Abstract
Background
Pectin methylesterase (PME) is a hydrolytic enzyme that catalyzes the demethylesterification of homogalacturonans and controls pectin reconstruction, being essential in regulation of cell wall modification. During fruit ripening stage, PME-mediated cell wall remodeling is an important process to determine fruit firmness and softening. Strawberry fruit is a soft fruit with a short postharvest life, due to a rapid loss of firm texture. Hence, preharvest improvement of strawberry fruit rigidity is a prerequisite for extension of fruit refreshing time. Although PME has been well characterized in model plants, knowledge regarding the functionality and evolutionary property of PME gene family in strawberry remain limited.
Results
A total of 54 PME genes (FvPMEs) were identified in woodland strawberry (Fragaria vesca ‘Hawaii 4’). Phylogeny and gene structure analysis divided these FvPME genes into four groups (Group 1–4). Duplicate events analysis suggested that tandem and dispersed duplications effectively contributed to the expansion of the PME family in strawberry. Through transcriptome analysis, we identified FvPME38 and FvPME39 as the most abundant-expressed PMEs at fruit ripening stages, and they were positively regulated by abscisic acid. Genetic manipulation of FvPME38 and FvPME39 by overexpression and RNAi-silencing significantly influences the fruit firmness, pectin content and cell wall structure, indicating a requirement of PME for strawberry fruit softening.
Conclusion
Our study globally analyzed strawberry pectin methylesterases by the approaches of phylogenetics, evolutionary prediction and genetic analysis. We verified the essential role of FvPME38 and FvPME39 in regulation of strawberry fruit softening process, which provided a guide for improving strawberry fruit firmness by modifying PME level.
Similar content being viewed by others
Background
Cell wall is a structural layer surrounding the plant cell membrane, providing protection and strength for plant growth. The construction, differentiation, maturation and degradation of cell wall creates a rigid but flexible outer surrounding for cell division, cell differentiation and multi-cellular organ patterning [1, 2]. The cell wall is composed of a cellulose-hemicellulose network with the cross-linked pectin [3]. Pectin is defined as a hetero-polysaccharides, predominantly containing homogalacturonan (HG), rhamnogalacturonan-I, rhamnogalacturonan-II, and xylogalacturonan components, contributing to wall porosity, wall hydration, and intercellular adhesion [4, 5].
Homeostasis of pectin is regulated by different kinds of pectin-modifying enzymes [6]. Pectin methylesterases (PME, EC 3.1.1.11) is one of the pectin-modifying enzymes, [7] which plays diverse roles in plant development [8]. PME catalyzes reactions on the demethylesterification of pectin to generate carboxyl groups during the release of methanol and hydrogen ions [9]. De-esterification of the major component, HG by PME leads to the generation of free carboxylic acid groups [9, 10]. A low level of HG methylesterification due to the low PME activity often results in an increase of wall rigidity, which influences diverse aspects of plant development, such as hypocotyl growth [11, 12], pollen tube elongation [13, 14], embryo development [15], and seed germination [16, 17]. In contrast, a high level of HG demethylesterification by a higher PME activity produces an opposite effect, that is, loosening of the cell wall. For example, application of high level PME onto the flower primordia induces the formation of ectopic primordia, which is caused by the loosening cell wall structure [18, 19].
Beside the above plant developmental processes, PME-controlled pectin modification is also involved in the regulation of fruit quality. During fruit ripening, a range of pectin-degrading enzymes are secreted into the cell wall, leading to the degradation of pectin polymers and decrease of pectin level [20]. The resultant fruit developmental process is called fruit softening. In strawberry (F. × ananassa, Duch. cv Aroma), PME activity has a close relationship with softening. It induces an abrupt increase of acid pectin during strawberry fruit ripening [21] and is reduced immediately after UV-C irradiation with the consequence of a firmer fruit [22]. FaPE1 is specifically expressed in strawberry fruit, showing an increasing expression during ripening process up to a maximum at turning stage [23]. In tomato fruits, the silence of PME enzyme is associated with an increased level of soluble solids and decreased level of soluble polyuronides in cell walls, which results in the increase of fruit rigidity [24]. In apple fruit ripening, the plant hormone ethylene and low temperature significantly increase PME activity to accelerate fruit softening [25, 26]. Thus, PME-mediated cell wall modification is an essential process to control fruit quality and rigidity.
Genome wide identification of PME genes has been widely studied in many plant species, such as Arabidopsis [27], rice (O. sativa subsp. Japonica cv.) [28], poplar (Populus spp.) [29], flax [30], and Asiatic cotton (Gossypium arboretum) [31]. All PME genes from those species contain a catalytically active zone PME domain, and some of them also comprise a pectin methylesterase inhibitor (PMEI) domain [27, 28, 30, 31]. With expression assay, those studies found some candidate PME genes which have tissue-specific expression patterns. For example, eight PMEs from cotton showed fiber predominant expression in secondary wall thickening, which provided an important basis for further research on the functions of PMEs in cotton fiber development [31]. Although PME has been well characterized in model plants, knowledge regarding the functionality and evolutionary property of PME gene family in Rosaceae plant species remain limited.
Fragaria vesca, the woodland strawberry, is emerging as a model plant of the Rosaceae plant species due to its small and sequenced genome, diploidy (2n = 14, 240 Mb genome) [32]. Strawberry fruit is a soft fruit with a short postharvest life, due to a rapid loss of firm texture. Hence, preharvest improvement of strawberry fruit rigidity is a prerequisite for extension of fruit refreshing time. In our study, we aim to improve strawberry fruit rigidity by manipulation of key cell wall degrading enzymes, PMEs, during fruit development. Firstly, through the global analysis of the genome sequence of strawberry PME (FvPME) genes, 54 unigenes were identified as candidate members of FvPMEs. Phylogenetics, gene structure, and predicted function were performed to characterize FvPMEs. Transcriptome analysis showed that FvPME38 and FvPME39 were particularly abundant in fruit ripening stage. Associated with the gradually decreased fruit firmness during ripening, transcripts of FvPME38 and FvPME39 were gradually increased. Further transient genetic manipulation of FvPME38 and FvPME39 in strawberry fruit by overexpression and silence approach supported the conclusion that FvPMEs were required for the regulation of pectin content and fruit firmness. Our study provided a preliminary knowledge for improving strawberry fruit firmness by modification of PME enzymes.
Methods
Plant materials
The 7th generation inbred lines of F. vesca accession, namely Ruegen (Ru F7–4, red-fruited) were used as wild-types in this study [33]. The plants were grown in a greenhouse (16 h/8 h light conditions at 22 °C, at a relative humidity of 65%). The samples, used for RNA isolation, were frozen in liquid nitrogen immediately after collection and then stored at − 80 °C.
Genome-wide identification of PME genes
The gene files of Arabidopsis thaliana were downloaded from TAIR (The Arabidopsis Information Resource, http://www.arabidopsis.org/). The gene files of Fragaria vesca (strawberry), Malus domestica (apple), Prunus mume (Chinese plum), Prunus persica (peach) and Rosa chinensis (rose) were downloaded from GDR database (Genome Database for Rosaceae: http://www.rosaceae.org/). The gene files of Pyrus bretschneideri (pear) were downloaded from the pear genome database (http://peargenome.njau.edu.cn/).
