Abstract
Background
The genus Libanotis Haller ex Zinn, nom. cons., a contentious member of Apiaceae, encompasses numerous economically and medicinally significant plants, comprising approximately 30 species distributed across Eurasia. Despite many previous taxonomic insights into it, phylogenetic studies of the genus are still lacking. And the establishment of a robust phylogenetic framework remains elusive, impeding advancements and revisions in the taxonomic system for this genus. Plastomes with greater variability in their genetic characteristics hold promise for building a more robust Libanotis phylogeny.
Results
During our research, we sequenced, assembled, and annotated complete plastomes for twelve Libanotis species belong to three sections and two closely related taxa. We conducted a comprehensive comparative analysis through totally thirteen Libanotis plastomes for the genus, including an additional plastome that had been published. Our results suggested that Libanotis plastome was highly conserved between different subclades, while the coding regions were more conserved than the non-coding regions, and the IR regions were more conserved than the single copy regions. Nevertheless, eight mutation hotspot regions were identified among plastomes, which can be considered as candidate DNA barcodes for accurate species identification in Libanotis. The phylogenetic analyses generated a robustly framework for Libanotis and revealed that Libanotis was not a monophyletic group and their all three sections were polygenetic. Libanotis schrenkiana was sister to L. sibirica, type species of this genus, but the remainders scattered within Selineae.
Conclusion
The plastomes of Libanotis exhibited a high degree of conservation and was effective in enhancing the support and resolution of phylogenetic analyses within this genus. Based on evidence from both phylogeny and morphology, we propose the recognition of "Libanotis sensu stricto" and provide taxonomic recommendations for other taxa that previously belonged to Libanotis. In conclusion, our study not only revealed the phylogenetic position and plastid evolution of Libanotis, but also provided new insights into the phylogeny of the family Apiaceae and phylogenetic relationships within the tribe Selineae.
Similar content being viewed by others
Background
Libanotis Haller ex Zinn, nom. cons., belonging to the tribe Selineae of the family Apiaceae, includes approximately 30 species distributed throughout Eurasia, with 19 species found in China [1,2,3,4,5,6,7,8,9,10,11,12]. Libanotis as an independent genus was supported by de Candolle [13], Schischkin [14], Korovin [15], Rechinger [16], Fu [17, 18], Shan, Watson and Sheh [1, 2, 11, 19]. They thought conspicuous calyx teeth, separated bracteoles, and hairy mericarps easily distinguished Libanotis from Seseli. But the genus then has been suggested to merge into Seseli L. to establish broad sense Seseli genus by Drude [20], Ball [21], Kljuykov and Pimenov [1, 22,23,24,25,26], because they think the above diagnostic features are not sufficient to distinguish them. The views of the above taxonomists are all based on morphology, and in the Chinese taxa the taxonomists are equally sharply divided between these two schools of thought, and some taxonomists all agree that Libanotis should be retained rather than merged [1,2,3, 12, 17,18,19, 27]. By 2015, new Libanotis taxa (L. laoshanensis W.Zhou & Q.X.Liu) were still being published [10]. Pimenov set aside the retention of the taxonomic status of Libanotis for this species untreated in the 2017 treatment of the Chinese Apiaceae taxa [24]. The above indicates that a thorough phylogenetic analysis of Libanotis is necessary. Regrettably, there has been no prior phylogenetic investigation conducted concerning this contentious genus Libanotis. Furthermore, all phylogenetic analyses have consistently demonstrated that the Seseli genus, in its broader sense, is polyphyletic, owing to the complex and perplexing variations in mericarps and vegetative body morphology. [26, 28,29,30,31,32,33,34,35,36,37]. Recently, Seseli s.s. was established and several phylogenetic studies using molecular fragments (nrITS and nrETS) robustly supported that L. sibirica C. A. Mey. (type species of Libanotis, L. montana Crantz ≡ Seseli libanotis W.D.J.Koch = L. sibirica) did not cluster with Seseli tortuosum L. (type species of Seseli) into a monophyletic branch [32, 33]. Hence, we believe that the taxonomic status of Libanotis needs to be discussed again, especially in China. Nevertheless, the delimitation of Libanotis genus still faced severely challenge. All previous phylogenetic studies showed that Libanotis was not a monophyletic group and members of this genus scattered in the Selineae tribe [6, 7, 35, 37, 38]. Due to limited sample and molecular fragments contained few informative loci, these studies all generated the phylogenetic framework with weak support and low resolution, which was insufficient to aid to the taxonomic revision of Libanotis members. Hence, it is imperative to establish a more comprehensive phylogenetic framework for Libanotis to address the controversy surrounding its evolutionary relationships and taxonomic status.
Due to the large number of species under the genus Libanotis, many taxonomists have established sections under the genus. Among the opinions in favor of the independence of Libanotis, de Candolle [13] was the first to group them, arguing that Libanotis could be divided into two sections, Sect. Eriotis and Sect. Eulibanotis: in which Sect. Eriotis are “Petals covered with short fascicular hairs on the outside leaves coriaceous, thickish, shiny.”; and Sect. Eulibanotis are “Petals dorsally glabrous or with sparse simple short hairs, leaves not coriaceous, not shiny.” After a series of species transfers and the publication of new species, Schischkin [14] added two sections: Sect. Pseudolibanotis and Sect. Schultziopsis, Sect. Pseudolibanotis were described by “Main stem not developed, the root neck bearing slightly leafy, sometimes nearly leafless shoots which spread along ground or ascend.” And the trait of Sect. Schultziopsis are special, their subcapitate umbel surrounded by the rounded sheaths of terminal leaves. The four-sections system was widely accepted by taxonomists that supported the independence of Libanotis [5, 15]. L. monstrosa (Willd.) DC., the only species in Sect. Schultziopsis, has been used as the type species for the establishment of the new monotypic genus Sajanella Soják [39] and is therefore excluded from this study, while the other all three sections are included for phylogenetic analysis (Fig. 1, Table 1). Based on these section characteristics, the newly published species L. jinanensis [3] and the newly transferred species L. grubovii [19] could be included in Sect. Eriotis according to their descriptions (Table 1, Table S10).
