valves; 33% (5/15) of the affected MVs and 36% (4/11) of the affected AVs were prosthetic valves (Table 3). This incidence of E. coli IE associated with prosthetic valves is slightly higher than the previously reported incidence (7–25%) [36]. A recent analysis of the International Collaboration on Endocarditis–Prospective Cohort Study (ICE-PCS) registry showed a rising incidence of non-HACEK gram-negative endocarditis in patients with implanted endovascular devices, including prosthetic valves, permanent pacemakers, and implantable cardioverter-defibrillators [33], and this may be applicable to patients with E. coli IE. However, 67% (22/33) of reviewed patients developed native valve E. coli IE [10,11,12,13,14,15,16,17,18,19,20,21, 24, 25, 27, 28, 30,31,32]. Among these 22 patients, degenerative valvular diseases were reported in 7 patients, accounting for no more than 32% (7/22) of cases of native valve E. coli IE. Conversely, 68% (15/22) of patients with native valve E. coli IE had no history of degenerative valvular disease. This contrasts with IE caused by common pathogens including streptococci, staphylococci, and enterococci because about 70% of such patients have degenerative valvular disease [37]. The reason why normal valves are more frequently affected in E. coli IE is unclear. In the present review, 52% (17/33) of patients had a urinary tract infection prior to or at the time of diagnosis of E. coli IE [12,13,14,15,16, 20, 21, 25, 28, 30,31,32]. Notably, 12 of these 17 patients were positive for E. coli in both the urine culture and blood culture, and 13 of the 17 patients had native valve IE [12,13,14,15,16, 20, 21, 25]. This suggests that virulence factors of extraintestinal pathogenic E. coli existing in the urinary tract may be strongly associated with the onset of E. coli IE. Notably, recent studies have indicated that extraintestinal pathogenic E. coli strains have a high prevalence of phylogenetic type B2, which has a variety of virulence factors that may enable the pathogens to invade cardiac endothelia [38, 39]. Moreover, the ICE-PCS registry reported that it takes ≥1 month from onset to clinical diagnosis in 90% of patients with non-HACEK gram-negative endocarditis [33]. This suggests that a prolonged duration of insufficient treatment resulting from a poor understanding of E. coli IE may increase the opportunity for E. coli to invade the endothelia of normal valves. Importantly, the initial foci of E. coli infection were unclear in 48% (16/33) of the reviewed cases, including our case. Therefore, a list of differential diagnoses for E. coli IE should be properly compiled when examining patients with E. coli bacteremia.
Complications during hospitalization
Peripheral embolization, congestive heart failure, and valve-ring abscesses have been reported as complications of non-HACEK gram-negative endocarditis [33]. Among all reviewed patients in the present report, eight (24%) had peripheral embolization [10, 11, 14, 16, 18, 19, 30, 32], and six (22%) had congestive heart failure [11,12,13, 16, 20, 25]. Intracardiac or valve-ring abscesses were reported in eight (18%) patients [12, 15, 16, 23, 30]. Infective myocarditis leading to left ventricular aneurysm formation was reported in two (6%) patients [13, 20]. Interestingly, newly observed atrioventricular block was reported in two (6%) patients: one with tricuspid valve endocarditis and the other with AV endocarditis and a ring abscess [21, 23]. These findings may suggest that potent invasive myocardial infection by E. coli leading to destruction of the myocardium can occur before detection of echocardiographic abnormalities. Renal failure was also reported in six (18%) patients [16, 25, 27]. Moreover, suppurative osteomyelitis or spondylodiscitis was observed in four (12%) patients [16, 19, 20]. Physicians should know the necessity of evaluating patients for orthopedic complications when treating them for E. coli IE.
