Abstract
Background
The anoxic redox control binary system plays an important role in the response to oxygen as a signal in the environment. In particular, phosphorylated ArcA, as a global transcription factor, binds to the promoter regions of its target genes to regulate the expression of aerobic and anaerobic metabolism genes. However, the function of ArcA in Plesiomonas shigelloides is unknown.
Results
In the present study, P. shigelloides was used as the research object. The differences in growth, motility, biofilm formation, and virulence between the WT strain and the ΔarcA isogenic deletion mutant strain were compared. The data showed that the absence of arcA not only caused growth retardation of P. shigelloides in the log phase, but also greatly reduced the glucose utilization in M9 medium before the stationary phase. The motility of the ΔarcA mutant strain was either greatly reduced when grown in swim agar, or basically lost when grown in swarm agar. The electrophoretic mobility shift assay results showed that ArcA bound to the promoter regions of the flaK, rpoN, and cheV genes, indicating that ArcA directly regulates the expression of these three motility-related genes in P. shigelloides. Meanwhile, the ability of the ΔarcA strain to infect Caco-2 cells was reduced by 40%; on the contrary, its biofilm formation was enhanced. Furthermore, the complementation of the WT arcA gene from pBAD33-arcA+ was constructed and all of the above features of the pBAD33-arcA+ complemented strain were restored to the WT level.
Conclusions
We showed the effect of ArcA on the growth, motility, biofilm formation, and virulence of Plesiomonas shigelloides, and demonstrated that ArcA functions as a positive regulator controls the motility of P. shigelloides by directly regulating the expression of flaK, rpoN and cheV genes.
Similar content being viewed by others
Background
Plesiomonas shigelloides, a gram-negative, rod-shaped bacterium that causes foodborne intestinal infections [1], can cause gastroenteritis, including acute secretory gastroenteritis, an invasive shigellosis-like disease, and a cholera-like illness [2,3,4]. Escobar et al. found that co-infections of P. shigelloides with either rotavirus or pathogenic Escherichia coli were 16.2-fold (95% confidence interval (CI) 5.5–62.3) and 13.8-fold (95% CI 3.3–69.3) more likely to result in diarrhea, respectively [5]. Extra intestinal infections, such as meningitis, bacteremia, and pseudoappendicitis, including skin and soft tissue infections, are also associated with P. shigelloides infection [6,7,8]. Fresh and estuarine water are considered the natural environments of P. shigelloides, which is often isolated from fish and other seafood [9].
P. shigelloides can grow under both aerobic and anaerobic conditions [10, 11]. The enzymes required for catabolism under aerobic and anaerobic conditions are substantially different; therefore, at the same time, to respond to the availability of oxygen, it is necessary to regulate the expression of genes related to cell functions, such as nutrient absorption and excretion systems, biosynthetic pathways, and macromolecule synthesis [12]. The Arc two-component signal transduction system, comprising the kinase sensor ArcB and its cognate response regulator ArcA, is one of the mechanisms that enable E. coli to adapt to changing oxygen availability [13, 14]. ArcB is activated in the form of a simplified electron carrier under conditions of hypoxia and energy provided by ATP. It has three cytoplasmic domains, and the autophosphorylation of His292 in the H1 domain, followed by transfer of the phosphate group to Asp576 in the D1 domain, then to His717 in the H2 domain [15], and finally to Asp54 in ArcA results in phosphorylation of ArcA [16], which activates ArcA to promote or repress the expression of Arc-regulated genes.
A previous study indicated that about 1139 genes in the E. coli K-12 genome are regulated either directly or indirectly by ArcA [17]. Under anaerobic conditions, ArcA inhibits the expression of genes required for aerobic metabolism, energy generation, amino acid transport, and fatty acid transport [18]. Another transcription factor involved in controlling anaerobic gene expression and facilitating bacterial adaptation to anaerobic conditions is FNR (fumarate and nitrate respiration) [19]. A comparison of the ArcA and FNR regulons showed that 303 genes were regulated by both proteins [17]. Jiang et al. found that citrate utilization in an anaerobic environment in E. coli is under direct control of FNR via the CitA-CitB system and under indirect control by ArcA [20]. A recent study showed that ArcA overexpression in aerobic conditions results in downregulation of respiratory pathways and enhanced growth rates on glycolytic substrates of E. coli, coinciding with acetate excretion and increased carbon uptake rates [21].
