Abstract
Background
Enhanced agricultural production is urgently required to meet the food demands of the increasing global population. Abundant genetic diversity is expected to accelerate crop development. In particular, the development of the CRISPR/Cas genome editing technology has greatly enhanced our ability to improve crop’s genetic diversity through direct artificial gene modification. However, recent studies have shown that most crop improvement efforts using CRISPR/Cas techniques have mainly focused on the coding regions, and there is a relatively lack of studies on the regulatory regions of gene expression.
Results
This review briefly summarizes the development of CRISPR/Cas system in the beginning. Subsequently, the importance of gene regulatory regions in plants is discussed. The review focuses on recent developments and applications of mutations in regulatory regions via CRISPR/Cas techniques in crop breeding.
Conclusion
Finally, an outline of perspectives for future crop breeding using genome editing technologies is provided. This review provides new research insights for crop improvement using genome editing techniques.
Similar content being viewed by others
Background
Enormous challenges in crop improvement will be faced in the coming decades to meet the food demands with the global population predicted to reach 10 billion by 2050 [1]. Up to 60% increase in crop production yield is needed to feed this global population [2]. However, global food production suffers from severe challenges due to climate change [3], reduced arable land [4], decreased water resources [5], and biotic and abiotic stresses [6]. Limited source of useful genetic variations for agriculture traits is also a significant obstacle in crop breeding. Therefore, scientific breakthroughs and technological innovations are urgently needed to improve crop production and ensure global food security.
Cross-breeding, mutation breeding and transgenic breeding are the major techniques used for crop improvement for decades [7]. Cross-breeding, which relies on genetic recombination, is labor-intensive and time-consuming [7, 8]. Transgenic breeding technique with genetically modified organisms can be achieved by randomly integrating exogenous DNA into plant genomes [9, 10]. In contrast to transgenic breeding, mutation breeding not only shortens breeding time but also has the advantage of being considered as non-GM [11]. Thus products generated by this approach can obtain governmental agency’s regulatory approval more easily, such as the Clearfield® [12, 13] and Provisia™ series [14] products. Nevertheless, the use of mutagenesis breeding remains limited owing to the random creation of unwanted off-target mutations and the low mutation frequency of on-target genes [15].
Since the elucidation of its biochemical mechanism in 2012 [16, 17], the CRISPR/Cas9 system has been successfully used to accelerate crop improvement and obtain desirable agronomic traits such as increased yield, improved quality, stress tolerance and herbicide resistance, owing to its simplicity, efficiency, and high specificity [18, 19].
This review provides a comprehensive overview of the applications of genome editing technology for crop improvement. The introduction briefly summarizes the development of CRISPR/Cas-mediated genome editing systems and the importance of gene expression regulatory elements. This review will focuses on the development and use of CRISPR/Cas-mediated genome editing techniques in crops through engineering regulatory regions to introduce genetic diversity. Finally, future research directions for the application of CRISPR/Cas-mediated genome editing in crop improvement are discussed.
Genome editing systems and DNA repair mechanisms
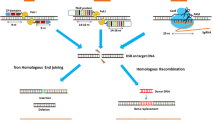
In plants, the CRISPR/Cas-based techniques introduce site-specific double strand DNA breaks (DSBs) that can be repaired via two main pathways: nonhomologous end-joining (NHEJ) and homology-directed repair (HDR) [20] (Fig. 1a). NHEJ is the main DSB repair pathway [21]which depends on an error-prone repair system that often introduces large or small sequence insertions, deletions (indels), or substitutions [22]. HDR-mediated genome editing can achieve precise mutations at a target site, but the efficiency is very low since it is a minor DNA repair pathway in most eukaryotic cells [23].
In addition to NHEJ-mediated and HDR-mediated genome editing, base editors have been developed to induce precise nucleotide substitutions at targeted sites without requiring double strand breaks (DSBs), donor DNA templates, or reliance on homology-dependent repair (HDR) [24, 25]. At present, cytosine base editors (CBEs) and adenine base editors (ABEs), have been developed to catalyze the conversion of C•G base pairs to T•A base pairs [26] and A•T base pairs to G•C base pairs [27], respectively (Fig. 1b). Recently, a novel and precise genome-editing technology, named prime editing, was also developed that enables the introduction of insertions, deletions and all 12 classes of point mutations without requiring DSBs or donor DNA templates (Fig. 1c); prime editor was initially developed in mammalian cells and was shown to function efficiently in plants [28, 29]. To date, these genome editing systems have been successfully used to obtain precise and predictable genome modifications in plants for trait improvement [30].