Treatment and outcome
The American Heart Association recommends combination antibiotic therapy with β-lactams (penicillins, cephalosporins, or carbapenems) and either an aminoglycoside or fluoroquinolone for 6 weeks in patients with non-HACEK gram-negative endocarditis [5]. Among 31 patients in whom the antibiotics used could be identified, 6 (20%) were treated with penicillins [10, 16, 17, 23, 26], 14 (45%) were treated with cephems and/or penicillins [11,12,13,14,15,16, 25, 28,29,30,31], and 11 (35%) were treated with carbapenems and/or other β-lactams [16,17,18,19,20,21,22, 24, 27, 30,31,32]. Although only six patients were positive for extended-spectrum β-lactamase (ESBL)-producing E.coli [21, 22, 24, 27, 30, 32], it should be noted that one-third of the reviewed patients were treated with carbapenems. Notably, five of the six ESBL-positive patients were successfully treated through the administration of carbapenems without surgical interventions. This may suggest the importance of prompt administration of carbapenems when needed because E. coli may potentially have resistance against β-lactams such as inducible β-lactamases, which can be overlooked in routine in vitro laboratory screening for antibiotic susceptibility [5]. Therefore, antibiotic stability against β-lactamases should be considered when choosing β-lactams for therapy of E. coli IE. Aminoglycosides and fluoroquinolones were administered with β-lactams or carbapenems in 21 and 7 patients, respectively [10, 11, 13, 15,16,17,18, 20, 21, 23, 25,26,27,28, 30, 31]. Four patients were treated with both aminoglycosides and fluoroquinolones besides β-lactams or carbapenems, and three of these four patients required surgical treatment despite ESBL-negative E. coli infection [16, 25, 27]. This suggests the importance of a thorough evaluation of patients with E. coli IE to assess the necessity of surgical intervention.
Among patients with non-HACEK gram-negative endocarditis, the mortality rate did not significantly differ between patients receiving medical therapy alone and those undergoing surgical treatment, and the in-hospital mortality and cardiac surgery rates were reportedly 24% and 51%, respectively [33]. Among these reviewed patients with E. coli IE, the mortality and surgical intervention rates were 21% (7/33) and 42% (14/33), respectively [10,11,12,13,14,15,16, 18, 20, 23, 25, 29, 30, 32]. Almost half of the patients (4/7) with fatal E. coli IE died within 1 month of hospitalization [11,12,13, 16, 29]. Meanwhile, the 14 patients who underwent surgical intervention included six (43%) patients with prosthetic valve endocarditis and eight (57%) patients with native valve endocarditis. Prosthetic valve replacement was performed in eight patients (four AV and four MV replacements) [14,15,16, 20, 23, 25], and MV annuloplasty was performed in one patient [16]. Resection of the infected valve leaflets without valve replacement was performed in two patients and was mainly done only in patients with tricuspid or pulmonary valve endocarditis [10, 18]. In three patients, details of the surgical procedure were unclear [30]. One patient died 9 months later [30], but in-hospital postoperative death was not reported among the reviewed patients, which may suggest the importance of the timing of the decision regarding surgical intervention. Limitations of our report include the lack of detailed observation of the patients’ MV on transesophageal echocardiography because of the patients’ refusal and the inability to statistically investigate the data of reviewed patients with E. coli IE because this report was based on a limited number of case reports. However, our findings suggest important characteristics of E. coli IE.
Conclusion
Our review shows that native valves without degenerative valvulopathy can be more susceptible to E. coli IE than prosthetic valves in patients with risk factors such as diabetes mellitus, malignancy, excessive alcohol consumption, and renal replacement therapy. Peripheral embolization, congestive heart failure, and valve-ring abscesses are major complications of E. coli IE; however, ventricular aneurysm and atrioventricular block resulting from infective myocarditis, which are less frequent complications, should also be noted. Cephems and carbapenems are frequently used for treatment, and the mortality and surgical intervention rates associated with this therapy are 21% and 42%, respectively. Physicians should be cognizant of the timing of surgical interventions when E. coli IE is resistant to antibiotic therapy.
Abbreviations
- AV:
-
Aortic valve
- CRP:
-
C-reactive protein
- E. coli :
-
Escherichia coli
- ESBL:
-
Extended-spectrum β-lactamase
- GM:
-
Gentamycin
- HACEK:
-
Haemophilus spp., Aggregatibacter spp., Cardiobacterium hominis, Eikenella corrodens, and Kingela spp.