The Hidden Markov Model (HMM) profiles of PF01095 (PME domain) and PF04043 (PMEI domain) were downloaded from PFam database (http://pfam.sanger.ac.uk/), and the HMMER software package [34] was used to detect PME genes with the best domain e-value cutoff of 1e− 10. These sequences were regarded as potential PME genes. To validate the HMM search, these sequences of candidate PME genes were used as queries to search the NCBI non-redundant protein database through blastp program of GenBank, and only the results with the best hits (an e-value less than 1e− 5) of ‘pectin methylesterases’ and ‘pectin methylesterases inhibitor’ were used for the following study.
Phylogenetics, gene structure and motif analyses
A rooted phylogenetic tree was constructed using MEGA X [35] with neighbor-joining (NJ) criteria and verified using the maximum likelihood (ML) method, and 1000 bootstrap replicates were performed based on the multiple alignments of the full-length amino acid sequences of all PME genes in Arabidopsis thaliana, Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica and Rosa chinensis using ClustalW [36]. Based on the alignments of CDS sequences with the corresponding full-length genomic sequences, the gene structures of the FvPME genes were displayed using an online website: Gene Structure Display Server (GSDS) [37]. Moreover, conserved motifs were detected in FvPME family members using the motif analysis tool Multiple Em for Motif Elicitation (MEME) [38] with the default parameters except for two: motif site distribution, any number of repetitions; maximum number of motifs, 30.
Synteny analysis
We used a modified method to perform the synteny analysis, based on the method which was used previously in the Genome Duplication Database (PGDD) [39]. Firstly, a BLASTP alignment was carried out across the whole genome to identify the candidate homologous gene pairs (E < 1e− 5, top 5 matches). The gene candidates were then uploaded to the software MCScanX with the default parameters [40, 41] to identify syntenic chains. We also used MCScanX for further distinguishing the WGD/segmental, dispersed, proximal, and tandem duplication events of the PME gene family.
Identification of cis-element on promoters of FvPME genes
Cis-elements on the 1.5 kb promoter sequences of FvPME genes were predicted by PlantCARE [42]. And the position of the cis-elements were displayed using an online website: Gene Structure Display Server (GSDS) [37].
Gene expression analyses
The RNA-Seq data of different strawberry varieties was obtained from NCBI (Neinongxiang, PRJNA438551; Toyonoka, PRJNA394190; Camarosa, PRJEB12420; Sweet Charlie, PRJNA263114; Benihoppe, PRJNA473417; Yellow wonder, SRA065786). The heatmaps were plotted in R using the heatmap.2 function based on the logarithmically (log2) transformed reads per kilobase per million (RPKM) values of each PME gene.
Texture analyses
TA.XT.plus Texture Analyser (Stable Micro Systems Ltd., Surrey, UK) along with the measuring probe P/5S (5 mm Spherical stainless steel, supplied with the Texture Analyser) were employed for texture determination. The system was equipped with texture profile analysis (TPA). Hardness was measured as the maximum penetration force (N) reached during tissue breakage. The maximum penetration force was set as 25 N. Other measurable parameters were: pretest speed 1 mm·s− 1; test speed 1 mm·s− 1 penetrating distance of 5 mm into the fruit. The measurement was triggered automatically at 0.04 N. The maximum force required for sample compression was calculated as an average of 10 measurements.
Plasmid construction
The primers used for plasmid construction are listed in Additional file 2: Table S1. The coding region of FvPME genes were amplified from the cDNA of ‘Ruegen’ using PrimerSTAR® GXL DNA Polymerase (TaKaRa, Japan), sub-cloned into pDONR221, and then inserted into the binary vector pK7WGF2 using Gateway® Technology. For RNAi, the partial coding sequences of FvPME38 (1–385 bp) and FvPME39 (1-361 bp) were sub-cloned into pDONR221, and then inserted into the binary vector pB7GWIWG2. The correct fusion constructs were transferred into Agrobacterium tumefaciens strain GV3101 by the freeze–thaw method.
Subcellular localization of FvPME
The leaves of 3-week-old Nicotiana benthamiana plants were infiltrated through their abaxial surfaces by Agrobacterium suspension (OD600 = 0.6). At 72 h post-infiltration, leaves were stained by FM4–64, and fluorescence signal was visualized using Zeiss LSM880 confocal microscope (Zeiss, Germany).
Histological analysis
Different developmental stages of ‘Ruegen’ fruit were fixed in formalin–acetic acid–alcohol (FAA) fixative [formaldehyde solution: glacial acetic acid: 70% ethyl alcohol (v/v); 5: 5: 90, v/v] at 4 °C for 1 week. Samples were dehydrated in an ethanol series solution and embedded in paraffin. Cross-section slicing (10 μm) was performed by Leica RM2255 ultramicrotome (Leica Mikrosysteme, Germany) and stained by 1% (w/v) Toluidine Blue O. After staining, the sections were observed using Nikon SMZ18 microscope.
Transiently transformed strawberry fruit flesh
Transiently transformed strawberry fruit flesh was carried out using agro-infiltration as previous described [43]. GV3101 strains which harbors FvPME38 and FvPME39 overexpression or RNAi constructs was infiltrated into the ‘Ruegen’ fruit flesh at 18 d after pollination (DAP) using syringes. Six plants or fruits were injected with each construct in triplicates. The transformed samples were placed in the dark at 22 °C overnight and then transferred to a phytotron (22 °C, 16-h of light and 8-h darkness) for 7 days before phenotype analysis.
Cell wall pectin content
Measurement of cell wall pectin content was carried out according to the previous study [44]. The alcohol-insoluble cell wall materials of fruits were first isolated with 96% ethanol, weighed and hydrolysed by incubating samples in concentrated H2SO4. The uronic acid content was determined colorimetrically using a microplate spectrophotometer Multiskan GO 1510 (Thermo Fisher, USA). Galacturonic acid was used as a calibration standard, thus the fruit pectin content was expressed as galacturonic acid equivalents (GaE).
Gene expression analysis by qRT-PCR
cDNA, used for quantitative reverse transcription–PCR (qRT-PCR) analysis, was synthesized using one-step genomic DNA removal and a cDNA synthesis kit (Transgen, China). qRT–PCR was performed using the MonAmp™ ChemoHS qPCR Mix (Monad, China). Primers are synthesized by Sangon Biotech Company (China) and shown in Additional file 2: Table S1. The analysis was performed using three biological samples and three technical repeats. Relative expression levels of each gene were normalized to internal control Fvactin and FvGAPDH by 2−ΔΔCp algorithm [45].
Hormonal treatment assay of strawberry fruits
The concentration of hormone solution is in accordance with previous study [46]. The concentration of stock solution was 5 mM in ethanol for nordihydroguaiaretic acid (NDGA) (Aladdin, China). The concentration of working solution for treatment were 100 μM for NDGA diluted in ddH2O. About 20 μL NDGA working solution was injected into the 15 DAP fruits.
Statistical analysis
Statistical analysis was done by one-way ANOVA in the Graphpad prism 8.0 software (Graphpad Software, USA). Significant differences between groups were calculated using P < 0.05 in one-way ANOVA analysis, Tukey’s HSD post hoc test.