Some flowering Libanotis species with diverse morphology. A, B- Sect. Pseudolibanotis (A) L. depressa R.H.Shan & M.L.Sheh (B) L. acaulis R.H.Shan & M.L.Sheh C, F, G, H, I- Sect. Eriotis (C) L. iliensis (Lipsky) Korovin (F) L. buchtormensis DC. (G) L. grubovii (V.M.Vinogr. & Sanchir) M.L.Sheh & M.F.Watson (H) L. lanzhouensis K.T.Fu ex R.H.Shan & M.L.Sheh (I) L. spodotrichoma K.T.Fu. D, E- Sect. Libanotis (D) L. seseloides (Fisch. & C.A. Mey. ex Ledeb.) Turcz. (E) L. sibirica C.A.Mey. Photograph by Liu Li-Jia
Additionally, many plants of Libanotis have important medicinal value and are used as traditional Chinese medicinal materials. For example, six Libanotis taxa (L. buchtormensis (Fisch.) DC., L. lancifolia K.T.Fu., L. spodotrichoma K.T.Fu., L. wannienchun K.T.Fu., L. lanzhouensis K.T.Fu ex R.H.Shan & M.L.Sheh, and L. sibirica) are all known as the "Changchun Seven" in the Qinling Seven medicines, which is used to treat common cold, toothache, headache, traumatic injury, inflammation, swelling, rheumatism, respiratory diseases, as well as symptomatic coughs and dyspnea [40,41,42]. However, due to morphological feature exhibiting highly similar in inter-species and significant divergence in intra-species, the accurate identification of Libanotis species was extremely difficult [40]. Due to their morphological similarity, instances of homonym or synonym in common names exist in various regions and markets, making it challenging to distinguish them during collection, acquisition, and clinical usage. They are often mistakenly interchanged. For example, the above 'Changchun Seven' consists of six different species and is divided into sixteen varieties in herbal medicine, causing confusion in the herbal market [40]. Therefore, the selection of reliable molecular markers for ensuring the accurate identification of medicinal Libanotis species is of utmost importance.
The plastome was highly conserved in flowering plant and harbored sufficient variable loci [43, 44]. Hence, plastome data have been widely used in phylogenetic analyses and development of special DNA barcode in Apiaceae, Poaceae, Lamiaceae, Rosaceae, Liliaceae, Allium, Artemisia, and other plant taxa [44,45,46,47,48,49,50,51,52]. Regrettably, despite the presence of two Libanotis plastomes in GenBank, there has been a lack of plastid phylogenomic analysis conducted for this genus. In this study, we filled this gap by sequencing the plastid genomes of twelve taxa of Libanotis. Together with two plastomes previously reported, we conducted comprehensive analyses to (1) reveal the plastid characteristics and evolution of Libanotis; (2) identify suitable mutation hotspots from plastomes to use as candidate barcodes for species identification of Libanotis; (3) investigate the genus boundary of Libanotis and provide new sights into the phylogenetic position of this genus taxa distributed in China.
Results
Plastome features of Libanotis and repeat sequence analyses
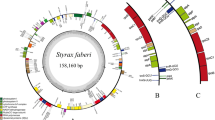
The complete plastomes newly sequenced of 12 Libanotis species have been fully characterized, with sizes ranging from 146,836 bp (L. sibirica) to 148,100 bp (L. depressa) (Table 2, Fig. 2). Compared to other Libanotis taxa, L. depressa was particularly unique, with a significantly expanded IR region of length 19,437 and the GC content of only 43.7%. The analysis of the twelve Libanotis plastomes revealed a collective inventory of 129 genes, including 84 PCGs, 36 tRNA genes, 8 rRNA genes, and one pseudogene (Table 2, Table S3, and Fig. 2). Of particular interest, the inversion of the trnY-trnD-trnE gene, previously observed in certain species of Angelica L. and Peucedanum L., was also detected in L. incana. [44, 53]. Additionally, these thirteen Libanotis plastomes exhibited no gene rearrangements or losses (Fig. 3). Additionally, a total of 1049 simple sequence repeats (SSRs) and 549 repeats belonging to four different types were identified (Fig. S1, Table S6, S7). Compared with other related taxa of Selineae, such as Seseli and Peucedanum, Libanotis was not much difference in the analysis of repeated sequences.
The gene map displays twelve newly sequenced Libanotis plastomes (L. sibirica was used as a representative). The inner circle's dark gray area indicates the GC content. Gene numbers and plastomes length are tagged inside. Genes outside the outer circle are transcribed clockwise, while inside are transcribed counterclockwise. Different gene functional groups are color-coded
Nucleotide diversity analyses and potential DNA barcodes
For these thirteen Libanotis, the nucleotide diversity (Pi) values for the protein-coding regions ranged from 3.58 × 10–4 (rps7 gene) to 0.01459 (ccsA gene), and the average value was 3.23 × 10–3 (Fig. 4, Table S8). The range of Pi values in non-coding regions and introns exhibits a considerable variation compared to coding regions. Among the protein-coding genes analyzed, only ccsA displayed a relatively high Pi value (> 0.01), whereas four other genes, namely matK, ycf2, ndhE, and ycf1, exhibited moderate levels of nucleotide diversity (0.007 < Pi < 0.01), making them viable alternatives for further investigation (Fig. 4A, Table S8). Furthermore, three non-coding regions and introns with high nucleotide diversity (Pi > 0 0.015) were identified: trnH-GUG-psbA, petA-psbJ, and ccsA-ndhD (Fig. 4B, Table S8). These eight highly variable regions (ccsA, matK, ycf2, ndhE, ycf1, trnH-GUG-psbA, petA-psbJ, and ccsA-ndhD) were selected as potential DNA barcodes.
Phylogenetic analyses
Seventy-nine single-copy plastome CDS from 57 plastomes were used to reconstruct the phylogeny of Libanotis (Fig. 5, Table S4). Our analyses robustly supported that the Libanotis taxa fell into one tribe (Selineae), and they were not clustered as a monophyletic group or divided into three sections but fell into seven groups (Subclades) (Fig. 5): (I) L. sibirica and L. schrenkiana clustered with Seseli glabratum Willd. ex Schult. (PP = 1.00, BS = 100); (II) L. buchtormensis and L. seseloides was sister to Saposhnikovia divaricata (Turcz.) Schischk. (PP = 0.99, BS = 84); (III) L. incana did not clustered with other Libanotis. However, within the phylogenetic analysis, L. incana and subclades I and II formed a robust clade with high support values (PP = 1.00, BS = 100). This clade indicated that L. incana diverged first from the rest of the taxa (PP = 1.00, BS = 99); (IV) L. iliensis, L. grubovii and L. acaulis formed a clade (PP = 1.00, BS = 100), clustered with I, II, III, and some Peucedanum species. (V) this clade contained L. jinanensis, L. lanzhouensis, L. spodotrichoma, and Seseli intramongolicum Y. C. Ma. (PP = 0.99, BS = 76); (VI) L. condensata was sister to Pachypleurum alpinum (PP = 1.00, BS = 95), and P. alpinum is type of Pachypleurum; (VII) L. depressa, along with other Ligusticopsis species, established a strong and clearly separated clade, displaying high support values (PP = 1.00, BS = 100), distinguishing it from the rest of the genus. In the nrDNA-based tree (Fig. 6, Table S5), that species in the subclades were clearly divergent except for Subclade V, which was better clustered into a single branch (PP = 1.00, BS = 100). L. sibirica and S. libanotis were clearly not clustered with Seseli s.s., S. tortuosum and some Seseli species were clustered with Kitagawia, Peucedanum, L. incana, and L. lancifolia, while L. sibirica, S. libanotis and some Libanotis were clustered with Stenocoelium popovii, and several Seseli species.