- ICE-PCS:
-
International Collaboration on Endocarditis–Prospective Cohort Study
- IE:
-
Infective endocarditis
- MRI:
-
Magnetic resonance imaging
- MV:
-
Mitral valve
- SBT/ABPC:
-
Sulbactam/ampicillin
References
Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, et al. International collaboration on endocarditis prospective cohort study (ICE-PCS) investigators. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829–35.
Micol R, Lortholary O, Jaureguy F, Bonacorsi S, Bingen E, Lefort A, et al. Escherichia coli native valve endocarditis. Clin Microbiol Infect. 2006;12:401–3.
Watanakunakorn C, Burket T. Infective endocarditis in a large community teaching hospital, 1980-1990. A review of 210 episodes. Medicine. 2013;72:90–102.
Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, et al. International collaboration on endocarditis prospective cohort study investigators. HACEK infective endocarditis: characteristics and outcomes from a large, multinational cohort. PLoS One. 2013;e63181:8.
Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2015;132:1435–86.
Fayyaz I, Rasheed MA, Ashraf M, Bukhsh A, Wadood A. Determination of bacterial etiological agents, sensitivity pattern and clinical outcome of patients with bacterial endocarditis at Punjab Institute of Cardiology. Lahore J Pak Med Assoc. 2014;64:1384–8.
Loubet P, Lescure FX, Lepage L, Kirsch M, Armand-Lefevre L, Bouadma L, et al. Endocarditis due to gram-negative bacilli at a French teaching hospital over 6-year period: clinical characteristics and outcome. Infect Dis (Lond). 2015;47:889–95.
Muñoz P, Cruz AF, Rodríguez-Créixems M, Bouza E. Gram-negative bloodstream infections. Int J Antimicrob Agents. 2008;32(suppl. 1):S10–4.
Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407.
Murray NH, Cheesman MG, Millar-Craig M. Echocardiographic demonstration of Escherichia coli endocarditis restricted to the pulmonary valve. Br Heart J. 1988;80:452–4.
Watanakunakorn C, Kim J. Mitral valve endocarditis caused by a serum-resistant strain of Escherichia coli. Clin Infect Dis. 1992;14:501–5.
Raymond NJ, Robertson MD, Land SD. Aortic valve endocarditis due to Escherichia coli. Clin Infect Dis. 1992;15:749–50.
Oosterbosch L, Oei F, Rogiers P, Vaerenberg M, Ranquin R, Nagler J. Escherichia coli endocarditis of a native valve with paraventricular aneurysm formation and fatal hemopericardium. Acta Cardiol. 1996;51:535–40.
Morrison DJ, Sperling LS, Schwartz DA, Felner JM. Escherichia coli endocarditis of a native aortic valve. Arch Pathol Lab Med. 1997;121:1292–5.
Soma J, Stakkevold TI, Henriksen AZ. Escherichia coli endocarditis of the aortic valve with formation of a paravalvular abscess cavity. Echocardiography. 2005;22:129–31.
Branger S, Casalta JP, Habib G, Collard F. Escherichia coli endocarditis: seven new cases in adults and review of the literature. Eur J Clin Microbiol Infect Dis. 2005;24:537–41.
Kulas T, Habek D. Infective puerperal endocarditis caused by Escherichia coli. J Perinat Med. 2006;34:342–3.
Foley JA, Augustine D, Bond R, Boyce K, Maciver D. Lost without Occam’s razor: Escherichia coli tricuspid valve endocarditis in a non-intravenous drug user. BMJ Case Rep 2010;pii:bcr0220102769.
Tsutsumi T, Hiraoka E, Kanazawa K, Akita H, Eron LJ. Diagnosis of E. coli tricuspid valve endocarditis: a case report. Hawaii Med J. 2010;69:286–8.
Lauridsen TK, Arpi M, Fritz-Hansen T, Fromodt-Moller N, Bruun NE. Infectious endocarditis caused by Escherichia coli. Scand J Infect Dis. 2011;43:545–6.