Accession numbers
Sequence data in this article can be found at GenBank with the following accession numbers: FvPME38 (MK775554); FvPME39 (MK775555).
Results
Identification of PME genes in rosaceous plants
To identify PME gene sequence of Rosaceous plants, PME candidate genes were searched from Rosaceous plant species of Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica and Rosa chinensis genome according to two strategies: Hidden Markov Model search (HMMsearch) using the HMM profiles PF01095 (PME domain) and PF04043 (PMEI domain); BLASTP search using PME proteins from Arabidopsis as queries. As a result, 54, 78, 79, 57,66 and 53 PME genes were individually identified from Fragaria vesca (strawberry), Malus domestica (apple), Pyrus bretschneideri (pear), Prunus mume (Chinese plum), Prunus persica (peach) and Rosa chinensis (rose) (Additional file 2: Table S2), named as FvPME, MdPME, PbPME, PmPME, PpPME, and RcPME (Table 1). Based on the domain structures, these PME genes were classified into two sub-families: type I PME contains only PME domain, and type II PME contains both PME and PMEI domains (Additional file 2: Table S3).
Phylogenetics analysis
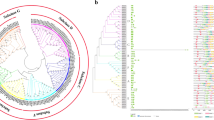
To explore the evolutionary relationships among Arabidopsis and six Rosaceous plants, we constructed a phylogenetic tree using MEGA X based on the multiple sequence alignments of 66 Arabidopsis PMEs (AtPME) and 387 PMEs of Rosaceous plants. The phylogenetic tree showed that the PME genes was split into four groups, which were strongly supported by bootstrap values (Fig. 1). Based on the classification of AtPMEs, the 387 PME genes with PME and PMEI domains were clustered in groups 1–3, and the rest which only contain PME domain belong to group 4. In addition, groups 1, 2 and 4 were divided into serval sub-groups (groups 1a-1 g, 2a-2b and 4a-4d). Based on this classification, we found that most of the clades/subclades are consisted of genes both from the Rosaceous plants and Arabidopsis, suggesting that AtPMEs and PME genes of Rosaceous plants are evolved from a common ancestor. Meanwhile, we also identified species-specific PME subclades, such as 1a-2, 1a-3, 1b-2, 1e-1, 1f-1, 2a-1, 3–1, 3–2, 4a-1, 4a-3, 4c-1, and 4d-2 subclades (Fig. 1), implying an independent evolutionary event happened among Rosaceous plants. Interestingly, in group 3, PME genes from apple and pear were clustered in subclade 3–1, which were entirely isolated from the PMEs of strawberry, rose, peach, and Chineses plum species. Moreover, in species-specific subclade 4a-1, the gene number of PMEs from peach and Chineses plum were intensely expanded, suggesting a particular function of PMEs in peach and Chinese plum.
Maximum likelihood phylogenetic analysis of PME family in Arabidopsis thaliana, Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica and Rosa chinensis. The full-length sequences of PME proteins were aligned using ClustalX, and the phylogenetic tree was generated using MEGA X with the NJ method. Those PME genes were divided into four main groups, among them, groups 1, 2 and 4 could be further divided into serval sub-groups (groups 1a-1 g, 2a-2b and 4a-4d). Different sub-groups were labelled and distinguished with each other by different branch color. Furthermore, PME genes from different species were labelled with different shape types
Different duplication events control the expansion of PME genes in rosaceous plants
To further understand how PME genes are evolved, gene duplication events were investigated in Arabidopsis and six Rosaceous plant species. As shown in Fig. 2, dispersed gene duplication represents the major event of gene expansion, as it accounted for 39.4% (26 of 66), 46.3% (25 of 54), 38.5% (30 of 78), 38.6% (22 of 57) and 45.3% (24 of 53) of PME genes in Arabidopsis, woodland strawberry, apple, Chinese plum and rose, respectively. In contrast, tandem gene duplication occurred more frequently in pear and peach, which is accounted for 38% (30 of 79) and 43.9% (29 of 66) (Additional file 2: Table S4). Besides, WGD/segmental duplication is another main driving force for PME gene family expansion in pear, apple and Arabidposis (Fig. 2 and Additional file 2: Table S4). These results implied that different duplication events controlled PME family expansion in Arabidopsis and six Rosaceous species.
Gene structure, conserved motif and physical distribution of FvPME genes
As mentioned above, strawberry fruit is highly perishable. To improve strawberry fruit rigidity and extend fruit refreshing time, we first analyzed strawberry PME genes by bioinformatics and genetic analysis. Gene structure and conserved motif analysis provided further evidence to support the phylogenetic topology groupings of gene families. Gene structure analysis of FvPMEs showed that most members in groups 1–3 contain one or two introns which is located in the conserved position, while group 4 members contain three to four introns whose positions are varied (Fig. 3a). To search for the potential conserved motifs of FvPMEs, we applied MEME tool to analyze the sequences of 54 FvPMEs. A total of 16 conserved motifs were detectable, named as motif 1–16 (Fig. 3b and S1). Most of the motifs are conserved. Particularly, motifs 14 and 16 are only present in groups 1 and 2 members, motif 8 only appears in groups 1–3 members, and motif 15 was exclusively found in group 4 members. Those special motifs might contribute to the diverse function of PME genes from different groups.
Analyses of gene structure and conserved motifs within each PME group in strawberry. a, Exon/intron structures of strawberry PME genes. White boxes represent the exons. Blue boxes represent UTRs. Black lines connecting two exons indicate introns. Sequence of purple boxes and green boxes code PME domain and PMEI domain, respectively. b, The distribution of conserved motif within each PME genes. Boxes in different colors represent different conserved motifs, and their relative position are displayed
Regarding the physical genome distributions, 54 FvPMEs are individually located in seven chromosomes. Among them, chromosome 7 contains the largest numbers of 12 FvPMEs, chromosome 6 contains 11 members and chromosome 1 contains 10 members. In contrast, only three FvPMEs are present on the chromosome 3 (Fig. 4). Interestingly, a higher density of FvPMEs was found on the particular regions of chromosomes, such as the top of chromosome 1, and the bottom of chromosomes 7.
Putative cis-element in FvPME gene promoters
To identify the possible gene responsiveness of FvPMEs, a 1.5 kb promoter region is captured and analyzed by PlantCARE (Additional file 2: Table S5). Bioinformatics analysis indicates that most of the cis-elements of FvPMEs’ promoters belong to the responsive elements of plant hormones, abiotic (e.g., dehydration and salt) and biotic stress (Additional file 2: Table S5). Particularly, a large number of transcription factor binding sites were present on the promoter of FvPME genes (Additional file 1: Fig. S2). These findings implied that the transcriptional level of FvPME genes are variously controlled by various factors including phytohormones and environmental stimuli during plant developmental processes.
Functional prediction of FvPME genes
In principle, homologous genes share similar gene structures and are clustered in the same clades, in which the genes possess similar functions [47]. Phylogenetic analysis allowed us to predict putative gene function of orthologous and paralogous PME genes in strawberry (Table 2). For example, group 1a is the biggest subgroup with diverse functions, involved in adventitious rooting, nematode defense and seedling development in Arabidopsis [48, 49], implying a similar role of FvPME in group 1a FvPME38 and two AtPME genes (AtPME9 and AtPME28) belong to subgroup 1 g-2. AtPME 9 and AtPME28 have been reported to participate in cell wall remodeling processes and response to abiotic stress [55, 56]. Possibly, the homologue strawberry gene FvPME38 is also involved in cell wall regulation.