Gene expression regulation elements
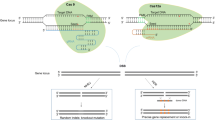
Plant growth and development requires precise gene regulation, controlled at the transcriptional, post-transcriptional and translational levels [31]. Up- or down- regulation of gene expression can be achieved by introducing mutations in different regulatory control elements, including promoters, introns, alternative splicing (AS) sites and untranslated regions (UTRs) [32,33,34,35]. These approaches for generating novel variations have been applied to crop improvement [36], for example, to accelerate plant domestication and achieve desirable traits by altering gene expression levels without the complete knockout of genes [37] (Fig. 2).
Effect of mutations in the gene coding region and regulatory sequences on gene expression level for crop improvement. (a) Expression levels of wild-type plants; (b) Coding region mutations have influence on gene expression; c, d, e, f, g, h. Mutations in regulatory control regions can affect gene expression levels or expression patterns; i. Ideal plants with various elite agronomic traits
UTRs contain highly conserved structural motifs involved in regulating gene expression. Gene expression and function are regulated by the production of multiple mRNA variants [38,39,40]. The regulation of gene expression at the translational level is mainly modulated by UTRs [41, 42]. From the cap site to the start codon (excluded) is the 5´UTR that may contain important regulatory elements such as ribosome entry site (RES), secondary structure, upstream ATGs, and upstream open reading frames (uORFs) [43, 44]. These elements may play major roles in translational regulation, including mRNA stabilization, folding, and ribosome interactions. Similarly, the 3´UTR spans from the stop codon (excluded) to the poly-A tail, containing numerous binding sites for regulatory factors [45]. Many regulatory factors involved in the post-transcriptional gene expression regulation regulate biological processes, such as mRNA turnover, localization, and translation efficiency [46, 47]. In addition, 3´UTRs were shown to mediate protein-protein interactions through facilitating alternative protein complex formation, resulting in the diversification of protein functions [48, 49].
Promoters are non-coding DNA sequences that activate gene transcription. Promoters are located at the 5’ upstream of the coding region, containing core promoter regions and upstream promoter response elements that recognize and bind RNA polymerase and transcriptional factors, thereby regulating downstream gene expression [50, 51]. The core promoter region is generally located near the transcription start site, which regulates the transcription initiation of genes, including the transcription start site, TATA-box, initiation factor, and downstream promoter element [52,53,54]. Promoter response elements, such as enhancers, CAAT-box, GC-box and G-box, are usually located upstream of the core promoter region, and determine the specificity, activity and efficiency of gene transcription [53].
Although introns are not directly involved in the translation of mRNA for protein synthesis, they play an important role in the regulation of gene expression [55]. In many cases, the presence of introns in eukaryotic gene coding regions can improve the gene expression. Introns are excised during post-transcriptional processing [56]. Alternative splicing sites lead to the formation of alternatively spliced mRNA variants, including some with intron retention, which affect gene expression levels or generate alternative protein molecules with different activities [57, 58]. For example, studies have identified a cis-regulatory element CME in the intron of FLC gene that can eliminate the “vernalization memory” derived from the parents [59, 60], indicating the importance of introns in the gene expression regulation.
CRISPR/Cas-mediated editing of regulatory elements for crop improvement
Through numerous studies on development and optimization, CRISPR/Cas systems have demonstrated their power in introducing genetic modification in various crops and potential in accelerating genetic improvement in crops, such as increased yield, improved quality, herbicide resistance, and disease and insect resistance.
Coding sequence editing for trait improvement
Increasing the crop yield is the primary purpose of crop breeding. The knockout of functional genes, such as Gn1a, DEP1, GS3, and IPA1, which negatively regulate yield parameters such as panicle architecture, grain number, grain size, and plant architecture, has been shown to improve rice yield [61,62,63,64]. Moreover, better quality requirements rather than higher yields have attracted increasing attention as people’s living standards improve. Mutations in the Rc and SBEII genes have been used to develop rice lines with increased anthocyanin and amylose contents, respectively [65, 66]. Knocking out the FAD2 gene in rape increased the oleic acid content in grains [67]. These mutants resulted in greatly improved crop quality.
Furthermore, genome editing techniques also have widespread applications for improving crop resistance traits, especially in herbicide resistance breeding. To date, various crop species that showed tolerance to ALS-inhibiting herbicides have been developed, including rice [68, 69], maize [70], wheat [71, 72], tobacco [73], watermelon [74], oilseed rape [75], tomato, and potato [76]. Similarly, crop resistance to ACCase inhibitors has been developed in wheat and rice [71, 77]. CRISPR/Cas9-directed mutagenesis has also been used to engineer resistance to crop diseases and pests. Tolerance to several catastrophic diseases, such as bacterial blight, blast, and tungro spherical virus, can be generated in rice by disrupting susceptibility genes using CRISPR/Cas9 [78,79,80,81]. Mutations in the cytochrome P450 gene CYP71A1 using the CRISPR/Cas9 technique resulted in resistance to brown planthoppers and borers by suppressing serotonin biosynthesis [82].