The plastome CDS-based phylogenetic tree constructed by Bayesian inference (BI) and maximum likelihood (ML) with the posterior probabilities (PP) of BI and the bootstrap values (BS) of ML above the branches. The topology of the tree is derived from the optimal tree of the maximum likelihood method, and the unaligned tree is labeled in the upper left corner. Respectively, (*) represents maximum support in both two analyses, (#) represents those nodes not occurring in the BI strict consensus tree. The red is the newly sequenced Libanotis in this study, and the orange is the Libanotis sequences downloaded from Genebank. Different subclades are colored differently. Details of the sections labeled with different symbols are shown in Table 1
The plastome nrDNA-based (ITS + ETS) phylogenetic tree constructed by Bayesian inference (BI) and maximum likelihood (ML) with the posterior probabilities (PP) of BI and the bootstrap values (BS) of ML above the branches. The topology of the tree is derived from the optimal tree of the bayesian inference method, and the unaligned tree is labeled in the upper left corner. (*) represents maximum support in both two analyses. Bolded are the sequences newly sequenced in this study, and the type species of Seseli and Libanotis are highlighted in red in the figure
In terms of morphological sections of CDS-based tree, only L. sibirica and L. schrenkiana (Subclade I) can be retained from the five species of the core group Sect. Libanotis, with the remaining three species each dispersed in three other branches (Subclade II, III, VI). The six species of Sect. Eriotis are also not monophyletic, with the exception of L. buchtormensis which is better concentrated in two branches (Subclade IV, V), and the two species of Sect. Pseudolibanotis are also separated, one clustered with Sect. Eriotis and one within the genus Ligusticopsis (Subclade IV, VII). On the nrDNA-based tree (Fig. 7), Sect. Libanotis except L. incana clustered together. It is noteworthy that these species of Libanotis sect. Eulibanotis included in Schischk [14] (Table 1) included in this tree (L. montana (≡ S. libanotis), L. sibirica, L. schrenkiana, L. condensata, L. seseloides, L. transcaucasica (≡ S. transcaucasicum)) clustered into a highly supported monophyletic clade (PP = 1.00, BS = 100). Sect. Eriotis apart from L. iliensis and L. lancifolia also clustered into a monophyletic clade (PP = 1.00, BS = 97), but within this clade were also included two narrowly-fielded Seseli species published in recent years. The two species of Sect. Pseudolibanotis are also separated.
Comparison of two trees constructed based on different datasets. The left one is a CDS-based phylogenetic tree, and the right one is an nrDNA-based (ITS + ETS) phylogenetic tree. The same species with different subclades in the two trees are connected to each other by a line representing the color of that subclade. Details of the sections labeled with different symbols are shown in Table 1
Comparative plastome analyses
The boundaries of these species were not too distinctly different or regular, either on the basis of phylogenetic subclades or on the basis of former taxonomic sections (Fig. S2). There is no doubt that the plastome structure of Libanotis is relatively conserved. The Relative Synonymous Codon Usage (RSCU) values across all codons exhibited a spectrum from 0.33 to 2.02, as depicted in Figure S3 and detailed in Table S9. Notably, L. depressa (Subclade VII) exhibited lower RSCU values for UGA termination codon (RSCU = 0.57), whereas L. condensata (Subclade VI) showed lower RSCU values for UAG termination codon (RSCU = 0.62) and higher values for UGA termination codon (RSCU = 0.74) compared to other subclades. The usage of specific codons within the remaining subclades, apart from the aforementioned individual subclades, shows no significant differences. (Fig. S3; Table S9).
The divergence analysis of thirteen Libanotis plastomes revealed that the coding regions exhibited higher conservation compared to the non-coding regions (Fig. 8). Compared with other taxa, L. schrenkiana was highly similar to the reference L. sibirica. Furthermore, the plastid divergence between Subclades I and II is relatively low, as is the divergence between IV and V, while the remaining three separate subclades exhibit distinct differences (Fig. 8). Interestingly, some subclades exhibit a certain degree of conservation when compared to the rest of the subclades, while others show significant differences. For instance, L. incana (Subclades III) displays significant distinctions from the rest of the sequences in the region from trnD-GUC to trnE-UUC, likely due to gene inversion, which aligns with the above analysis. It also exhibits noticeable differences from other sequences in the region from psbL-psbF-psbE.
mVISTA-based sequence identity plots for the thirteen plastomes with L. sibirica as the reference. The different colors and Roman numerals correspond to the different subclades separated by the plastome CDS-based phylogenetic tree in Fig. 5
Discussion
Comparison of the Libanotis plastomes and Potential DNA barcodes
In this study, we sequenced and assembled twelve plastomes of Libanotis and performed comprehensive comparative analyses of these plastomes with one other published plastomes of this genus obtained from GeneBank. All Libanotis plastomes exhibited the typical quadripartite structure with various features displaying similarity. And the genome length (146,836 BP- 148,100 bp), IR/ SC borders and gene numbers and arrangements (129) of each Subclades formed by Libanotis species were not significantly different. These results suggested that Libanotis plastomes were highly conserved between different subclades, while the coding regions were more conserved than the non-coding regions, the IR regions were more conserved than the single copy regions. Nevertheless, we identified eight mutation hotspot regions, each spanning over 200 bp, with elevated Pi values. These regions, including the matK gene, ycf2 gene, ccsA gene, ndhE gene, ycf1 gene, trnH-GUG-psbA, petA-psbJ, and ccsA-ndhD, were selected as potential DNA barcodes for the purposes of phylogenetic analysis and species identification within the Libanotis genus.
Phylogeny analyses and taxonomic inference
We have reconstructed the phylogenetic relationships using 13 Libanotis species plastomes sample (Fig. 5). This work provides a solid and high-resolution phylogenetic tree of Libanotis, revealing inconsistencies between molecular systematics and traditional taxonomic studies. According to the current research results, the genus Libanotis is obviously polyphyletic, and L. sibirica (type species) and L. schrenkiana (Subclade I) formed a monophyletic clade with strong supports. Meanwhile, the clade could be recognized by leaf segments ovate-rhombic or lanceolate, surfaces glaucous on the back of the leaves and sparsely puberulent; bracts absent or few, subulate to linear, small, easy to loss; bracteoles several, linear; petals abaxially glabrous; calyx teeth conspicuous, triangular-lanceolate; fruit ovoid-ellipsoid, dorsally compressed, densely pubescent when young, becoming sparsely puberulent or glabrous; ribs subequal, shortly keeled; vittae 1 in each furrow, 2 on commissure [1, 2]. As Pimenov argues [22], Libanotis s.s. and Seseli s.s. do not differ in fruit morphology up to the genus level, and their main morphological differences are in less commonly used morphologies (characteristics of bracteoles, bracts and leaf segments, stem branching, stem and petiole pubescent etc.). But the results of the phylogeny suggest that we cannot simply merge them because the monophyly of the genera is not supported, and we cannot rule out the effects of homoplasy or reversals. Just like the concept of cryptic species, Libanotis is in a sense a cryptic genus. Therefore, we propose to accept this genus in narrow sense, namely Libanotis s.s., and identify only two members for the time being. According to the type specimens and literature records, other possible members of Libanotis s.s. are Seseli junatovii V. M. Vinogradova and Seseli salsugineum A.Duran & Lyskov. However, due to the limited sampling and the lack of sufficient reliable morphological information, we would not make taxonomic treatments for now. The results of the comparison between nrDNA-based tree and the CDS-based tree (Fig. 7) showed that there was nucleoplasmic conflict in Libanotis, these may be due to incomplete lineage sorting and introgression. In these conflicts, we found that species with similar leaf morphology tended to cluster more in the nrDNA-based tree: in the branch where L. sibirica located, the four Libanotis species (L. sibirica, L. schrenkiana, L. seseloides, and L. condensata) all have green, thin, papery leaf blades, and leaf abaxial surfaces sometimes gray-green; whereas the eight Libanotis species (L. abolinii, L. grubovii, L. buchtormensis, L. laticalycina, L. spodotrichoma, L. wannienchun, L. jinanensis, and L. lanzhouensis) in the branch beneath them have leathery to fleshy leaf blades, the leaf blades mostly blue-green or gray-green overall. The rest of the dispersed species have distinctive vegetative body morphology. Meanwhile, species with similar morphology of mericarps tended to cluster together (such as subclades I and V) in the CDS-based tree (Table S10).