In addition, gene clusters with similar function were observed for other PME genes in subgroups 1c-1,1c-2 and 4c-2, among which the homologue genes from Arabidopsis (AtPME36, AtPME17, AtPME37 and AtPME64) are known to be involved in stress pathways, such as drought, salt and pathogen response [51,52,53, 66]. Some members of AtPMEs from groups 2b-1, 4a and 4b-1 are contributed to pollen development [58,59,60,61,62,63,64]. Therefore, we predicted that FvPME genes of group 1c and 4c-2 might be involved in stress-related pathways, and group 2b-1, 4a and 4b-1 homologues might join in reproductive development. Particularly, in 4a clade, 4a-1 and 4a-3 group members were identified as species-specific subclades. Therefore, we suppose that the Rosaceous PME genes from 4a-1 and 4a-3 might develop distinct evolutionary story and functionality which should be verified by further experiment.
FvPME38 and FvPME39 are involved in regulation of fruit softening
According to the phylogenetic analysis and functional prediction, we aim to study the functionality of FvPMEs during strawberry fruit softening. The spatial and temporal expression patterns of FvPMEs showed that FvPME 7, 38, 39, 42 and 54 were expressed in the strawberry fruit among all FvPME members. FvPME7 was preferentially detectable in embryo, ghost and wall tissues, and FvPME38 and FvPME39 had higher expression level in pith and cortex than other tissues (Fig. 5). In line with the previous phylogenetic analysis that the orthologous of FvPME38, AtPME9 has been reported to participate in cell wall remodeling [55]. FvPME38 and FvPME39 are good candidates to study PME’s role during strawberry fruit development.
Expression heatmap of strawberry PME family genes at developmental stages of fruit. The expression levels of PME genes were measured by RNA-seq analysis at different stages of fruit in five varieties. The color scale at the left represents RPKM normalized by log2. Blue indicates a low expression level, yellow indicates a medium, and green indicates a high level. Four PME genes, which are marked by dotted line, don’t have expression level during any developmental stages. And FvPME 7, 38, and 39 expressed abundantly during fruit development which are filled green color
To understand the strawberry fruit developmental process, we firstly conducted morphological and physiological characterization according to the fruit developmental stages. We used diploid woodland strawberry F. vesca, ‘Ruegen’ fruit as the study model and divided it into five stages: small green (SG, 7–8 d after pollination [DAP]), big green (BG, 12–14 DAP), turning red (TR, 18–20 DAP), start red (SR, white flesh with red achenes, 22–25 DAP), and full red (FR, 28–30 DAP) stages (Fig. 6a). To examine cell wall structure of fruit, paraffin sections of fruit at different stages were stained by Toluidine Blue O which is commonly employed in polychromatic staining of paraffin embedded plant cell walls [67]. The cell wall texture of the SG, BG and TR stages was more compact than those in SR and FR stages. Moreover, greater cell-cell adhesion and smaller intercellular spaces were present in SG, BG and TR stages, in comparison of larger intercellular spaces with loosely organized cells in SR and FR stages (Fig. 6 a). Along with the gradual expanded fruit size, fruit firmness was significantly decreased from SG to FR stages (Fig. 6 b-c). Particularly, the firmness suddenly became very low level at TR and SR stages (Fig. 6c). The above morphological analysis indicated that fruit swelling and cell wall loosening are probably correlated with the decrease of firmness and tissue integrity during fruit ripening.
Developmental stages of ‘Ruegen’ fruit. a, Five stages of ‘Ruegen’ fruit development. Top row shows each receptacle fruit in different stages. Second row shows longitudinal sections of fruit in five stages. Third row is higher magnifications of sections in second row. b and c, The changes of diameter and firmness in ‘Ruegen’ fruit during five developmental stages. Values are mean ± SD of ten fruits in (B) and fifteen fruits in (C). DAP, day after pollination. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test)
RNA-seq data showed that FvPME 7, 38, 39, 42 and 54 were expressed in the strawberry fruit (Fig. 5). The five FvPME genes were selected to verify their expression in achenes and receptacles at five stages of fruit development for ‘Ruegen’ by qRT-PCR (Fig. 7). In receptacles, expression of FvPME7 and FvPME42 were absent; FvPME54 expression was detected mainly at early stages, and the expression level of FvPME38 and FvPME39 was opposite to that of FvPME54 which was expressed mainly in ripening stage. In achenes, FvPME7 was expressed abundantly in early stages, and the expression levels of FvPME38, FvPME39 and FvPME42 increased at late stages. The expression levels of FvPME38 and FvPME39 in receptacles were opposite to the change of fruit firmness which had a sudden drop at SR and FR stages, implying that FvPME38 and FvPME39 serve as negative regulators to control fruit rigidity.
Spatial and temporal expression level of FvPME genes during development in achenes and receptacles. Developmental stages were showed in Fig. 6 a. Error bars represent SD of three independent replicates. Rec, receptacle; Ach, achene. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test)
To examine if FvPME38 and FvPME39 proteins function as pectin-modifying enzymes which should be localized on the cell wall, we transiently expressed 35S::GFP-FvPME38 and 35S::GFP-FvPME39 vectors into tobacco leaves. Green fluorescence signals of GFP-FvPME38 and GFP-FvPME39 were detectable specifically in the cell walls which were differentiated from the red fluorescence signals staining by the plasma membrane dye FM4–64 (Fig. 8a).