However, these mutations occur in the coding region, which may have deleterious pleiotropic effects, and lead to loss-of-function mutants with undesirable plant phenotypes [83]. Thus, engineering gene expression regulatory regions may be a preferred approach for crop improvement (Table 1).
Disruption of promoters to improve crop traits
Rice production is threatened by several diseases caused by plant pathogens [84]. Bacterial blight, caused by Xanthomonas oryzae pv. Oryzae (Xoo), is a destructive bacterial disease and can reduce rice yield by up to 75%. SWEET genes have been reported to be susceptibility genes for bacterial blight [85]. Rice lines with broad-spectrum resistance to bacterial blight were generated by targeting and mutating the promoter region of OsSWEET genes [86,87,88]. Similarly, promoter mutations in the Xa13 gene, a fully recessive allele related to bacterial blight, using the CRISPR/Cas9 system can also result in bacterial-blight-resistant rice [89]. Moreover, editing the PthA4 effector binding CREs in the promoter of the susceptibility gene CsLOB1 in citrus showed enhanced resistance to citrus canker caused by Xanthomonas citri subsp. citri [90,91,92].
Amylose content (AC) is a major physiochemical property that determines the eating and cooking quality (ECQ) of rice [93]. AC is governed by the Waxy (Wx) gene that encodes granule-bound starch synthase I (GBSSI), an enzyme that controls amylose synthesis in the endosperm [94]. Recently, novel Wx alleles generated using CRISPR/Cas9-mediated gene knockout resulted in fine-tuned grain AC and produced glutinous rice. Editing promoter or 5´UTR intronic splicing site of the Wx gene fine-tuned gene expression at transcriptional and post- transcriptional levels, produced various ACs (amylose contents) by generating diverse quantitative trait alleles in rice and improved the rice quality [95]. Moreover, amylose content can be fine-tuned by creating several novel Wx alleles in the japonica cultivar Nipponbare by editing key cis-acting elements that regulate gene expression in the Wxb promoter using CRISPR/Cas9 technology [96].
Additionally, scientists have identified a wide range of cis-regulatory mutations through targeting the putative SlWUS CArG element and SlCLV3 promoter. A series of mutants were generated with different fruit sizes and quantitative trait variation for elite crop breeding and domestication of wild relatives [97, 98]. Engineering the promoters for quantitative variation of yield-related CLE gene using CRISPR-Cas9 genome editing demonstrated the potential of promoter mutations for the modification of grain yield-related trait genes to enhance crop yields [99]. Mutating the key regulatory elements of the main QTL SLG7 promoter in rice improved SLG7 gene expression, created new allelic variations with reduced chalkiness, thus providing a new strategy for the rapid improvement of appearance quality of rice [100].
Interestingly, promoter insertion and swapping have great potential for crop improvement via CRISPR/Cas9-mediated HDR. Insertion of the GOS2 promoter into the 5´UTR or replacement of the promoter of ARGOS8 gene by CRISPR/ Cas9 technology resulted in increased expression of ARGOS8 and created drought-resistant maize [101]. Moreover, editing the promoter region may be an effective strategy for reducing the pleiotropic effects. For example, a 54 bp deletion in the promoter of IPA1, a gene that showed a trade-off effect between tiller number and panicle size, can simultaneously increase tiller number and panicle size through overcoming their tradeoff, which greatly enhanced grain yield [102]. Mutagenesis in the promoter of WOX9 gene in tomatoes resulted in multiple pleiotropic phenotypes in both vegetative and inflorescence development, suggesting that targeted promoter mutations using genome editing can reveal conserved gene functions, which can reduce undesirable effects in crop improvement [103].
Regulating genes expression by mutating UTRs
Untranslated regions (UTRs) are known to regulate gene expression and protein function by producing multiple mRNA variants [40]. Thus, mutations in the UTRs may lead to the development of desirable phenotypes, for instance, deleting the 5´UTR of CRTISO gene reduced gene expression, and subsequently causing changes in fruit color in tomato [104].
In plants, about 30% of mRNAs contain uORFs located in the 5´UTR [105], suggesting uORFs are widespread regulatory elements and may play important roles in regulating the downstream mORF (main open reading frame) via translational repression [106, 107]. Therefore, engineering uORFs by genome editing can also be an effective approach for crop improvement [108]. In certain cases, it is easier to upregulate gene expression by altering the uORF than by changing the coding sequences and promoters.