ArcA also controls chemotaxis and motility, contributing to the pathogenicity of E. coli [22]. Kato et al. determined that the ΔarcA mutant displayed a motility-defective phenotype and ArcA is necessary for the expression of fliA [23]. Furthermore, in Salmonella enterica sv. Typhimurium, the ΔarcA mutant was also non-motile and lacked flagella [24]. Biofilms are sessile bacterial communities that predominate in nature, and may form wherever a solid surface is in contact with a liquid [25]. Many opportunistic pathogens are capable of biofilm formation. E. coli dominates biofilms found on urethral catheters, and has also been isolated from percutaneous trans-hepatic catheters [26, 27]. Previous studies on certain enterobacteria and non-enterobacteria have also reported the relationship between ArcA and biofilms. For example, Hengge proposed that ArcA has a regulatory role between the sigma factor RpoS and biofilm formation [28]. Xi et al. found that the response regulator ArcA enhances biofilm formation in a vpsT-dependent manner under anaerobic conditions in Vibrio cholerae [29]. In addition, studies on Actinobacillus pleuropneumoniae and Haemophilus parasuis also suggested that ArcA regulates the formation of biofilms positively [30, 31]. However, in Porphyromonas gingivali, Wu et al. showed that ArcA inhibits FimA production and inhibits biofilm formation [32].
In addition to ArcA being related to cell metabolism, biosynthesis, and motility, many studies have provided evidence that ArcA is related to virulence. For example, a recent study found that ArcA of E. coli K12, which causes human meningitis, downregulates the expression of sRNA-17 to benefit bacterial survival in blood and the penetration of the blood-brain barrier [33]. Moreover, ArcA is also required for the toxicity of Salmonella typhimurium, Vibrio cholerae, Haemophilus influenzae, and Actinobacillus pleuropneumoniae [34,35,36,37,38].
The effects of ArcA in P. shigelloides are unknown; therefore, the present study aimed to determine the correlation between ArcA and growth, motility, biofilm formation, and virulence in P. shigelloides.
Results
Phylogenetic analysis of ArcA
The two-component system response regulator ArcA of P. shigelloides is comprised of 238 amino acids. A phylogenetic tree based on ArcA amino acid sequences was constructed using the neighbor-joining method and plotted by MEGA 6.0. Bootstrap analysis was carried out based on 1000 replicates. The RopD protein of P. shigelloides was selected as the outgroup control. A dendrogram consisting of 17 species of bacteria, including some common human gut bacteria, was constructed. The comparison results showed that ArcA is conserved in all the selected bacteria. ArcA of P. shigelloides is relatively close to those from Proteus and Aeromonas, but far from those from Actinobacillus and Pseudomonas (Fig. 1).
Identification of the deletion and complementation of arcA
A schematic illustration of the overlap-extension PCR method used for deletion of arcA is shown in Fig. 2A. The deletion and identification of arcA is showed in Fig. 2B, in which SX (800 bp) and S-arcA-X (1517 bp) are the controls for ArcA− and ArcA+, respectively. The ΔarcA isogenic deletion mutant strain was obtained (Lane 4 in Fig. 2B). To further confirm the result, we designed arcA identification primers, arcA-F and arcA-R, to amplify the arcA gene from the genomes of ΔarcA and the WT, respectively. The PCR reaction generated a negative signal with ΔarcA and a positive one with the WT (717 bp).