FvPME38 and FvPME39 involved in fruit softening. a, FvPME38 and FvPME39 were cell wall localized as determined by GFP-FvPME38 and GFP-FvPME39 fusion protein in tobacco leaf epidermal cells. Plasma membrane was identified by FM4–64 staining. b, Phenotypes of fruits were agro-infiltrated with FvPME38 and FvPME39 overexpression and RNAi constructs, respectively. DAI, day after infiltration; RNAi, RNA interference; OE, overexpression. c, QRT-PCR analysis of transcript levels for FvPME genes in overexpression and RNAi fruits at three days after injection. Error bars represent SD of three independent replicates. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test). d-e, Pectin content of cell wall (D) and fruit size (E) at seven days after injection. Error bars represent SD of 15 fruits. f, Sections of overexpression and RNAi fruits at seven days after injection. g, QRT-PCR analysis of transcript levels for ripening-related genes in fruits of transient overexpression or silencing of FvPMEs. Relative expression levels of each gene were normalized to internal control Fvactin. Error bars represent SD of three independent replicates. PL, pectate lyase; PG, polygalacturonase; CEL, cellulose; XYL1, beta-xylosidase1; EXP1, expansin1; CHS, chalcone synthase; CHI, chalconeisomerase; F3H, flavanone 3-hydroxylase; DFR, dihydroflavonol 4-reductase; UFGT, UDP-glucose flavonoid 3-O-glycosyltransferase; ANS, anthocyanidin synthase; SS, sucrose synthase; SUT1, sucrose transporter1; SPS3, sucrose phosphate synthase3; HXK2, hexokinase2. h, Texture analysis of fruits were agro-infiltrated with FvPME38 and FvPME39 overexpression and RNAi constructs, respectively. i-j, Firmness value of overexpression and RNAi fruits at same ripening stages after infiltration. SR stage (I); FR stage (J). The number in bracket of x-axis labels represents the number of days after injection. Error bars represent SD of 15 fruits. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test)
To further elucidate the roles of FvPME38 and FvPME39, overexpression and RNA interference (RNAi) constructs were agro-infiltrated into ‘Ruegen’ fruit at 18 DAP. After infiltration, we observed a dramatically delay of fruit ripening in FvPME38 or FvPME39 RNAi fruits, but FvPME38 or FvPME39 overexpressions accelerated ripening process (Fig. 8b and c). FvPME38 and FvPME39 RNAi fruits turned full red at 13 days after infiltration (DAI), which is longer than control (11 days), but FvPME38 and FvPME39 overexpressing fruits only need 7 days to turn full red (Fig. 8b). Furthermore, in FvPME38 and FvPME39 RNAi fruits, pectin content of fruits were significantly higher than the control, and fruits were smaller than the control at 7 DAI; while overexpression of FvPME38 or FvPME39 reduced fruit pectin content (Fig. 8d and e). We further examine cell wall texture by paraffin sections combined with Toluidine Blue O staining. The result showed that fruit sections of FvPME38 or FvPME39 RNAi exhibited greater cellular adhesion and smaller intercellular spaces, and cell wall structure of parenchymal cell was broken in FvPME overexpressors at 7 DAI (Fig. 8f). Beside of the above morphological phenotypes, we also assessed the transcriptional level of downstream genes which indicated fruit ripening, including the softening-related genes polygalacturonase (PG), and beta-xylosidase1 (XYL1); the anthocyanin biosynthesis genes dihydroflavonol 4-reductase (DFR) and UDP-glucose flavonoid 3-O-glycosyltransferase (UFGT); and the sugar-related genes hexokinase (HXK2) [68,69,70,71]. QRT-PCR analysis showed that the transcript levels of PG, XYL1, DFR, UFGT, and HXK2 were significantly downregulated in the FvPME-RNAi fruits, but upregulated in the overexpressor fruits, compared with the control (Fig. 8g and Additional file 1: Figure S3). Apparently, FvPME38 and FvPME39 significantly influenced fruit ripening. Texture analysis showed that in FvPME38 and FvPME39 RNAi fruits, fruit firmness value was significantly higher than the control fruits at 5, 7, 9, and 11 DAI, respectively (Fig. 8h). And FvPME38 or FvPME39 overexpressing fruits were clearly softer than the control at 5 and 7 DAI (Fig. 8h). However, the differences in texture were possibly caused by different ripening stages of fruit. Therefore, we measured the texture of fruit at the same ripening stages (SR and FR stages). The firmness value of FvPME-RNAi fruits was significantly higher than that of control, and overexpressing fruits were softer (Fig. 8i and j). Altogether, these results demonstrated that FvPME38 and FvPME39 play important roles for regulation of strawberry fruit ripening and softening.
Abscisic acid regulation of FvPME 38 and FvPME 39
In previous study, abscisic acid (ABA) has been proved essentially for the onset of ripening process in strawberry fruit [72]. Endogenous ABA content is low at the small white stage and gradually increases through fruit ripening [73], which is consistent with the expression levels of FvPME 38 and FvPME 39 genes during receptacle development (Fig. 7). Based on the prediction of the cis-element in FvPME 38 and FvPME 39 promoter region, there are some ABRE-elements (Additional file 1: Figure S2 and Additional file 2: Table S5) which belong to the conserved element responding to ABA signal [74]. We speculated that FvPME 38 and FvPME 39 may perform downstream of ABA signaling. To verify this hypothesis, we applied nordihydroguaiaretic acid (NDGA), an ABA inhibitor, on the fruits at TR stage to block ABA production. The treated fruits were harvested after five days for qRT-PCR analysis. A significant reduction in the amount of FvPME 38 and FvPME 39 transcripts was observed, comparing with the control (Fig. 9). This result indicates that FvPME 38 and FvPME 39 expression could be activated by ABA.
Hormonal regulation of FvPMEs expression. QRT-PCR analysis of FvPME38 and FvPME39 expression in strawberry fruits after 5 days treatment. CK, 15 DAP fruits injected with water; NDGA, 15 DAP fruits injected with NDGA (100 μM). Error bars represent SD of three independent replicates. Asterisk indicates values that were determined by the t-test to be significantly different from the control (*, P < 0.05; ***, P < 0.001)
Discussion
The evolutionary histories of PME have slight differences among rosaceous species
As one of the pectin-modifying enzymes, the functionality of PME genes has been widely studied in many plant species, such as Arabidopsis [27], rice [28], flax [30], and cotton [31]. Here, 54 PME genes were identified in strawberry, and 53, 57, 66, 78 and 79 PME genes were found from rose, Chinese plum, peach, apple and pear, respectively (Additional file 2: Table S2). The number of PME homologues in Rosaceous species was similar to that in Arabidopsis, suggesting a conserved function between Rosaceous plants and Arabidopsis. For gene family evolution, a major mechanism to generate new models for evolutionary innovation is the gene duplication, such as tandem gene duplication, WGD/segmental duplication [75]. Following the genome data of six Rosaceous species, all of them experienced one WGD event from one Rosaceous ancestor [76]. And a more recent WGD event could be dated to approximately 40 million years ago in apple and pear, but not in woodland strawberry, rose, peach, and Chinese plum [32, 77,78,79,80,81]. By duplication modes analysis, we found that the tandem duplication events of PME gene in peach happen more frequently than those in rose, woodland strawberry and Chinese plum, as shown by the expansion of PME gene number in peach than in other species. In apple and pear, WGD/segmental duplication is another main driving force for PME gene family expansion. Therefore, larger numbers of PME genes were identified in apple and pear, possibly due to twice WGD events happen in these two species. In addition, PME genes of peach only undergo dispersed, tandem and proximal duplications, without WGD/segmental duplication. It suggested that duplication modes of PME gene family in those Rosaceous species were diversified. Previous studies have illustrated that a gene family may have common non-random patterns of origin, with conserved duplication modes in different species [82, 83]. However, our results indicated that the main duplication modes of PME gene family in the six Rosaceous species were not always strictly conserved, and non-random patterns in different origins were common.