Zhang et al. edited the uORF of several genes, AtBRI1, AtVTC2, LsGGP1 and LsGGP2, and obtained enhanced mRNA translation of these genes with increased level of the encoded proteins. Moreover, they obtained mutants with paraquat tolerance by designing sgRNAs to modify the two uORFs of LsGGP1 and LsGGP2, respectively [109]. Similarly, editing the highly conserved SC-uORF of the transcription factor gene FvebZIPs1.1 in strawberries demonstrated a way to increase the sugar content without causing unfavorable agronomic performance [110]. uORF mutations in SlGGP1 gene that encodes a vitamin C-biosynthetic enzyme substantially increased foliar ascorbic acid levels in wild tomatoes [98]. Recently, Xue et al. generated a suite of uORFs via editing the 5´UTR of OsDLT gene involved in the brassinosteroid transduction pathway and obtained various plant heights and tiller numbers in rice [111].
RNA splicing affects gene expression
Protein expression level is also regulated at the post-transcriptional level. Alternative splicing (AS) of precursor mRNAs (pre-mRNAs) occurs in over 60% of intron-containing genes and plays a critical role in gene expression regulation [112]. The base editing technology is a powerful tool for manipulating plant gene splicing in gene expression regulation and functional studies.
Xue et al. mutated the 5′ splice sites of four genes: HAB1, T30G6.16, RS31A and Act2 in Arabidopsis, and obtained a G-to-A or G-to-C conversion at the desired G of the 5′ splice site and the neighboring G, demonstrating that disruption of the 5’ splice site affected abscisic acid synthesis, toxin response, and intron-mediated enhancement (IME), respectively [113].
Improving the β -carotene content in crops is also an important target of plant breeding. Splice variants in the orange (Or) gene play crucial roles in the effective accumulation of β-carotene. Endo et al. changed the splicing junction of Or gene at the third exon and intron by genome editing and realized the accumulation of β -carotene in rice callus, and then developed the golden rice lines [114].
Conclusions and perspectives
During the past decade, CRISPR/Cas-based genome editing technologies coupled with functional genomics have greatly promoted the genetic improvement of crops and have made important progress in improving crop’s yield, quality, and resistance to herbicides, diseases, and pests. However, several challenges remain to be addressed.
At present, unlike mutation in the protein-coding region which often results in the loss of gene function, editing of the gene regulatory regions leads to phenotypic outcomes that are quantitative and may be difficult to assess [115]. In addition, non-homologous end joining (NHEJ), an error-prone repair system that causes random insertions or deletions (indels) near the target sequence, is the predominant repair pathway, which does not support precise modification and often generates unexpected mutations [21, 22]. Although precision genome editors are continuously being developed, especially PE systems that can achieve template-free replacement of bases, the editing efficiency remains low [116, 117]. To overcome these limitations, it is necessary to optimize editing of regulatory region, develop an efficient editing system for precise editing to introduce desirable traits and expand the molecular breeding resources. The newly developed CRISPR-Cas12a promoter editing (CAPE) system introduced quantitative trait variation (QTV) continuums for starch content and grain size by targeting the promoters of OsGBSS1 and OsGS3, respectively, providing an effective strategy for realizing the QTV of important agronomic traits in crops [118].
Moreover, the CRISPR/Cas-mediated mutagenesis of regulatory regions provides a method for inducing subtle phenotypic changes by achieving desirable level of gene expression. Currently, it is difficult to predict the expression levels of edited genes because of many factors, including secondary structure, regulatory elements and upstream and downstream sequences [119]. Therefore, developing a method for predictable endogenous gene expression would be necessary to obtain desired traits for crop improvement. For example, an efficient and easy method has been developed for down-regulating protein translation to predictable and desired levels by engineering uORFs, generating a series of mutants with varied heights and tiller numbers, as predicted, in rice [111]. However, precise prediction tools for other expression regulatory regions (such as promoters, 5′UTR and 3′UTR) need further exploration and development.
Finally, although genome editing technology has been used in various plants, only a few edited products have been successfully commercialized. Though the edited plants may be considered nontransgenic, their social acceptance remains uncertain. The regulatory framework for gene-edited products has not yet been established in many countries. Hence, more efforts are needed to ensure a more favorable environment for regulatory support and public acceptance of genetically edited products.
Data availability
Not applicable.
References
Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. PNAS. 2011;108(50):20260–64.
Sprinmann M, Clark M, Mason-D´Croz, Marco S, Wiebe K, Bodirsky BL, et al. Options for keeping the food system within environmental limits. Nature. 2018;562(7728):519–25.
Vermeulen SJ, Campbell BM, Ingram JSI. Climate change and food systems. Annu Rev Environ Resour. 2012;37(1):195–222.
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko L, Senior RA, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520(7545):45–9.