When considering the outcomes of morphological sections within the phylogenetic tree, it becomes evident that the alignment is less than ideal. None of the three sections of taxa appear to be monophyletic. This situation is not unique among Apiaceae family. The Apiaceae, located on the upper echelons of angiosperms, signify a taxon along the path of divergence, and belong to the most complicated families of flowering plants, also in terms of species identification [54,55,56]. The reliability of diagnostic features between and within genera may be affected by homoplasy and reversals, and that traditional printed dichotomous keys in large “Floras” are far from satisfying. Recent times have witnessed a rapid reconfiguration of species within the tribe Selineae, marked by the revision of established genera and the independence of new ones [57,58,59]. In our assessment, we propose that all thirteen species in this study, except L. sibirica and L. schrenkiana, should be transferred and revised, but not transferred to Seseli to further confuse the polygenetic genus. It is worth noting that Peucedanum, Saposhnikovia and Kitagawia, which are close relatives of Libanotis and Seseli, also suffer from the problems mentioned above. Comprehensive sampling of Seseli and Peucedanum, two the world-wide complex genera with a mass of species, will be crucial to the taxonomic system of Libanotis and the entire tribe Selineae.
Except for them, the members of Libanotis were scattered among the branches, and the phylogenetic positions of L. condensata and L. depressa are particularly noteworthy. L. condensata (Subclade VI) and Pachypleurum alpinum Ledeb. (Type species of Pachypleurum) clustered together. Their morphology is similar in vegetative body which both have solitary stem with branched above or simple, hollow, glabrous, and striate, base densely clothed with fibrous leaf remains, and oblong leaf blade, but quite different in mericarps especially ribs all winged, subequal in Pachypleurum. Thus, L. condensata may be more closely related to Pachypleurum than Seseli s.s. or Libanotis s.s., but its transfer to Pachypleurum seems inappropriate unless the definition of Pachypleurum is reconstructed. Other molecular evidence [7] also supports the view that L. condensata does not belong to Libanotis. In nrDNA sequence (ITS) phylogenetic results [7], L. condensata is obviously separated from above genera, and is located in Pilopleura Schischk. While our nrDNA tree (ITS + ETS) showed that L. condensata was inserted into Libanotis s.s.. Unfortunately, due to the chloroplast genome and ETS sequence of Pilopleura absented, we could not confirm the relationship between Pilopleura and L. condensata. L. depressa clustered with Ligusticopsis species. However, we found L. depressa develops few bracts, lanceolate and very unequal bracteoles, and mericarps with few vittae in the furrow (1) and commissure (2), not strongly compressed and marginal ribs not winged, which are distinguishable from Ligusticopsis. Consequently, L. depressa should be treated as an independent taxon distinct from Ligusticopsis or Libanotis. Pimenov [24] argued that L. depressa should be transferred to Stenocoelium. Our results showed that Stenocoelium popovii clustered with some Seseli species and was far away from L. depressa. L. depressa also does not conform to the unique mericarp morphology of Stenocoelium that ribs are thick-obtuse, very prominent, irregularly denticulate especially along ribs and furrows are narrow. Due to conflicting and partial lack of morphological data sampling, we will detailedly discuss its taxonomic status in future research. The other species of Libanotis (Subclade II, III, IV, V) were clustered into some relatively single clades: L. incana (Subclade III) was alone; L. seseloides and L. buchtormensis were gathered in one branch (Subclade II) and then sister to Saposhnikovia, but the shape of mericarps and the numbers of vittae are quite different among them; L. acaulis and L. grubovii, L. iliensis clustered together (Subclade IV); L. lanzhouensis, L. jinanensis, and L. spodotrichoma formed a clade (Subclade V). Compared with Libanotis s.s., they belong to Sect. Eriotis or Sect. Pseudolibanotis, and petals are densely coated with soft hairs or stem not developed, which is easy to distinguish. In conclusion, our results showed that Libanotis s.s. has a need to be retained, but other eleven species that thought to be attributed to Libanotis should be transferred out Libanotis genus but their taxonomic status needs to be further studied by adding more species.
Conclusion
This study marks the inaugural endeavor to conduct a comprehensive exploration of plastome characteristics and to deduce the phylogeny of the Libanotis genus, encompassing a total of thirteen Libanotis species. In the course of this investigation, we conducted the fresh sequencing, assembly, and annotation of complete plastomes for twelve Libanotis species along with two closely related taxa. These results suggested that Libanotis plastomes were conserved between different subclades, while the coding regions were more conserved than the non-coding regions, and the IR regions were more conserved than the single copy regions. Nevertheless, eight mutation hotspot regions (matK gene, ycf2 gene, ccsA gene, ndhE gene, ycf1 gene, trnH-GUG -psbA, petA-psbJ, ccsA-ndhD) longer than 200 bp with high Pi values were chosen as potential DNA barcodes for the purpose of both phylogenetic investigation and species identification used in materia medica of Libanotis. 78 common single-copy CDS from fifty-seven plastomes sequences and 144 nrDNA (72 ETS + 72 ITS) sequences were used to perform the phylogenetic analysis of Libanotis. Plastid phylogenomic analyses confirmed the efficacy of plastome data in enhancing the support and resolution of Libanotis phylogeny, firmly showing that Libanotis belong to Selineae and not a monophyletic genus, and the species within the sections in the original morphological framework are also polyphyletic. We finished the delimitation of Libanotis by establishing Libanotis s.s. and provided some taxonomic suggestions for other species in the genus, especially L. depressa and L. condensata. In short, our study can provide new insights into the plastome evolution of Libanotis and promoted the improvement of taxonomic system for Aipaceae family.