FvPME 38 and FvPME 39 accelerate ripening and softening in fruit
Previous studies have well characterized the general role of PMEs for fruit ripening, whereas PMEs execute different roles in the different plant species. For example, the firmness of F. × ananassa fruit reduced at the late ripening stages which was concomitant to the high transcript level of PME and PG [84]. In different variety of apple, PME activity generally increases during fruit softening; whereas it differed at different stages in different cultivar, which is regulated by ethylene and temperature [26]. Exceptionally, a PME gene in apple, called Malus domestica PME (MdPME2), showed an atypical role [85]. High expression of MdPME2 in fruit flesh prevents apple fruit mealiness [85]. In some apricot varieties, PME also performs differently in different varieties. ‘San Castrese’ is an apricot variety which maintained the fruit firmness during ripening, and ‘Ceccona’ is another variety which showed rapid softening associated with ripening [86]. During fruit development, PME activity gradually declined in ‘Ceccona’, but it slightly increased in ‘San Castrese’ [86]. Those studies indicated that PME may activate or repress fruit softening in different varieties of same specious, suggesting the various function of PME. The FvPME38 and FvPME39 identified in our study displays a typical PME role during fruit ripening, which promotes the softening of strawberry fruits. In previous study, FaPE1 (AY324809 in the GenBank database), namely FvPME7 in our study, was specifically expressed in F. × ananassa fruit, with higher levels during final development stage, coinciding with the beginning of the ripening process [23]. Although our results showed that expression of FvPME7 was detected mainly at early stages of achenes and clearly absent at all stage of receptacles in F. vesca (Fig. 7), which was not coincided with the results of that study. Southern blot analysis showed that FaPE1 was a single-copy gene in the diploid species F. vesca, but elevated allele polymorphism was detectable in the octoploid species F. × ananassa [23]. It suggested that the number of FaPE1 allele was extended in F. × ananassa, which might execute different roles during fruit development between diploid and octoploid strawberry.
Interestingly, beside of the regulation of fruit softening, overexpression of FvPMEs accelerates the process of fruit ripening, implying an additional role of FvPME for regulation of fruit ripening. The function of FvPME38 and FvPME39 was similar with other cell wall-modifying genes, such as FvXTH9, FvXTH6 and FaβGAL4. Overexpression of the genes led to faster ripening by modification of cell wall components in strawberry fruits [87, 88]. Whereas, in tomato fruit, PME activity is only associated with the level of firmness but do not interfere with ripening [24]. Therefore, PMEs in different plant species show diverse functions, which might be caused by the diversity of fruit developmental processes. On the other hand, because of the specific hormonal requirement during fruit ripening, such as climactic fruit and non-climactic fruit [73], PME-mediated pectin degradation and cell wall reconstruction in these two types of fruits might be very different. In our study, FvPME38 and FvPME39 had a wide role to affect other genes and overall phenotype. Maybe the PMEs involved in the modification of some molecular signal. In tomato, an increase of PME activity is associated with a corresponding increase of methanol content during fruit ripening [89]. Methanol is crucial in control of plant growth and response to stresses [90,91,92]. Pectin demethylesterification by PME is the main source of plant-derived methanol [93]. Therefore, we speculate that cell wall pectin demethylesterification may determine the production of methanol, in turn regulate fruit ripening.
Conclusions
Our work aims to improve strawberry fruit rigidity by genetic manipulation of key cell wall-modifying enzymes, pectin methylesterases (FvPMEs) during fruit development. Through analysis of the gene evolution in Rosaceous plants, we found that tandem and dispersed duplication events played important roles for the gene expansion of FvPME family. Further genetic manipulation of fruit-specific FvPME38 and FvPME39 by overexpression and RNAi-silencing showed that FvPMEs significantly influences the fruit firmness, pectin content and cell wall structure, indicating a functional requirement of PME for strawberry fruit softening. These results offer a preliminary understanding of the function and evolution of strawberry PME genes, meanwhile provide a knowledge guide for improving fruit firmness by modifying PME level.
Availability of data and materials
All Arabidopsis protein sequences were downloaded from The Arabidopsis Information Resource (TAIR) (https:// www.arabidopsis.org). The gene files of apple, strawberry, Chinese plum, peach and rose were downloaded from GDR database (Genome Database for Rosaceae: http://www.rosaceae.org/). The gene files of pear were downloaded from the pear genome database (http://peargenome.njau.edu.cn/). The RNA-Seq data of different strawberry varieties was obtained from NCBI (Neinongxiang, PRJNA438551; Toyonoka, PRJNA394190; Camarosa, PRJEB12420; Sweet Charlie, PRJNA263114; Benihoppe, PRJNA473417; Yellow wonder, SRA065786).
Abbreviations
- ABA:
-
Abscisic acid
- ANS:
-
Anthocyanidin synthase
- BG:
-
Big green stage
- BLAST:
-
The basic local alignment search tool
- CEL:
-
Cellulose
- CHI:
-
Chalconeisomerase
- CHS:
-
Chalcone synthase
- DAI:
-
Day after infiltration
- DAP:
-
Day after pollination
- DFR:
-
Dihydroflavonol 4-reductase
- EXP1:
-
Expansin1
- F3H:
-
Flavanone 3-hydroxylase
- FR:
-
Full red stage
- HG:
-
Homogalacturonan
- HMM:
-
Hidden markov model
- HXK2:
-
Hexokinase2
- MEGA:
-
Molecular evolutionary genetics analysis
- MEME:
-
Multiple em for motif elicitation
- ML:
-
Maximum likelihood
- NDGA:
-
Nordihydroguaiaretic acid
- NJ:
-
Neighbor-joining
- OE:
-
Overexpression
- Pfam:
-
Protein family
- PG:
-
Polygalacturonase
- PL:
-
Pectate lyase
- PME:
-
Pectin methylesterase
- QRT-PCR:
-
Quantitative reverse transcription–PCR
- RNAi:
-
RNA interference
- RNA-seq:
-
Ribose nucleic acid sequencing
- RPKM:
-
Reads per kilobase per million reads
- SG:
-
Small green stage
- SMART:
-
A simple modular architecture research tool
- SPS3:
-
Sucrose phosphate synthase3
- SR:
-
Start red stage
- SS:
-
Sucrose synthase
- SUT1:
-
Sucrose transporter1
- TR:
-
Turning red stage
- UFGT:
-
UDP-glucose flavonoid 3-O-glycosyltransferase
- WGD:
-
Whole-genome duplication
- XYL1:
-
Beta-xylosidase1
References
Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6(11):850–61.
Braybrook SA, Jonsson H. Shifting foundations: the mechanical cell wall and development. Curr Opin Plant Biol. 2016;29:115–20.
Cosgrove DJ. Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot. 2016;67(2):463–76.
Daher FB, Braybrook SA. How to let go: pectin and plant cell adhesion. Front Plant Sci. 2015;6:523.
Anderson CT. We be jammin': an update on pectin biosynthesis, trafficking and dynamics. J Exp Bot. 2016;67(2):495–502.
Wang D, Yeats TH, Uluisik S, Rose JKC, Seymour GB. Fruit softening: revisiting the role of pectin. Trends Plant Sci. 2018;23(4):302–10.
Wang M, Yuan D, Gao W, Li Y, Tan J, Zhang X. A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS One. 2013;8(8):e72082.
Senechal F, Wattier C, Rusterucci C, Pelloux J. Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. J Exp Bot. 2014;65(18):5125–60.
Pelloux J, Rusterucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007;12(6):267–77.
Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6(9):414–9.
Derbyshire P, McCann MC, Roberts K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol. 2007;7:31.
Pelletier S, Van Orden J, Wolf S, Vissenberg K, Delacourt J, Ndong YA, Pelloux J, Bischoff V, Urbain A, Mouille G, et al. A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 2010;188(3):726–39.
Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220(4):582–92.
Bosch M, Hepler PK. Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta. 2006;223(4):736–45.
Levesque-Tremblay G, Muller K, Mansfield SD, Haughn GW. HIGHLY METHYL ESTERIFIED SEEDS is a pectin methyl esterase involved in embryo development. Plant Physiol. 2015;167(3):725–37.