Wada Y, Beek LPH, Kempen CM, Reckman JWTM, Vasak S, Bierkens MFP. Global depletion of groundwater resources. Geophys Res Lett. 2010;37:1–5.
Sundström JF, Albihn A, Boqvist S, Ljungvall K, Marstorp H, Martiin C, et al. Future threats to agricultural food production posed by environmental degradation, climate change, and animal and plant diseases-a risk analysis in three economic and climate settings. Food Sect. 2014;6:201–15.
Scheben A, Wolter F, Batley J, Puchta H, Edwards D. Towards CRISPR/Cas crops-bringing together genomics and genome editing. New Phytol. 2017;216(3):682–98.
Chen K, Wang Y, Zhang R, Zhang H, Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol. 2019;70:667–88.
Ramessar K, Capell T, Twyman RM, Quemada H, Christou P. Trace and traceability-a call for regulatory harmony. Nat Biotechnol. 2008;26(9):975–8.
Stein AJ, Rodríguez-Cerezo E. International trade and the global pipeline of new GM crops. Nat Biotechnol. 2010;28(1):23–5.
Dong H, Huang Y, Wang K. The development of herbicide resistance crop plants using CRISPR/Cas9-mediated gene editing. Genes. 2021;12(6):912.
Sudianto E, Beng-Kah S, Ting-Xiang N, Saldain NE, Scott RC, Burgos NR. Clearfield rice: its development, success, and key challenges on a global perspective. Crop Prot. 2013;49:40–51.
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL. Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci. 2005;61(3):246–57.
Dauer J, Hulting A, Carlson D, Mankin L, Harden J, Mallory-Smith C. Gene flow from single and stacked herbicide-resistant rice (Oryza sativa): modeling occurrence of multiple herbicide-resistant weedy rice. Pest Manag Sci. 2018;74(2):348–55.
Endo M, Toki S. Creation of herbicide-tolerant crops by gene targeting. J Pestic Sci. 2013;38(2):49–59.
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–7.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Islam W. CRISPR-Cas9; an efficient tool for precise plant genome editing. Mol Cell Probes. 2018;39:47–52.
Svitashev S, Schwartz C, Lenderts B, Young JK, Cigan AM. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat Commun. 2016;7:13274.
Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38(7):824–44.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Belhaj K, Chaparro-Garcia A, Kamoun S, Nekrasov. Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods. 2013;9(1):39.
Mao Y, Botella JR, Liu Y, Zhu J. Gene editing in plants: progress and challenges. Natl Sci Rev. 2019;6(3):421–37.
Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;169(3):559.
Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19(12):770–88.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420–4.
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A.T to G.C in genomic DNA without DNA cleavage. Nature. 2017;551:464–71.
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149–57.
Lin Q, Zong Y, Xue C, Wang S, Jin S, Zhu Z, et al. Prime genome editing in rice and wheat. Nat Biotechnol. 2020;38(5):582–5.
Gao C. Genome engineering for crop improvement and future agriculture. Cell. 2021;184(6):1621–35.
Zhang T, Wu A, Yue Y, Zhao Y. uORFs: important cis-regulatory elements in plants. Int J Mol Sci. 2020;21(17):6238.
Wray GA, Hahn MW, Abouheif E, Balhoff JP, Pizer M. The evolution of transcriptional regulation in eukaryotes. Mol Biol Evol. 2003;20(9):1377–419.
Haberle V, Stark A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat Rev Mol Cell Bio. 2018;19(10):621–37.
Rose AB, Carter A, Korf I, Kojima N. Intron sequences that stimulate gene expression in Arabidopsis. Plant Mol Biol. 2016;92(3):337–46.
Shaul O. How introns enhance gene expression. Int J Biochem Cell Biol. 2017;91:145–55.
Li X, Xie Y, Zhu Q, Liu Y. Targeted genome editing in genes and cis-regulatory regions improves qualitative and quantitative traits in crops. Mol Plant. 2017;10(11):1368–70.
Meyer RS, Purugganan MD. Evolution of crop species: genetics of domestication and diversification. Nat Rev Genet. 2013;14(12):840–52.
Iacono M, Mignone F, Pesole G. uAUG and uORFs in human and rodent 5′untranslated mRNAs. Gene. 2005;349:97–105.
Tsuzaka K, Itami Y, Kumazawa C, Suzuki M, Setoyama Y, Yoshimoto K, et al. Conservative sequences in 3′UTR of TCRzeta mRNA regulate TCRzeta in SLE T cells. Biochem Biophy Res Commun. 2008;367(2):311–7.
Srivastava AK, Lu Y, Zinta G, Lang Z, Zhu JK. UTR-dependent control of gene expression in plants. Trends Plant Sci. 2018;23(3):248–59.