Fordyce CB, Leather RA, Partlow E, Swiggum EA. Complete heart block associated with tricuspid valve endocarditis due to extended spectrum β-lactamase-producing Escherichia coli. Can J Cardiol. 2011;263:e17–20.
Modi HH, Modi SH, Siddiqui BR, Andreoni JM. A rare case of prosthetic valve endocarditis caused by extended-spectrum β-lactamase producing Escherichia coli. J Glob Infect Dis. 2011;3:99–101.
Senel AC, Ondrush J. A case of Echerichia coli endocarditis after hemorrhoidectomy performed by a herbalist. Balkan Med J. 2012;29:201–2.
Lupse M, Flonta M, Straut M, Usein CR, Tantau M, Serban A. Recurrent infective endocarditis of the native aortic valve due to ESBL-producing Escherichia coli (E.coli) after therapeutic ERCP. J Gastrointestin Liver Dis. 2012;21:217–9.
Rangarajan D, Ramakrishnan S, Parto KC, Devaraj S, Krishnamurthy V, Kothari Y, et al. Native valve Escherichia coli endocarditis following urosepsis. Indian J Nephrol. 2013;23:232–4.
Gupta SK, Nanda V, Malviya P, Jacobs N, Naheed Z. An unusual case of early onset persistent Escherichia coli septicemia associated with endocarditis. Am J Perinatal Rep. 2013;3:105–6.
Spaleniak S, Romejko-Ciepielewska K, Lubas A, Ryczek R, Niemczyk S. Infective endocarditis in the course of urosepsis E. coli ESBL(+) in a patient with Goodpasture’s syndrome. Kardiol Pol. 2015;73:670.
Chen CA, Lin ZZ, Yu WL, Wu WS. Escherichia coli endocarditis of native aortic valve and mitral valve. J Formos Med Assoc. 2015;114:893–4.
Tsai MH, Leu JG, Fang YW, Hsieh SC. Necrotizing fasciitis and infective endocarditis caused by Escherichia coli in a hemodialysis patient. Hemodial Int. 2015;19:E41–4.
Loubet P, Lescure FX, Lepage L, Kirsch M, Armand-Lefevre L, Bouadma L, et al. Endocarditis due to gram-negative bacilli at a French teaching hospital over a 6-year period: clinical characteristics and outcome. Infect Dis (Lond). 2015;47:889–95.
Menon T, Balakrishman N, Somasundaram S, Dhandapani P. Native valve endocarditis caused by Escherichia coli. J Clin Diag Res. 2017;11:DD05–6.
Kim CJ, Yi JE, Kim Y, Choi HJ. Emphysematous endocarditis caused by AmpC beta-lactamase-producing Escherichia coli. Medicine (Baltimore). \2018;97:e9620.
Raza SS, Sultan OW, Sohail MR. Gram-negative bacterial endocarditis in adults: state-of-the-heart. Expert Rev Anti-Infect Ther. 2010;8:879–85.
Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7:2058–68.
Chaudry MS, Carison N, Gislason GH, Kamper AL, Rix M, Fowler VG Jr, et al. Risk of infective endocarditis in patients with end stage kidney disease. Clin J Am Soc Nephrol. 2017;12:1814–22.
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30.
Pierce D, Calkins BC, Thornton K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician. 2012;85:981–6.
Micenková L, Bosák J, Vrba M, Ševčíková A, Šmajs D. Human extraintestinal pathogenic Escherichia coli strains differ in prevalence of virulence factors, phylogroups, and bacteriocin determinants. BMC Microbiol. 2016;16:218.
Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181:1753–4.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Availability of data materials
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NA collected the data and drafted the manuscript. MK edited the manuscript, participated in the study design and coordination, and helped to draft the manuscript. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of this written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Akuzawa, N., Kurabayashi, M. Native valve endocarditis due to Escherichia coli infection: a case report and review of the literature. BMC Cardiovasc Disord 18, 195 (2018). https://doi.org/10.1186/s12872-018-0929-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-018-0929-7