Confirmation of the deletion and complementation of arcA in P. shigelloides. A Graphical process of the deletion of the arcA gene. B PCR detection of the product. 1, DL2000 DNA marker; 2, PCR fragment of SX; 3, PCR amplicon of S-arcA-X from the WT genomic DNA; 4, PCR amplificon of SX from the ΔarcA genome DNA; 5, PCR amplification of arcA from the ΔarcA genome DNA; 6, PCR amplification of arcA from the WT genome DNA. C 1, DL2000 DNA marker; 2, PCR amplification of pBAD33-UD from the pBAD33 plasmid; 3, PCR amplification of pBAD33-U-arcA-D from the arcA+ complementation strain; 4, PCR amplification of arcA from the genomic DNA of the complementation strain
The complementation of arcA is shown in Fig. 2C. The pBAD33-UD (529 bp) is a negative control. After complementation, pBAD33-U-arcA-D (1246 bp) and arcA (717 bp) were both amplified with the correct sizes.
ArcA affected the microaerobic growth of P. shigelloides
In this study, we used LB liquid medium and M9 minimal medium with only glucose as a carbon source to verify the role of ArcA in the growth and reproduction of P. shigelloides. When grown in LB liquid, the growth of ΔarcA slightly lagged behind that of the WT in the lag and log phases before 6 h, and the growth was completely restored to the WT level upon complementation with arcA (Fig. 3A). When grown in the M9 minimal medium with only glucose as the carbon source, the growth difference between ΔarcA and WT were obvious, and the ΔarcA mutants lagged behind the growth of WT before WT entered the stable phase at 12 h. Growth in M9 plus glucose was completely restored to the WT level upon complementation with arcA (Fig. 3B), which indicated that ArcA affects the uptake and utilization of glucose by P. shigelloides. In addition, the colony forming units were counted for the WT, ΔarcA and pBAD33-arcA+ strains at OD600 = 0.6, which showed that there was a 2.6-fold reduction for ΔarcA compared with that for the WT (Fig. 3C).
Deletion of arcA affected the growth of P. shigelloides in either LB medium or M9 medium under microaerobic conditions. A Bacterial strains were grown in LB and B M9 medium containing only glucose as a carbon source under microaerobic conditions, and the optical density at 600 nm (OD600) was monitored. C Bacterial strains were grown to OD600 = 0.6, and the plate colony counting method was used to count the three strains separately. The experiments were performed three times in quadruplicate. Significant differences were indicated by asterisks (***P < 0.001)
ArcA controls the motility of P. shigelloides by directly regulating the expression of flaK, rpoN and cheV genes
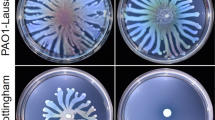
In addition to ArcA being related to the growth and metabolism of P. shigelloides, we also found that ArcA is related to motility. The WT, ΔarcA and pBAD33-arcA+ strains were freshly cultured, transferred to both swimming and swarming agar plates, and incubated at 25 °C for 24–72 h. When grown in swimming agar plates, the motility of the ΔarcA strain was markedly reduced compared with the WT. There was almost no obvious movement traces after the ΔarcA strain was grown for 24 h, and it spread by 2.8 cm when cultured for 72 h (Fig. 4B). In contrast, the WT and pBAD33-arcA+ strains had overgrown the plates under the same conditions at 72 h (Fig. 4A and C). The movement data of the strains in swimming agar plates are listed in Table 1. Moreover, when grown in swarming agar plates, the motility of the ΔarcA strain was totally lost, and there was no significant change even it was cultured for 72 h. Interestingly, the WT and pBAD33-arcA+ strains showed irregular trajectories similar to radials when grown in swarming agar plates (Fig. 4D), which was rarely mentioned in previous studies. The flagella produced by the WT, ΔarcA, and pBAD33-arcA+ strains were observed by TEM. Compared to the ΔarcA mutant strain with a single flagellum, the WT and pBAD33-arcA+ strains showed the typical three-four flagella (Fig. 4E). TEM results indicated that the lack of ArcA attenuates the flagella synthesis in Plesiomonas shigelloides.