Vogel C, Abreu RS, Ko D, Le S, Shapiro BA, Burns SC, et al. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Syst Biol. 2010;6:400.
Quattrone A, Dassi E. Introduction to bioinformatics resources for post-transcriptional regulation of gene expression. Methods Mol Biol. 2016;1358:3–28.
Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science. 2016;352(6292):1413–6.
Wilkie GS, Dickson KS, Gray NK. Regulation of mRNA translation by 5′-and 3′-UTR-binding factors. Trends Biochem Sci. 2003;28(4):182–8.
Hernando CE, Romanowski A, Yanovsky MJ. Transcriptional and post-transcriptional control of the plant circadian gene regulatory network. Biochim Biophys Acta Gene Regul Mech. 2017;1860(1):84–94.
Mignone F, Gissi C, Liuni S, Pesole G. Untranslated regions of mRNAs. Genome Biol. 2002;3(3):1–8.
Mercer TR, Dagmar W, Dinger ME, Soldà G, Korbie DJ, Glazov EA, et al. Expression of distinct RNAs from 3′ untranslated regions. Nucleic Acids Res. 2010;39(6):2393–403.
Mayr C. Evolution and biological roles of alternative 3′UTRs. Trends Cell Biol. 2015;26(3):227–37.
Berkovits BD, Mayr C. Alternative 3´UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015;522(7556):363–7.
Wei W, Pelechano V, Järvelin AI, Steinmetz LM. Functional consequences of bidirectional promoters. Trends Genet. 2011;27(7):267–76.
Butler JEF, Kadonaga JT. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 2002;16(20):2583–92.
Kadonaga JT. Perspectives on the RNA polymerase II core promoter. Wiley Interdiscip Rev Dev Biol. 2012;1:40–51.
Peremarti A, Twyman RM, Gómez-Galera S, Naqvi S, Farré G, Sabalza M, et al. Promoter diversity in multigene transformation. Plant Mol Biol. 2010;73(5):363–78.
Dikstein R. The unexpected traits associated with core promoter elements. Transcription. 2011;2(5):201–6.
Herz MAG, Kornblihtt AR. Alternative splicing and transcription elongation in plants. Front Plant Sci. 2019;10:309.
Barbazuk WB, Fu Y, Mcginnis KM. Genome-wide analyses of alternative splicing in plants: opportunities and challenges. Genome Res. 2008;18(9):1381–92.
Kalyna M, Simpson CG, Syed NH, Lewandowska D, Marquez Y, Kusenda B, et al. Alternative splicing and nonsense-mediated decay modulate expression of important regulatory genes in Arabidopsis. Nucleic Acids Res. 2012;40(6):2454–69.
Chang C, Lin W, Tu S. Genome-wide analysis of heat-sensitive alternative splicing in physcomitrella patens. Plant Physiol. 2014;165(2):826–40.
Yuan W, Luo X, Li Z, Yang W, Wang Y, Liu R, et al. A cis cold memory element and a trans epigenome reader mediate polycomb silencing of FLC by vernalization in Arabidopsis. Nat Genet. 2016;48(12):1527–34.
Tao Z, Hu H, Luo X, Jia B, Du J, He Y. Embryonic resetting of the parental vernalized state by two B3 domain transcription factors in Arabidopsis. Nat Plants. 2019;5(4):424–35.
Wang Y, Geng L, Yuan M, Wei J, Jin C, Li M, et al. Deletion of a target gene in Indica rice via CRISPR/Cas9. Plant Cell Rep. 2017;36(8):1333–43.
Shen L, Hua Y, Fu Y, Li J, Liu Q, Jiao X, et al. Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci China Life Sci. 2017;60(5):506–15.
Zhou J, Xin X, He Y, Chen H, Li Q, Tang X, et al. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep. 2019;38(4):475–85.
Li M, Li X, Zhou Z, Wu P, Fang M, Pan X, et al. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front Plant Sci. 2016;7:377.
Zhu Y, Lin Y, Chen S, Liu H, Chen Z, Fan M, et al. CRISPR/Cas9-mediated functional recovery of the recessive rc allele to develop red rice. Plant Biotechnol J. 2019;17(11):2096–105.
Sun Y, Jiao G, Liu Z, Zhang X, Li J, Guo X, et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front Plant Sci. 2017;8:298.
Okuzaki A, Ogawa T, Koizuka C, Kaneko K, Inaba M, Imamura J, et al. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol Biochem. 2018;131:63–9.
Zhang R, Chen S, Meng X, Chai Z, Wang D, Yuan Y, et al. Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci China Life Sci. 2020;64(10):1624–33.
Wang F, Xu Y, Li W, Chen Z, Wang J, Fan F, et al. Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. Crop J. 2020;9:305–12.