Muller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013;161(1):305–16.
Scheler C, Weitbrecht K, Pearce SP, Hampstead A, Buttner-Mainik A, Lee KJ, Voegele A, Oracz K, Dekkers BJ, Wang X, et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015;167(1):200–15.
Peaucelle A, Louvet R, Johansen JN, Hofte H, Laufs P, Pelloux J, Mouille G. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol. 2008;18(24):1943–8.
Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Hofte H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol. 2011;21(20):1720–6.
Airianah OB, Vreeburg RA, Fry SC. Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method. Ann Bot. 2016;117(3):441–55.
Draye M, Van Cutsem P. Pectin methylesterases induce an abrupt increase of acidic pectin during strawberry fruit ripening. J Plant Physiol. 2008;165(11):1152–60.
Pombo MA, Dotto MC, Martínez GA, Civello PM. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol Technol. 2009;51(2):141–8.
Castillejo C, de la Fuente JI, Iannetta P, Botella MA, Valpuesta V. Pectin esterase gene family in strawberry fruit: study of FaPE1, a ripening-specific isoform. J Exp Bot. 2004;55(398):909–18.
Tieman DM, Harriman RW, Ramamohan G, Handa AK. An antisense pectin Methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell. 1992;4(6):667–79.
Dheilly E, Gall SL, Guillou MC, Renou JP, Bonnin E, Orsel M, Lahaye M. Cell wall dynamics during apple development and storage involves hemicellulose modifications and related expressed genes. BMC Plant Biol. 2016;16(1):201.
Wei J, Ma F, Shi S, Qi X, Zhu X, Yuan J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol Technol. 2010;56(2):147–54.
Louvet R, Cavel E, Gutierrez L, Guenin S, Roger D, Gillet F, Guerineau F, Pelloux J. Comprehensive expression profiling of the pectin methylesterase gene family during silique development in Arabidopsis thaliana. Planta. 2006;224(4):782–91.
Jeong HY, Nguyen HP, Lee C. Genome-wide identification and expression analysis of rice pectin methylesterases: implication of functional roles of pectin modification in rice physiology. J Plant Physiol. 2015;183:23–9.
Geisler-Lee J, Geisler M, Coutinho PM, Segerman B, Nishikubo N, Takahashi J, Aspeborg H, Djerbi S, Master E, Andersson-Gunneras S, et al. Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol. 2006;140(3):946–62.
Pinzon-Latorre D, Deyholos MK. Characterization and transcript profiling of the pectin methylesterase (PME) and pectin methylesterase inhibitor (PMEI) gene families in flax (Linum usitatissimum). BMC Genomics. 2013;14:742.
Li W, Shang H, Ge Q, Zou C, Cai J, Wang D, Fan S, Zhang Z, Deng X, Tan Y, et al. Genome-wide identification, phylogeny, and expression analysis of pectin methylesterases reveal their major role in cotton fiber development. BMC Genomics. 2016;17(1):1000.
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al. The genome of woodland strawberry (Fragaria vesca). Nat Genet. 2011;43(2):109–16.
Slovin JP, Schmitt K, Folta KM. An inbred line of the diploid strawberry f semperflorens for genomic and molecular genetic studies in the Rosaceae. Plant Methods. 2009;5:15.
Eddy SR. Accelerated profile HMM searches. PLoS Comput Biol. 2011;7(10):e1002195.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8.
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–8.
Lee TH, Tang H, Wang X, Paterson AH. PGDD: a database of gene and genome duplication in plants. Nucleic Acids Res. 2013;41(Database issue):D1152–8.
Tang H, Wang X, Bowers JE, Ming R, Alam M, Paterson AH. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008;18(12):1944–54.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Pi M, Gao Q, Kang C. Transient Expression Assay in Strawberry Fruits. Bio-Protocol. 2019;9(11).
Geng X, Horst WJ, Golz JF, Lee JE, Ding Z, Yang ZB. LEUNIG_HOMOLOG transcriptional co-repressor mediates aluminium sensitivity through PECTIN METHYLESTERASE46-modulated root cell wall pectin methylesterification in Arabidopsis. Plant J. 2017;90(3):491–504.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Gu T, Jia S, Huang X, Wang L, Fu W, Huo G, Gan L, Ding J, Li Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening. Planta. 2019.
Fitch WM. Distinguishing homologous from analogous proteins. Syst Zool. 1970;19(2):99–113.
Qu T, Liu R, Wang W, An L, Chen T, Liu G, Zhao Z. Brassinosteroids regulate pectin methylesterase activity and ☆. Cryobiology. 2011;63(2):111–7.
Guénin S, Hardouin J, Paynel F, Müller K, Mongelard G, Driouich A, Lerouge P, Kermode AR, Lehner A, Mollet JC. AtPME3, a ubiquitous cell wall pectin methylesterase of Arabidopsis thaliana, alters the metabolism of cruciferin seed storage proteins during post-germinative growth of seedlings. J Exp Bot. 2017;68(5):1083–95.
Sénéchal F, Graff L, Surcouf O, Marcelo P, Rayon C, Bouton S, Mareck A, Mouille G, Stintzi A, Höfte H. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann Bot-London. 2014;114(6):1161.
Huang YC, Wu HC, Wang YD, Liu CH, Lin CC, Luo DL, Jinn TL. PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 2017;174(2):748.
Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007;143(4):1871–80.
Hongo S, Sato K, Yokoyama R, Nishitani K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell. 2012;24(6):2624–34.
Lakshmanan V, Castaneda R, Rudrappa T, Bais HP. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta. 2013;238(11):657–68.
Vatén A, Dettmer J, Wu S, Stierhof YD, Miyashima S, Yadav SR, Roberts C, Campilho A, Bulone V, Lichtenberger R. Callose biosynthesis regulates Symplastic trafficking during root development. Dev Cell. 2011;21(6):1144–55.
Creighton MT, Kolton A, Kataya ARA, Maple-Grodem J, Averkina IO, Heidari B, Lillo C. Methylation of protein phosphatase 2A-influence of regulators and environmental stress factors. Plant Cell Environ. 2017;40(10):2347–58.
Turbant A, Fournet F, Lequart M, Zabijak L, Pageau K, Bouton S, Wuytswinkel OV. PME58 plays a role in pectin distribution during seed coat mucilage extrusion through homogalacturonan modification. J Exp Bot. 2016;67(8):2177–90.
Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell. 2005;17(2):584–96.
Tian GW, Chen MH, Zaltsman A, Citovsky V. Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol. 2006;294(1):83–91.
Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science. 1994;264(5164):1458–60.
Kirk EF, Sandy YL, Gregory PC. Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiol. 2006;142(3):1004–13.
Kirk EF, Sandy YL, Benjamin DH, Alexandra LB, Luke EB, Gregory PC. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. P Natl Acad Sci USA. 2007;104(10):3913–8.
Rhee SY, Somerville CR. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Protoplasma. 2010;15(1):79–88.
Lu P, Han X, Qi J, Yang J, Wijeratne AJ, Li T, Ma H. Analysis of Arabidopsis genome-wide variations before and after meiosis and meiotic recombination by resequencing Landsberg erecta and all four products of a single meiosis. Genome Res. 2012;22(3):508–18.