Motility of the WT, ΔarcA and pBAD33-arcA+ strains. A The WT, B ΔarcA, and C pBAD33-arcA+ strains grown in swimming agar plates for 24 h, 48 h and 72 h, respectively. D From left to right, the WT, ΔarcA and pBAD33-arcA+ strains grown in swarming agar plates for 72 h. E TEM visualization of the flagella produce by the WT, ΔarcA and pBAD33-arcA+ strains from left to right. The hollow bacterial flagella were pointed by the colored arrows
A previous search for the putative ArcA binding sites at the flagella gene cluster promoter region was performed using Virtual Footprint 3.0. The analysis predicted the presence of ArcA binding sites in the promoter regions of flaK, rpoN and cheV genes (see Fig. S1A to C). To confirm a direct interaction between ArcA and the predicted binding sites, ArcA-His6 fusion protein was expressed and purified (Fig. S1D), three genes promoter region were generated by PCR and used to perform EMSA with phosphorylated ArcA (ArcA-P) and non-phosphorylated ArcA (non-ArcA-P) as the negative control. The complex of protein and DNA with ArcA-P were observed when incubated with flaK, rpoN and cheV promoter fragments (Fig. 5A, B and C). The negative control (non-ArcA-P) generated no shifts even at high protein concentration (2.0 μg). Then we performed the qRT-PCR and found that the expression of flaK, rpoN and cheV decreased approximately 5.6-, 4.3-, and 2.7-fold in the ΔarcA mutant compared to the WT (Fig. 5D). The data indicated that ArcA functions as a positive regulator controls the motility of P. shigelloides by directly regulating the expression of flaK, rpoN and cheV genes.
ArcA controls the motility of P. shigelloides by directly binding the promoter regions of flaK, rpoN and cheV genes. A The EMSA of phosphorylated ArcA protein and the flaK promoter. B The EMSA of phosphorylated ArcA protein and the rpoN promoter. C The EMSA of phosphorylated ArcA protein and the cheV promoter. The concentration of phosphorylated ArcA protein was increased gradually with the non-phosphorylated ArcA as a negative control (non-ArcA-P). D The mRNA level of flaK, rpoN and cheV of the WT and ΔarcA mutant. Significant differences were indicated by asterisks (**P < 0.01)
ArcA negatively regulates P. shigelloides biofilm formation
The biofilm formation assays were performed by both glass-tubes and 24-well plates. When the WT, ΔarcA and pBAD33-arcA+ strains were cultured in a glass-tube, the results showed that the WT could not form a biofilm. By contrast, the ΔarcA strains could form a biofilm circle at the surface of liquid, which was visible to the naked eyes. After arcA was complemented in the deletion strains, the biofilm formation ability disappeared (Fig. 6A). Furthermore, purple crystal violet staining was observed for the residue in the tubes containing the ΔarcA strain but in not the glass tubes that had contained the other two strains (Fig. 6B). In addition, we also quantitatively measured the biofilm formation ability and the results indicated that biofilm formation of ΔarcA (OD570 approximately 0.35) was 21.56-fold higher than that in the WT (Fig. 6C). In addition, for the bacteria were cultured in the 24-well culture plates, with LB only as the negative control. Compared to the ΔarcA strain, which formed an obvious biofilm at the bottom of the wells, only a small amount of residues was observed for the WT and pBAD33-arcA+ strains after being stained (Fig. 6D). The quantitative measurement results showed that biofilm formation ability of the ΔarcA (OD595 approximately 7.86) was 23.01-fold higher than that in the WT (Fig. 6E). The data of the above two biofilm formation assays indicated that ArcA fundamentally inhibits biofilm formation in P. shigelloides.
Biofilm formation assays with both glass tubes and 24-well culture plates. A The biofilm formation of the WT, ΔarcA and pBAD33-arcA+ strains cultured in the glass tubes. B The biofilm mass determined by staining surface-attached cells with crystal violet. C The biofilm formation was measured at OD570. D The biofilm formation of the WT, ΔarcA and pBAD33-arcA+ strains cultured in 24-well plates. E The biofilm formation was measured at OD595. Significant differences were indicated by asterisks (***P < 0.001)
ArcA enhances the invasion of Caco-2 cells in P. shigelloides
Compared with the P. shigelloides WT, the ΔarcA mutant showed a 40% reduction in its capacity to invade Caco-2 cells. In contrast to the biofilm results, the pBAD33-arcA+ complementation strain could restore the invasive ability only partially, failing to reach the same level as the WT (Fig. 7). The assay was repeated four times and the difference in invasion capabilities between the WT and ∆arcA was statistically significant (p = 0.0186). The data demonstrated that ArcA could enhance the ability to invade eukaryotic cells in P. shigelloides.