Methods
Taxon sampling and DNA sequencing
A total of 57 plastomes from 56 taxa and 144 nrDNA sequences (72 ITS + 72 ETS) from 67 taxa were used in this study, of which 48 plastomes and 96 nrDNA originated from us (Table S4, S5). We collected fresh and fully developed leaves from twelve different Libanotis species, which included the type species L. sibirica (L.) C.A.Mey. and then dried with silica gel (Table S1). These sections of Libanotis species reference FRPS [2], including all three sections (one previous section has been used to create the new monotypic genus Sajanella) to establish a more complete phylogenetic framework (Table 1, Fig. 1). Additionally, we expanded our sampling efforts for Pachypleurum alpinum Ledeb. and Stenocoelium popovii V.M.Vinogr. & Fedor., based on prior experimentation and taxonomic studies [24, 60]. In addition to the 14 plastomes, we newly measured 24 ETSs as well as 24 ITSs containing 17 species of Libanotis and two closely related species (Table S2). The formal identifications of all collected samples were identified by Liu Li-Jia and Professor He Xing-Jin from Sichuan University. Specimens vouchering the mentioned taxa were stored in the herbarium of Sichuan University (SZ) and the herbarium of the Kunming Institute of Botany (KUN), and the details of these vouchers can be found in Table S1 and S2. In order to distinguish them from other genera, all the scientific names of Libanotis species and sections in this study were based on the taxonomic treatment of FOC and IPNI [1, 61], but the scientific names in Table 1 followed the authors' original records. The newly published species L. jinanensis and the newly transferred species L. grubovii are included in Sect. Eriotis according to their morphologic descriptions (Table 1, Table S10).
We began by extracting total DNA from approximately 20 ~ 30 mg of silica gel-dried leaves using the CTAB (Cetyl trimethylammonium bromide) method [62]. We conducted Polymerase chain reactions (PCRs) to amplify ITS (Internal Transcribed Spacer) and ETS (External Transcribed Spacer) sequences using the following primers: ITS-4, ITS-5 [63], 18S-ETS [64], and Umb-ETS [65]. Each PCR reaction had a 30 µL volume with 2 µL plant DNA, 1.5 µL forward primer,1.5 µL reverse primer, 15 µL of 2 × Taq MasterMix (cwbio, Beijing, China), and 10 µL of ddH2O. We used Geneious v2023.0.4 [66] for sequence editing and assembly. The newly acquired sequences have been officially submitted in GenBank (accession numbers in Table S2). For plastomes, we fragmented the genomic DNA into 150 bp fragments to create a pair-end library, adhering to the manufacturer's instructions provided by Illumina in San Diego, CA, USA. The sequencing of these libraries took place on the Illumina NovaSeq platform at Personalbio in Shanghai, China. We applied fastP v0.15.0 [67] to filter the raw data, and these high-quality reads were then assembled for the whole plastomes using GetOrganelle v1.7.7.0 [68].
Genomic annotation and feature analyses
We utilized the Plastid Genome Annotator (PGA) [69] for the annotation of plastomes, employing L. buchtormensis (MZ707534) and L. spodotrichoma (MZ707535) as our reference sequences. Subsequently, we performed manual refinements using Geneious v2023.0.4 [66]. The newly acquired plastome sequences for the twelve Libanotis taxa, along with two additional sequences, have been officially submitted in GenBank (accession numbers in Table S1). To visualize the circular plastome maps for the twelve newly sequenced Libanotis taxa, we employed the online tool Organellar Genome DRAW (OGDRAW) [70]. Furthermore, we identified gene rearrangements among the thirteen Libanotis taxa including one previously published sequence, using Mauve Alignment [71] within Geneious v2023.0.4 [66].
Repeat sequence and nucleotide diversity analyses
We employed the online REPuter program [72] to identify repeat sequences in the plastomes of the thirteen Libanotis taxa and the parameters used for this analysis referred to Cai et al. [33]. Furthermore, we utilized the Perl script MISA [73], available at http://pgrc.ipk-gatersleben.de/misa/sleben.de/misa/, to detect simple sequence repeats (SSRs) within the plastomes of the thirteen Libanotis taxa. For the assessment of nucleotide diversity (Pi) within protein-coding genes, noncoding regions, and introns, we turned to DnaSP version 6.12.03 [74]. This analysis aimed to pinpoint regions with elevated mutation rates, potentially serving as valuable molecular markers for future research. Regions meeting or exceeding a length of 200 base pairs were singled out for this purpose, as described previously [33].
Sequences selection and alignment
In accordance with initial experiments and prior taxonomic assessments [24, 60], we carefully curated two dataset consisting of 57 complete plastomes derived from 56 taxa and 144 nrDNA (72 ITS + 72 ETS) from 67 taxa for the purpose of constructing phylogenetic trees. Notably, 13 plastomes of these sequences, which include 11 Libanotis species (including the type species L. sibirica) and two related taxa, Stenocoelium popovii and Pachypleurum alpinum, were being introduced for the first time into our analysis. And among these nrDNAs, all ETSs of 17 Libanotis and Stenocoelium popovii were sequenced for the first time. In recognition of the intricate relationship between Seseli and Libanotis, we incorporated Seseli into our study. To establish the root of our phylogenetic tree, we selected three species from the Tordylieae tribe: Heracleum moellendorffii Hance, Heracleum yungningense Hand.-Mazz., and Semenovia transiliensis Regel & Herder, as recommended by Wen et al. [75]. Our main clade designations were based on the contributions of Downie et al. [76] and Wen et al. [75]. We further assembled a dataset comprising 78 common single-copy coding sequences (CDSs) extracted from the 57 complete plastomes. This dataset was concatenated using PhyloSuite v1.2.2 [77]. To ensure accuracy, we aligned the sequences using MAFFT v7.221 [78] and performed inspection and manual refinements with the assistance of MEGA7 [79]. It's worth noting that all sequences data utilized in our phylogenetic analyses are readily accessible in GenBank (Table S4, S5).
Phylogenetic analyses
To elucidate phylogenetic relationships, we employed both maximum likelihood (ML) and Bayesian inference (BI) methods. For ML analyses, we utilized RAxML v8.2.10 [80] with the GTRGAMMA model, accompanied by 1000 rapid bootstrap replicates to assess node support. In the case of BI analyses, we first determined the best-fitting substitution model using MrModeltest v2.4 [81]. Subsequently, we conducted Bayesian inference with MrBayes v3.2.7 [82], employing the selected GTR + I + G parameters. The parameter settings for the BI analysis refer to previous research about Apiaceae [33, 83, 84]. Finally, we visualized and edited the resulting phylogenetic trees using FigTree v1.4 [85].
Comparative analyses of plastomes
We visualized the variations in size between the inverted repeat (IR) border regions in the plastomes of the thirteen Libanotis species using IRscope [86]. Any necessary manual adjustments were made to ensure accuracy. Subsequently, we conducted a sequence divergence analysis of these thirteen plastomes, using mVISTA [87] in Shuffle-LAGAN mode, with L. sibirica serving as the reference species. For codon usage analysis, we employed codonW [88]. To reduce the impact of sampling bias [63, 89], we selected 53 coding sequences (CDSs) from the thirteen plastomes, excluding CDSs shorter than 300 base pairs and repetitive sequences. These selected CDSs were then concatenated using PhyloSuite v1.2.2 [77]. To visualize the relative synonymous codon usage (RSCU) [90] values across the thirteen plastomes, we utilized TBtools [91].