Alexis P, Romain L, Jorunn NJ, Fabien S, Halima M, Françoise F, Katia B, Françoise G, Hf H, Patrick L. The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Dev. 2011;138(21):4733–41.
Yan J, He H, Fang L, Zhang A. Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem Biophys Res Commun. 2018;496(2).
O'Brien TP, Feder N, MEJP MC. Polychromatic staining of plant cell walls by toluidine blue O. 1964;59(2):368–73.
Jia HF, Lu D, Sun JH, Li CL, Xing Y, Qin L, Shen YY. Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J Exp Bot. 2013;64(6):1677–87.
Han Y, Dang R, Li J, Jiang J, Zhang N, Jia M, Wei L, Li Z, Li B, Jia W. SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6, an ortholog of OPEN STOMATA1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol. 2015;167(3):915–30.
Wei L, Mao W, Jia M, Xing S, Ali U, Zhao Y, Chen Y, Cao M, Dai Z, Zhang K, et al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J Exp Bot. 2018.
Guo J, Wang S, Yu X, Dong R, Li Y, Mei X, Shen Y. Polyamines regulate strawberry fruit ripening by Abscisic acid, Auxin, and ethylene. Plant Physiol. 2018;177(1):339–51.
Cherian S, Figueroa CR, Nair H. Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. J Exp Bot. 2014;65(17):4705–22.
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB. Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot. 2012;63(13):4741–50.
Mukherjee K, Choudhury AR, Gupta B, Gupta S, Sengupta DN. An ABRE-binding factor, OSBZ8, is highly expressed in salt tolerant cultivars than in salt sensitive cultivars of indica rice. BMC Plant Biol. 2006;6:18.
Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154(1):459–73.
Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, et al. Ancestral polyploidy in seed plants and angiosperms. Nat. 2011;473(7345):97–100.
Verde I, Abbott AG, Scalabrin S, Jung S, Shu S, Marroni F, Zhebentyayeva T, Dettori MT, Grimwood J, Cattonaro F, et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet. 2013;45(5):487–94.
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, et al. The genome of the domesticated apple (Malus x domestica Borkh.). Nat Genet. 2010;42(10):833–9.
Zhang Q, Chen W, Sun L, Zhao F, Huang B, Yang W, Tao Y, Wang J, Yuan Z, Fan G, et al. The genome of Prunus mume. Nat Commun. 2012;3:1318.
Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S, Khan MA, Tao S, Korban SS, Wang H, et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013;23(2):396–408.
Raymond O, Gouzy J, Just J, Badouin H, Verdenaud M, Lemainque A, Vergne P, Moja S, Choisne N, Pont C, et al. The Rosa genome provides new insights into the domestication of modern roses. Nat Genet. 2018;50(6):772–7.
Rodgers-Melnick E, Mane SP, Dharmawardhana P, Slavov GT, Crasta OR, Strauss SH, Brunner AM, Difazio SP. Contrasting patterns of evolution following whole genome versus tandem duplication events in Populus. Genome Res. 2012;22(1):95–105.
Wang Y, Wang X, Tang H, Tan X, Ficklin SP, Feltus FA, Paterson AH. Modes of gene duplication contribute differently to genetic novelty and redundancy, but show parallels across divergent angiosperms. PLoS One. 2011;6(12):e28150.
Severo J, Tiecher A, Chaves FC, Silva JA, Rombaldi CV. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa Food Chem. 2011;126(3):995–1000.
Segonne SM, Bruneau M, Celton JM, Le Gall S, Francin-Allami M, Juchaux M, Laurens F, Orsel M, Renou JP. Multiscale investigation of mealiness in apple: an atypical role for a pectin methylesterase during fruit maturation. BMC Plant Biol. 2014;14:375.
Botondi R, DeSantis D, Bellincontro A, Vizovitis K, Mencarelli F. Influence of ethylene inhibition by 1-methylcyclopropene on apricot quality, volatile production, and glycosidase activity of low- and high-aroma varieties of apricots. J Agric Food Chem. 2003;51(5):1189–200.
Witasari LD, Huang FC, Hoffmann T, Rozhon W, Fry SC, Schwab W. Higher expression of the strawberry xyloglucan endotransglucosylase/hydrolase genes FvXTH9 and FvXTH6 accelerates fruit ripening. Plant J. 2019.
Paniagua C, Blanco-Portales R, Barcelo-Munoz M, Garcia-Gago JA, Waldron KW, Quesada MA, Munoz-Blanco J, Mercado JA. Antisense down-regulation of the strawberry beta-galactosidase gene FabetaGal4 increases cell wall galactose levels and reduces fruit softening. J Exp Bot. 2016;67(3):619–31.
Frenkel C, Peters JS, Tieman DM, Tiznado ME, Handa AK. Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (Lycopersicon esculentum) fruit. J Biol Chem. 1998;273(8):4293–5.
Hasunuma T, Fukusaki E, Kobayashi A. Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. J Biotechnol. 2004;111(3):241–51.
Dorokhov YL, Komarova TV, Petrunia IV, Frolova OY, Pozdyshev DV, Gleba YY. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012;8(4):e1002640.
Sheshukova EV, Komarova TV, Pozdyshev DV, Ershova NM, Shindyapina AV, Tashlitsky VN, Sheval EV, Dorokhov YL. The Intergenic interplay between aldose 1-Epimerase-like protein and pectin Methylesterase in abiotic and biotic stress control. Front Plant Sci. 2017;8:1646.
Dorokhov YL, Sheshukova EV, Komarova TV. Methanol in plant life. Front Plant Sci. 2018;9:1623.
Acknowledgements
The authors would like to thank anonymous reviewers for comments on this manuscript.
Funding
This work was supported by the International Joint Research Grant of Fujian Agriculture and Forestry University (KXGH170102) and the Fok Ying Tung Education Foundation (161027) to Xu Chen and the National Natural Science Foundation of China (31901983) and China Postdoctoral Science Foundation (2019 M662218) to Cheng Xue.
Author information
Authors and Affiliations
Contributions
CX and XC perceived and planned the study. CX and SCG performed most of the experiments and analysis. CJW and JFC helped planting strawberry and extracted total RNAs from samples for QRT-PCR. JQC helped in run bioinformatics software and data arrangement. CX and XC wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Logo of conserved motifs in Fig. 2. Figure S2. Putative cis-elements in the 1.5 kb promoter region of FvPMEs. Figure S3. QRT-PCR analysis of transcript levels of ripening-related genes in fruit of transient overexpression or silencing of FvPMEs.
Additional file 2: Table S1.
Primers used for this study. Table S2. Pectin methylesterases were identified in Arabidopsis thaliana, Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica and Rosa chinensis. Table S3. Conserved domains were predicted by CDD. Table S4. Gene duplication modes of PME genes in Arabidopsis thaliana, Fragaria vesca, Malus domestica, Pyrus bretschneideri, Prunus mume, Prunus persica and Rosa chinensis. Table S5. Cis-elements were predicted by PlantCARE in promoter sequences of FvPME genes.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xue, C., Guan, SC., Chen, JQ. et al. Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol 20, 13 (2020). https://doi.org/10.1186/s12870-019-2225-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-019-2225-9