Discussion
As a facultative anaerobe, P. shigelloides can obtain energy under anaerobic or aerobic conditions through phosphorylation reactions related to electron transfer. The ArcAB binary regulatory system and the global regulatory protein FNR (ferric nitrate reductase) have been proven to play a major regulatory role in the metabolic process in response to changes in oxygen [39, 40]. Most of the known ArcA target genes of E. coli are related to aerobic respiration metabolism, and the DNA binding activity of ArcA is regulated by the reversible phosphorylation of ArcB [41]. Park et al. identified a total of 229 differentially expressed operons under anaerobic growth conditions by ChIP, among which ArcA has a direct regulatory effect on 85 of them by bioinformatic analysis [42]. At present, the role of ArcA in bacterial energy metabolism is not very clear. However, based on our comparison of the growth of P. shigelloides and the ΔarcA strain in the two media (LB and M9), it can be seen that ArcA has an impact on the metabolism of nutrients. When the ΔarcA strain was grown in M9 minimal medium with glucose as the carbon source, the glucose utilization rate was significantly lower than that of the WT before reaching the stable period. These results indicated that there is a certain connection between ArcA and the nutrition and energy metabolism of P. shigelloides.
In addition to the regulation of oxidative metabolism in bacteria, our data also confirmed that ArcA is related to bacterial motility. P. shigelloides is the unique member of the Enterobacteriaceae family that is able to produce polar flagella when grown in liquid medium and lateral flagella when grown in solid or semisolid media [43]. Previous studies have shown that P. shigelloides contained two different gene clusters, one exclusively for the lateral flagella biosynthesis and the other one containing the biosynthetic polar flagella genes [44]. The P. shigelloides polar flagella gene regions occupy higher similarity to those reported in Vibrio Parahemolyticus and Aeromonas hydrophila than the regions in E. coli or S. typhimurium [44, 45]. The primary regulatory factor of the polar flagella region of P. shigelloides is FlaK, not the FlhDC in E. coli. P. shigelloides lateral gene cluster is almost identical to the one of A. hydrophila [46]. However, no LafK ortholog could be detected in P. shigelloides even though the lafK gene has been reported in all the lateral gene clusters in the Enterobacteriaceae [46, 47]. In addition, we found that the trajectory of P. shigelloides in swarming agar plates was radial rather than circular, which was also different from the swarming motion shape of P. dendritiformis type-C [48] and Pseudomonas aeruginosa [49]. We suggest that the higher agar concentration of the swarming agar plates induced the production of lateral flagella in P. shigelloides, and resulted in a radial movement trajectory. Taken together, polar and lateral flagella transcriptional hierarchy in the P. shigelloides could represents a different Gammaproteobacteria model. Here, we provide evidence that ArcA could control the motility of P. shigelloides by directly regulating the expression of flaK, rpoN and cheV genes, and next we will focus on the flagella regulation mechanism of P. shigelloides in the future study.