Availability of data and materials
The fourteen newly sequenced plastomes have been submitted into NCBI with accession numbers: OR529367- OR529372, OR529374- OR529379, PP078851 and OQ685947, and details of the 48 newly sequenced ETS and ITS sequences are attached.
Abbreviations
- BI:
-
Bayesian inference
- bp:
-
Base pair
- BS:
-
Bootstrap value
- CDS:
-
Coding sequences
- CTAB:
-
Cetyl trimethylammonium bromide
- ETS:
-
External transcribed Spacer
- FRPS:
-
Flora Republicae Popularis Sinicae
- FOC:
-
Flora of China
- GC:
-
Guanine-cytosine
- IR:
-
Inverted repeat
- ITS:
-
Internal transcribed spacer
- KUN:
-
Herbarium, Kunming Institute of Botany, Chinese Academy of Sciences
- LSC:
-
Large single copy
- ML:
-
Maximum Likelihood
- PCGs:
-
Protein-coding genes
- PCR:
-
Polymerase chain reaction
- Pi:
-
Nucleotide diversity
- PP:
-
Posterior probability
- rRNA:
-
Ribosomal RNA
- RSCU:
-
Relative synonymous codon usage
- s.s.:
-
Sensu stricto
- SSC:
-
Small single copy
- SSR:
-
Simple sequence repeat
- SZ:
-
Herbarium, College of Life Sciences, Sichuan University
- tRNA:
-
Transfer RNA
References
Sheh Ml, Pimenov MG, Kljuykov EV, Watson MF. LIBANOTIS Haller ex Zinn, Cat. Pl. Hort. Gott. 226. 1757, nom. cons., not Hill (1756). In: Flora of China Edited by Wu ZY, Raven PH, Hong DY, vol. 14. Beijing: Science Press & St. Louis: Missouri Botanic Garden Press; 2005.
Shan RH, Sheh MI. In: Libanotis Hill. In: Flora Republicae Popularis Sinicae Edited by Shan R-h, Sheh M-l, vol. 55. Beijing: Science Press; 1985. p. 160–81.
Xu L-c, Xu M-d. A New species of Libanotis from Shan Dong. Bull Bot Res. 1989;9(1):37–9.
Sprague TA. Generic names published in Zinn’s Catalogus. Bull Miscellaneous Inform (Royal Botanic Gardens Kew). 1934;1934(5):217–9.
Tamamschian SG. On the nomenclature history of the Genus Libanotis. Taxon. 1960;9(7):210–2.
Zhou J, Gong X, Downie SR, Peng H. Towards a more robust molecular phylogeny of Chinese Apiaceae subfamily Apioideae: additional evidence from nrDNA ITS and cpDNA intron (rpl16 and rps16) sequences. Mol Phylogenet Evol. 2009;53(1):56–68.
Zhou J, Gao Y-z, Wei J, Liu Z-W, Downie SR. Molecular phylogenetics of Ligusticum (Apiaceae) based on nrDNA ITS sequences: Rampant Polyphyly, Placement of the Chinese endemic species, and a much-reduced circumscription of the Genus. Int J Plant Sci. 2020;181(3):306–23.
Stephan R. 682) Proposal zur Konservierung von post 6052 Libanotis Haller ex Zinn, 1757, vs. Libanotis Hill, 1756, und Dela Adanson, 1763 (Umbelliferae. Taxon. 1982;31(4):755–6.
Jarvis CE, Knees SG. Linnaean names in the Genus Athamanta L. (Umbelliferae: Apioideae) and their typification. Taxon. 1988;37(2):472–7.
Zhou W, Liu Q, Song C, Wu B. Libanotis laoshanensis (Apiaceae), a new species in China. J Plant Resour Environ. 2015;24(3):107–8.
Shan R-H, Sheh M-L, Yuan C-C, Pu F-T. New Taxa of the Chinese Umbelliferae (I). Acta Phytotaxonomica Sinica. 1983;21(1):79–88.
Ma C-L, Wang X-Z. Studies on morphology of some species of Libanotis. J Changwei Teachers Coll. 1999;18(2):18–20.
de Candolle AP. Prodromus Systematis Naturalis Regni Vegetabilis, sive, enumeratio contracta ordinum generum specierumque plantarum huc usque cognitarium, juxta methodi naturalis, normas digesta. Volume 4. Parisii: Sumptibus Sociorum Treuttel et Würtz; 1830.
Schischkin BK. Genus 1023. Libanotis L. In: Flora URSS (Flora Unionis Rerumpublicarum Sovieticarum Socialisticarum) Edited by Schischkin BK, vol. 6. Moscow: Leningrad: Academy of Sciences; 1950. p. 471.
Korovin EP. Flora Kazakhstana. Volume 6. Alma-Ata: A Kazakhsk SSR Press; 1963.
Rechinger KH. . In: Rechinger KH, editor. Libanotis. In: Flora Iranica. Wien: Naturhistorisches Museum Wien; 1987. p. 16:2351-355.
Fu KT. 24. Libanotis Crantz. In: Flora Tsinlingensis Edited by sinicae IBB-Oa. Beijing: Science Press; 1981;1. p. 408–412.
Fu K-T. On the Genus Libanotis Crantz. From Tsingling Range. Acta Phytotaxonomica Sinica. 1975;13(2):57–61.
Watson MF, Sheh ML, Pu FD, Pan ZH. Nomenclatural novelties in the Apiaceae (Umbelliferae) for the Flora of China. Acta Phytotaxonomica Sinica. 2004;42:561–5.
Drude CGO. Naturlichen Pflanzenfamilien. Volume 3, 8 ed. Leipzig: Wilhelm Engelmann; 1898.
Ball PW. Seseli L. (incl. Libanotis Hill). In: Flora Europaea Edited by Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DW, Walters SM, Webb DA. Cambridge: Cambridge University Press; 1968;2. p. 334–338.
Pimenov MG, Sdobnina L. On the taxonomy of the genus Seseli L. I. Revision of the genus Libanotis Hill (Umbelliferae). Botanicheskii Zhurnal. 1975;60(8):1108–22.
Pimenov MG, Kljuykov EV. New nomenclatural combinations for Chinese Umbelliferae. Feddes Repertorium. 1999;110:481–91.
Pimenov MG. Updated checklist of Chinese Umbelliferae: nomenclature, synonymy, typification, distribution. Turczaninowia. 2017;20(2):106–239.
Pimenov MG. De generis Seseli L. notulae systematicae. II. Adumbratio Specierum Florae URSS. Novosti Sist Vyssh Rast. 1978;15:188–200.
Pimenov MG. The identity of Himalayan Seseli sibiricum (Umbelliferae). Kew Bull. 1993;48(4):781–5.
Pimenov MG, Kljuykov EV. Floristic novelties in the Umbelliferae of Xinjiang, China. Acta Phytotaxonomica Sinica. 2001;39(3):193–202.
Pimenov MG. The identity of Ligusticum Thomsonii C. B. Clarke (Umbelliferae). Kew Bull. 1995;50(2):413–5.
Pimenov MG. Inclusion of Eriocycla into Seseli (Umbelliferae) and description of some new sections and subsections within the genus Seseli. Botanicheskii Zhurnal. 2000;85(10):96–109.