Li Y, Zhu J, Wu H, Liu C, Huang C, Lan J, et al. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. Crop J. 2020;8(3):449–56.
Zhang R, Liu J, Chai Z, Chen S, Bai Y, Zong Y, et al. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat Plants. 2019;5(5):480–5.
Zong Y, Song Q, Li C, Jin S, Zhang D, Wang Y, et al. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat Biotechnol. 2018;36(10):950–3.
Kang BC, Woo JW, Kim ST, Bae SJ, Choi M, Kim JS, et al. Guidelines for C to T base editing in plants: base-editing window, guide RNA length, and efficient promoter. Plant Biotechnol Rep. 2019;13(5):533–41.
Tian S, Jiang L, Cui X, Zhang J, Guo S, Li M, et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018;37(9):1353–6.
Wu J, Chen C, Xian G, Liu D, Lin L, Yin S, et al. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9‐mediated cytosine base‐editing. Plant Biotechnol J. 2020;18(9):1857–9.
Veillet F, Perrot L, Chauvin L, Kermarrec MP, Guyon-Debast A, Chauvin JE, et al. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int J Mol Sci. 2019;20(2):402.
Li C, Zhang R, Meng X, Chen S, Zong Y, Lu C, et al. Targeted, random mutagenesis of plant genes with dual cytosine and Adenine base editors. Nat Biotechnol. 2020;38(7):875–82.
Kim YA, Moon H, Park CJ. CRISPR/Cas9-targeted mutagenesis of Os8N3 in rice to confer resistance to Xanthomonas oryzae Pv. Oryzae. Rice. 2019;12(1):67.
Liu D, Chen X, Liu J, Ye J, Guo Z. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J Exp Bot. 2012;63(10):3899–911.
Wang F, Wang C, Liu P, Lei C, Hao W, Gao Y, et al. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE. 2016;11(4):e0154027.
Macovei A, Sevilla NR, Cantos C, Jonson GB, Slamet-Loedin I, Čermák T, et al. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to rice tungro spherical virus. Plant Biotechnol J. 2018;16(11):1918–27.
Lu H, Luo T, Fu H, Wang L, Tan Y, Huang J, et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat Plants. 2018;4(6):338–44.
Morineau C, Bellec Y, Tellier F, Gissot L, Kelemen Z, Nogué F, et al. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol J. 2017;15(6):729–39.
Marie AF, Roosens NHC, Taverniers I, Loose MD, Deforce D, Herman P. Biotech rice: current developments and future detection challenges in food and feed chain. Trends Food Sci Technol. 2016;52:66–79.
Chen L, Hou B, Lalonde S, Takanaga H, Hartung ML, Qu X, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468(7323):527–32.
Olive R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A, Li T, et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol. 2019;37(11):1344–50.
Xu Z, Xu X, Gong Q, Li Z, Li Y, Wang S, et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol Plant. 2019;12(11):1434–46.
Zafar K, Khan MZ, Amin I, Mukhtar Z, Yasmin S, Arif M, et al. Precise CRISPR-Cas9 mediated genome editing in super basmati rice for resistance against bacterial blight by targeting the major susceptibility gene. Front Plant Sci. 2020;11:575.
Li C, Li W, Zhou Z, Chen H, Xie C, Lin Y. A new rice breeding method: CRISPR/Cas9 system editing of the Xa13 promoter to cultivate transgene-free bacterial blight-resistant rice. Plant Biotechnol J. 2020;18(2):313–5.
Jia H, Orbović V, Wang N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol J. 2019;17(10):1928–37.
Jia H, Orbovic V, Jones JB, Wang N. Modification of the PthA4 effector binding elements in type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccDpthA4: dCsLOB1.3 infection. Plant Biotechnol J. 2016;14(5):1291–301.
Peng A, Chen S, Lei T, Xu L, He Y, Wu L, et al. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol J. 2017;15(12):1509–19.
Li H, Prakash S, Nicholson TM, Fitzgerald MA, Gilbert RG. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016;196:702–11.
Sano Y. Differential regulation of waxy gene expression in rice endosperm. Theor Appl Genet. 1984;68:467–73.
Zeng D, Liu T, Ma X, Wang B, Zheng Z, Zhang Y, et al. Quantitative regulation of Waxy expression by CRISPR/Cas9-based promoter and 5’UTR‐intron editing improves grain quality in rice. Plant Biotechnol J. 2020;18(12):2385–7.
Huang L, Li Q, Zhang C, Chu R, Gu Z, Tan H, et al. Creating novel wx alleles with fine-tuned amylose levels and improved grain quality in rice by promoter editing using CRISPR/Cas9 system. Plant Biotechnol J. 2020;18(11):2164–6.
Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB. Engineering quantitative trait variation for crop improvement by genome editing. Cell. 2017;171(2):470–80.
Li T, Yang X, Yu Y, Si X, Zhai X, Zhang H, et al. Domestication of wild tomato is accelerated by genome editing. Nat Biotechnol. 2018;36(12):1160–3.
Liu L, Gallagher J, Arevalo ED, Chen R, Skopelitis T, Wu Q, et al. Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat Plants. 2021;7(3):287–94.
Tan W, Miao J, Xu B, Zhou C, Wang Y, Gu X, et al. Rapid production of novel beneficial alleles for improving rice appearance quality by targeting a regulatory element of SLG7. Plant Biotechnol J. 2023. https://doi.org/10.1111/pbi.14041.
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, et al. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J. 2017;15(2):207–16.
Song X, Meng X, Guo H, Cheng Q, Jing Y, Chen M, et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat Biotechnol. 2022;40(9):1403–11.
Hendelman A, Zebell S, Rodriguez-Leal D, Dukler N, Robitaille G, Wu X, et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell. 2021;184(7):1724–39.
Jayaraj KL, Thulasidharan N, Antony A, John M, Augustine R, Chakravartty N, et al. Targeted editing of tomato carotenoid isomerase reveals the role of 5´UTR region in gene expression regulation. Plant Cell Rep. 2021;40(4):621–35.
Arnim AG, Jia Q, Vaughn JN. Regulation of plant translation by upstream open reading frames. Plant Sci. 2014;214:1–12.
Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci. 2009;106(18):7507–12.
Zhang H, Dou S, He F, Luo J, Wei L, Lu J. Genome-wide maps of ribosomal occupancy provide insights into adaptive evolution and regulatory roles of uORFs during Drosophila development. PLoS Biol. 2018;16(7):e2003903.
Si X, Zhang H, Wang Y, Chen K, Gao C. Manipulating gene translation in plants by CRISPR–Cas9-mediated genome editing of upstream open reading frames. Nat Protoc. 2020;15(2):338–63.
Zhang H, Si X, Ji X, Fan R, Liu J, Chen K, et al. Genome editing of upstream open reading frames enables translational control in plants. Nat Biotechnol. 2018;36(9):894–8.
Xing S, Chen K, Zhu H, Zhang R, Zhang H, Li B, et al. Fine-tuning sugar content in strawberry. Genome Biol. 2020;21(1):230.
Xue C, Qiu F, Wang Y, Li B, Zhao K, Chen K, et al. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat Biotechnol. 2023. https://doi.org/10.1038/s41587-023-01707-w.
Reddy ASN, Marquez Y, Kalyna M, Barta A. Complexity of the alternative splicing landscape in plants. Plant Cell. 2013;25(10):3657–83.
Xue C, Zhang H, Lin Q, Fan R, Gao C. Manipulating mRNA splicing by base editing in plants. Scienc China Life Sci. 2018;61(11):1293–300.
Endo A, Saika H, Takemura M, Misawa N, Toki S. A novel approach to carotenoid accumulation in rice callus by mimicking the cauliflower Orange mutation via genome editing. Rice. 2019;12(1):81.
Ren Q, Sretenovic S, Liu S, Tang X, Huang L, He Y, et al. PAM-less plant genome editing using a CRISPR-SpRY toolbox. Nat Plants. 2021;7(1):25–33.
Jin S, Lin Q, Gao Q, Gao C. Optimized prime editing in monocot plants using PlantPegDesigner and engineered plant prime editors (ePPEs). Nat Protoc. 2023;18(3):831–53.
Zong Y, Liu Y, Xue C, Li B, Li X, Wang Y, et al. An engineered prime editor with enhanced editing efficiency in plants. Nat Biotechnol. 2022;40(9):1394–402.
Zhou J, Liu G, Zhao Y, Zhang R, Tang X, Li L, et al. An efficient CRISPR–Cas12a promoter editing system for crop improvement. Nat Plants. 2023. https://doi.org/10.1038/s41477-023-01384-2.
Bowman EK, Deaner M, Cheng J, Evans R, Oberortner E, Yoshikuni Y, et al. Bidirectional titration of yeast gene expression using a pooled CRISPR guide RNA approach. Proc Natl Acad Sci USA. 2020;117(31):18424–30.
Acknowledgements
None.
Funding
This work was supported by Project of Hainan Yazhou Bay Seed Laboratory (B22C10213 ).
Author information
Authors and Affiliations
Contributions
H D designed the manuscript, wrote the initial manuscript, and approved to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, H. Application of genome editing techniques to regulate gene expression in crops. BMC Plant Biol 24, 100 (2024). https://doi.org/10.1186/s12870-024-04786-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04786-2