Bacterial biofilms are bacteria that adhere to the surface of non-biological or active tissues in order to adapt to the living environment, and are coated in the mucus heterogeneous polymer matrix produced by themselves, forming a bacterial group that grows in a different way from planktonic bacteria [50]. Bacterial adhesion is the first step of bacterial biofilm formation. Previous studies reported that the groEL operon is related to adhesion and cell toxicity in P. shigelloides [51]. Edward et al. compared the genome sequence of 11 strains of Plesiomonas shigelloides and found that some strains contained biofilm forming proteins PgaA, PgaB and PgaC. However, subsequent experiments proved that Plesiomonas shigelloides strain EE2 can be formed even without these proteins. This indicated that P. shigelloides uses other mechanisms to regulate the formation of biofilms [52]. We found pgaC in the genome sequence of the P. shigelloides strain used in this experiment, but did not find pgaA and pgaB. At the same time, the WT showed almost no biofilm formation ability. However, after the arcA gene was deleted, the biofilm formation ability of the ΔarcA mutant strain was significantly enhanced, which indicated that ArcA has a relatively strong ability to inhibit the formation of P. shigelloides biofilms under normal conditions. Therefore, it is necessary to explore the relationship between ArcA and biofilm formation in subsequent studies. In the present study, our data also showed that ArcA is related to the virulence of P. shigelloides. Compared with the WT, the ΔarcA mutant showed a 40% reduction in infectivity of Caco-2 cells. However, the specific regulation mechanism is remains unclear. In addition, flagella [53,54,55], adhesin [56], Type 1 fimbriae [57], and curled fimbriae [58,59,60,61] are also essential for bacterial biofilm formation and virulence. They mediate the adhesion, movement, and chemotaxis of bacteria to help them seek advantages and avoid harm.
Conclusions
In this work, we report the roles of ArcA in P. shigelloides, and the data showed that ArcA could control the motility of P. shigelloides by directly regulating the expression of flaK, rpoN and cheV genes. And, the phenotype experiments in this study is significant for further discovering the specific links between ArcA and P. shigelloides in terms of growth, metabolism, biofilm formation, and virulence. Our results also laid a foundation to reveal the pathogenic mechanisms of P. shigelloides.
Materials and methods
Bacterial strains, growth conditions, and plasmids
The bacterial strains, as well as the plasmids used, are listed in Table 2. Bacteria were grown in tryptic soy broth (TSB), tryptic soy agar (TSA); and Luria-Bertani (LB) liquid, solid, and semi-solid medium at 37 °C statically or in a shaking incubator, or at 25 °C statically. If necessary, media were supplemented with ampicillin (25 μg/ml), chloramphenicol (25 μg/ml) or kanamycin (50 μg/ml).
Deletion and complementation studies of arcA
In this study, an effective and precise conjugate transfer process mediated by the suicide vector pRE112 was used to make deletion mutations in the arcA gene of P. shigelloides [65]. The complementation strains was constructed by introducing the recombinant vector pBAD33-arcA+ into the ΔarcA strain via electroporation. DNA sequencing were used to confirm the presence of the correct deletion mutations and complementation strains. And all primers used in this study are shown in Table 3.
RNA isolation and quantitative real time PCR (qRT-PCR)
Total RNA was extracted using TRIzol® Reagent (Invitrogen, Waltham, MA, USA #15596-018) according to the manufacturer’s protocol. qRT-PCR analysis was conducted on an Applied Biosystems ABI 7500 sequence detection system with SYBR green fluorescence dye. The P. shigelloides 16S rRNA gene was used as the internal control for qRT-PCR, and relative expression levels were calculated as fold change values using the 2-△△CT method. Each experiment was carried out in triplicate.
Electrophoretic mobility shift assay (EMSA)
E. coli BL21 (DE3) with pET28a-arcA+ was grown in 200 ml of LB medium for 5 h at 30 °C, and protein expression was induced by adding 0.1 mM isopropyl beta-D-1-thio-galactopyranoside (IPTG). The ArcA-His6 fusion protein was purified using an Ni-NTA-Sefinose Column (Sangon Biotech, Shanghai, China #C600791) in accordance with the protocol provided by the manufacturer. Phosphorylation reactions of ArcA were carried out as described previously [20]. EMSAs were performed by adding increasing amounts of purified and phosphorylated ArcA-His6 fusion protein (0, 0.4, 0.8, 1.2,1.6 and 2.0 μg) to the DNA probe (50 ng) in binding buffer (100 mM Tris-HCl pH 7.5, 10 mM MgCl2, 2 mM DTT, 100 mM KCl, 10% glycerol) for 30 min at 37 °C. DNA–protein complexes were separated by 6% PAGE in 0.5 × TBE buffer (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA, pH 8.0) at 160 V for 1 h. Gels were stained with GelRed for 10 min and imaged using a gel imaging system (GE Healthcare, Chicago, IL, USA).