Degtjareva GV, Valiejoroman CM, Pimenov MG. Preliminary results of Seseli (Umbelliferae-Apioideae-Apieae) molecular taxonomic analysis, based on nrDNA ITS sequence variation, vol. 1. In Proceedings of the 7th International Apiales Symposium; 2 August 2011. Sydney: The National Herbarium of New South Wales, The Royal Botanic Garden Trust; 2011. p. 2–2.
Dogan Guner E, Duman H. The revision of genus Seseli (Umbelliferae) in Turkey. Turkish J Bot. 2013;37:1018–37.
Pimenov M, Degtjareva G, Ostroumova T, Samigullin T, Zakharova E. What is Seseli diffusum? A comparative morphological and molecular appraisal of a critical species of the Umbelliferae. Plant Syst Evol. 2019;305(1):49–59.
Cai J, Qin HH, Lei JQ, Liu CK, He XJ, Zhou SD. The phylogeny of Seseli (Apiaceae, Apioideae): insights from molecular and morphological data. BMC Plant Biol. 2022;22(1):534.
Lyskov D, Degtjareva G, Zarre S, Terentieva E, Samigullin T. Neither Seseli nor Eriocycla: a new Iranian relict genus Shomalia (Apiaceae), related to Azilia. Plant Syst Evol 2022;308(3):21.
Downie SR, Watson MF, Spalik K, Katz-Downie DS. Molecular systematics of Old World Apioideae (Apiaceae): relationships among some members of tribe Peucedaneae Sensu Lato, the placement of several island-endemic species, and resolution within the apioid superclade. Can J Bot. 2000;78(4):506–28.
Spalik K, Reduron JP, Downie SR. The phylogenetic position of Peucedanum Sensu Lato and allied genera and their placement in tribe Selineae (Apiaceae, subfamily Apioideae). Plant Syst Evol. 2004;243(3–4):189–210.
Valiejo-Roman CM, Shneyer VS, Samigullin TH, Terentieva EI, Pimenov MG. An attempt to clarify taxonomic relationships in “Verwandtschaftskreis der Gattung Ligusticum” (Umbelliferae-Apioideae) by molecular analysis. Plant Syst Evol. 2006;257(1–2):25–43.
Choi H-K, Kim C, Shin H. Molecular reexamination of Korean umbelliferae based on internal transcribed spacer sequences of rDNA:Ligusticum tenuissimum (Nakai) Kitagawa andLibanotis coreana (Wolff) kitagawa. J Plant Biology. 2000;43(3):128–35.
Soják J. Sajanella Soják. Časopis Národního Muzea v Praze Rada Přírodovědna. 1980;148(3–4):209.
Shuilong M, Weige M. Study on Easy Identification of 16 Kinds of Confusing Chinese Medicine for Common Use in TaibaiMountain of Qinling and Expanding Medicinal Parts of Libanotis buchtormensis (Fisch.) DC. In: The 10th National Symposium on Natural Medicnal Material Resources Proceedings and Abstracts: 2012-08-14; Gan Su, China. 2012. p. 373–9.
Shi K, Xie Q, Meng Q. Survey and evaluation of Medicinal plants of the Umbelliferae Family in the Northern Slopes of Qinling Mountains, Shaanxi. Shaanxi J Traditional Chin Med. 2015;36(2):226–8.
Li Y-l, Liu J, Fan W-d. Ex situ conservation of Libanotis spodotrichoma in Shaanxi. Shaanxi for Sci Technol. 2019;47(6):108–10.
Wicke S, Schneeweiss GM, de Pamphilis CW, Muller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76(3–5):273–97.
Liu C-K, Lei J-Q, Jiang Q-P, Zhou S-D, He X-J. The complete plastomes of seven Peucedanum plants: comparative and phylogenetic analyses for the Peucedanum Genus. BMC Plant Biol. 2022;22(1):101.
Zhang SD, Jin JJ, Chen SY, Chase MW, Soltis DE, Li HT, Yang JB, Li DZ, Yi TS. Diversification of Rosaceae since the late cretaceous based on plastid phylogenomics. New Phytol. 2017;214(3):1355–67.
Saarela JM, Burke SV, Wysocki WP, Barrett MD, Clark LG, Craine JM, Peterson PM, Soreng RJ, Vorontsova MS, Duvall MR. A 250 plastome phylogeny of the grass family (Poaceae): topological support under different data partitions. PeerJ. 2018;6:e4299.
Zhao F, Chen YP, Salmaki Y, Drew BT, Wilson TC, Scheen AC, Celep F, Brauchler C, Bendiksby M, Wang Q, et al. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol. 2021;19(1):2.
Jin G, Li W, Song F, Yang L, Wen Z, Feng Y. Comparative analysis of complete Artemisia subgenus Seriphidium (Asteraceae: Anthemideae) chloroplast genomes: insights into structural divergence and phylogenetic relationships. BMC Plant Biol. 2023;23(1):136.
Li J, Cai J, Qin HH, Price M, Zhang Z, Yu Y, Xie DF, He XJ, Zhou SD, Gao XF. Phylogeny, Age, and evolution of Tribe Lilieae (Liliaceae) based on whole plastid genomes. Front Plant Sci. 2022;12:699226.
Peng C, Guo X-L, Zhou S-D, He X-J. Backbone phylogeny and adaptive evolution of Pleurospermum s. l.: new insights from phylogenomic analyses of complete plastome data. Front Plant Sci. 2023;14:1148303.
Lei JQ, Liu CK, Cai J, Price M, Zhou SD, He XJ. Evidence from Phylogenomics and Morphology Provide Insights into the Phylogeny, Plastome Evolution, and Taxonomy of Kitagawia. Plants. 2022;11(23):3275.
Li Z-X, Guo X-L, Price M, Zhou S-D, He X-J. Phylogenetic position of Ligusticopsis (Apiaceae, Apioideae): evidence from molecular data and carpological characters. AOB Plants. 2022;14(2):plac008.
Wang M, Wang X, Sun J, Wang Y, Ge Y, Dong W, Yuan Q, Huang L. Phylogenomic and evolutionary dynamics of inverted repeats across Angelica Plastomes. BMC Plant Biol. 2021;21(1):26.
Pimenov MG, Leonov MV, Ostroumova TA. Taxonomic and phytogeograpical databases in systematics of the flowering plant family Umbelliferae/Apiaceae. In: 1st International Conference on Information Technologies in the Research of Biodiversity: 2019 Sep 11–14 2018. Irkutsk. 2019. p. 28–36.
Baczyński J, Miłobędzka A, Banasiak Ł. Morphology of pollen in Apiales (Asterids, Eudicots). Phytotaxa. 2021;478(1):1–32.
Kljuykov E, Zakharova E, Ostroumova T, Tilney P. Most important carpological anatomical characters in the taxonomy of Apiaceae. Bot J Linn Soc. 2020;195(3):532–44.
Wen J, Yu Y, Xie D-F, Peng C, Liu Q, Zhou S-D, He X-J. A transcriptome-based study on the phylogeny and evolution of the taxonomically controversial subfamily Apioideae (Apiaceae). Ann Botany. 2020;125(6):937–53.
Clarkson JJ, Zuntini AR, Maurin O, Downie SR, Plunkett GM, Nicolas AN, Smith JF, Feist MAE, Gutierrez K, Malakasi P, et al. A higher-level nuclear phylogenomic study of the carrot family (Apiaceae). Am J Bot. 2021;108(7):1252–69.
Danderson CA, Downie SR, Hermann M. Rampant polyphyly in the Arracacia clade (Apiaceae) and an assessment of the phylogenetic utility of 20 noncoding plastid loci. Mol Phylogenet Evol. 2018;118:286–305.
Korovin EP. Pachypleurum condensatum (L.) Korovin. In: Flora Kazakhstana Edited by Pavlov NV. Alma-Ata: A Kazakhsk SSR Press; 1963: 310.
The Royal Botanic Gardens. K, Harvard University Herbaria & Libraries and Australian National Herbarium: International Plant Names Index 2023. https://www.ipni.org/.
Doyle JJ, Doyle JL, Doyle JA, Doyle FJ. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. 1987.
White T, Bruns T, Lee S, Taylor J, Innis M, Gelfand D, Sninsky J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: PCR Protocols: A Guide to Methods and Applications Edited by Innis MA, Gelfand DH, Sninsky JJ, White TJ. New York: Academic Press; 1990;31:315–322.
Baldwin BG, Markos S. Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Mol Phylogenet Evol. 1998;10(3):449–63.
Logacheva MD, Valiejo-Roman CM, Degtjareva GV, Stratton JM, Downie SR, Samigullin TH, Pimenov MG. A comparison of nrDNA ITS and ETS loci for phylogenetic inference in the Umbelliferae: an example from tribe Tordylieae. Mol Phylogenet Evol. 2010;57(1):471–6.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9.
Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884-890.
Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21(1):241.
Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50.
Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007;52(5–6):267–74.
Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–403.
Kurtz S, Choudhuri J, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the Manifold Applications of Repeat Analysis on a genomic scale. Nucleic Acids Res. 2001;29:4633–42.
Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33(16):2583–5.
Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sanchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34(12):3299–302.
Wen J, Xie D-F, Price M, Ren T, Deng Y-Q, Gui L-J, Guo X-L, He X-J. Backbone phylogeny and evolution of Apioideae (Apiaceae): new insights from phylogenomic analyses of plastome data. Mol Phylogenet Evol. 2021;161:107183.
Downie SR, Spalik K, Katz-Downie DS, Reduron J-P. Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Divers Evol. 2010;128(1–2):111–36.
Zhang D, Gao F, Jakovlic I, Zou H, Zhang J, Li WX, Wang GT. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2020;20(1):348–55.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3.
Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–8.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42.
Deng J-J, Peng C, Liu C-K, Xie D-F, Gui L-J, Zhou S-D, He X-J. Cortiella yatungense, a new species of Cortiella (Apiaceae) from Xizang, China. Phytotaxa. 2022;566(2):189–99.
Xu XR, Guo XL, Price M, He XJ, Zhou SD. New insights into the phylogeny and taxonomy of Chinese physospermopsis (Apiaceae). PhytoKeys. 2021;175:67–88.
Rambaut A, Drummond A. FigTree v.1.4.4. 2018. https://tree.bio.ed.ac.uk/software/figtree/. Accessed 9 Feb 2023.
Amiryousefi A, Hyvonen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34(17):3030–1.
Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32(Web Server issue):W273-279.
Peden JF. Analysis of codon usage. Nottingham: University of Nottingham; 2000. p. 73–4.
Yang Y, Zhu J, Feng L, Zhou T, Bai G, Yang J, Zhao G. Plastid Genome comparative and phylogenetic analyses of the Key Genera in Fagaceae: highlighting the Effect of Codon Composition Bias in phylogenetic inference. Front Plant Sci. 2018;9: 82.
Sharp PM, Li W-H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24(1):28–38.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative Toolkit developed for interactive analyses of big Biological Data. Mol Plant. 2020;13(8):1194–202.
Acknowledgements
We are grateful to Zhou Xin-Xin, Peng Chang, Lei Jia-Qing, Qin Huan-Huan for their help in samples collection. We thank Li Wen-Jun of XJBI Herbarium for providing consultation on the Libanotis specimens from Xinjiang Province, and we thank Mariya Sheludyakova of LE Herbarium and Rachel Webster of MANCH Herbarium for providing information on the type specimens.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32070221, 32170209), and Survey on the Background Resources of Chengdu Area of Giant Panda National Park (Project No. 510101202200376). The funders were not involved in the design of the research, col‑ lection, analysis and interpretation of data, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
S-DZ and X-JH designed the research. L-JL, J-JD, and JC collected and analyzed the data; L-JL and C-KL prepared the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All samples collected fully adhere to national and local legal requirements. The plant samples used in the study were neither listed as nationally protected nor gathered from national parks or natural reserves. No specific permissions were necessary for their collection according to national and local laws.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Analyses of repeats in the thirteen Libanotis plastomes. (A, B) Total number of SSRs,(C) Total number of four repeat types.See Table S6, S7 for specific values. Fig. S2. Comparing LSC, SSC, and IR region boundaries among the thirteen Libanotis plastomes, with gene positions indicated by different boxes. Fig.S3. The relative synonymous codon usage (RSCU) values of 53 CDSs for 13Libanotis plastomes. (*) to denote the terminator codons. See Table S9 for specific values. Table S1. The newly sequenced plastomes in the present study with taxa, source, voucher and GenBank accession numbers. Table S2. The newly sequenced nrDNA in the present study with taxa, source, voucher and GenBank accession numbers. Table S3. List of unique genes identified in plastomes of twelve Libanotis newly sequenced. Table S4. Plastomes included in phylogenetic analyses with GenBank accession and length. Bolded are newly sequenced sequences. (*) to denote the sequences from us. Table S5. nrDNA (ITS and ETS) included in phylogenetic analyses with GenBank accession. Bolded are newly sequenced sequences. (*) to denote the sequences from us. Table S6. Simple sequence repeats (SSRs) distribution in the thirteen Libanotis plastomes. These data were visualized in Figure S1. Table S7. The repeat sequences distribution in the thirteen Libanotis plastomes. These data were visualized in Figure S1. Table S8. Nucleotide diversity (Pi) values of thirteen Libanotis, while coding and non-coding regions were listed on the left and right, respectively. These data were visualized in Figure 4. Table S9. Codon usage and relative synonymous codon usage (RSCU) values of protein-coding genes of the thirteen plastomes. These data were visualized in Figure S3. Table S10. The morphological comparision of different Libanotis in this study. Data based on FOC (2005), JSTOR, CVH and sampled specimens.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, LJ., Liu, CK., Cai, J. et al. The complete plastomes of thirteen Libanotis (Apiaceae, Apioideae) plants: comparative and phylogenetic analyses provide insights into the plastome evolution and taxonomy of Libanotis. BMC Plant Biol 24, 106 (2024). https://doi.org/10.1186/s12870-024-04784-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04784-4