Dynamic growth of the WT, ΔarcA and pBAD33-arcA + strains
The WT, ΔarcA and pBAD33-arcA+ bacterial strains were cultured overnight at 37 °C with shaking into sterile LB medium and until they reached an OD600 = 0.6. Then, the bacterial solution was added to five wells of a 96-well cell plate containing 200 μl of LB at a ratio of 1:200 per well. Fresh LB was added to the surrounding wells as a control. Finally, the prepared 96-well cell plate was placed in a Molecular Devices Spectra MAX 190 full-wavelength microplate reader (Molecular Devices, San Jose, CA, USA) to carry out the Dynamic growth experiment. The dynamic growth experiment for the WT, ΔarcA and pBAD33-arcA+ strains was also carried out in M9 medium, which contains only glucose as a carbon source. The temperature was controlled at 37 °C throughout the whole process. We conducted the experiments at three time points with five repetitions for each time.
Motility assays
The motility assays were performed as described previously [66]. Freshly grown bacterial colonies were transferred using a sterile toothpick into the center of swarming agar or swimming agar plates. The swimming agar plates were incubated for 24–72 h at 25 °C and motility was examined by the migration of bacteria through the agar from the center toward the plate periphery. Additionally, according to experimental requirements, the swarming agar plates were incubated up for 72 h at 25 °C. We conducted the experiments at three time points with six repetitions for each time.
Transmission electron microscopy (TEM)
TEM and negative staining used to visualize the flagella of the WT, ΔarcA, and pBAD33-arcA+ strains was as previously described [24].
Biofilm assay
In this study, we carried out the biofilm formation assay as described previously [67, 68] with some modifications. The WT, ΔarcA, and pBAD33-arcA+ strains were grown overnight in TSB. The next day, the overnight bacterial solution was transferred to fresh TSB and the bacteria were grown to OD600 = 0.6. The bacteria were then subcultured in fresh LB liquid medium at 1:100 and inoculated into 10 × 75 mm borosilicate glass test tubes containing 3 ml of sterile LB, and incubated at 37 °C for 20 h without shaking. Subsequently, the tubes were rinsed with phosphate-buffered saline (PBS) and filled with 0.1% crystal violet stain. After 5 min, the tubes were rinsed and then photographed. The biofilm-associated crystal violet was resuspended in dimethyl sulfoxide (DMSO), and the OD570 of the resulting suspension was measured. In addition, we also applied a 24-well tissue culture plate for the biofilm formation assay [52] on the WT, ΔarcA and pBAD33-arcA+ strains. All experiments were performed at three time-points independently and each individual samples were assayed in four repetitions.
Invasion assays
The invasion assay was carried out as described previously [69], with some modifications. Briefly, approximately 5 × 107 bacterial cells were layered onto confluent monolayers of approximately 1 × 105 Caco-2 cells (suspended in Dulbecco’s modified Eagle’s medium (DMEM)) per well in 24-well plates. The plates were centrifuged at 1000×g for 30 s to promote the sinking of bacteria, followed by incubation at 37 °C in 5% CO2 for 1 h. The monolayer washed extensively with PBS, and fresh, pre-warmed DMEM containing gentamycin (100 μg/ml) was added to kill extracellular bacteria. After 1 h of incubation, the monolayer was washed with PBS twice, and the cells were lysed with 0.1% Triton X-100 for 10 min. The released intracellular bacteria were enumerated using the plate counting method. The invasive ability was expressed as the percentage of the inoculum that survived the gentamycin treatment. We conducted the assay at four time points with six repetitions for each time.
Statistical analysis
Statistical analysis of the data was performed using analysis of variance (ANOVA). A probability value (P) ≤ 0.05 was considered statistically significant (*** p ≤ .001; ** p ≤ .01; * p ≤ .05; ns indicates not significant). The construction of the ArcA evolutionary tree used the Molecular Evolutionary Genetics Analysis (MEGA 6.0) software package [70].
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ArcA:
-
Anoxic redox control cognate response regulator
- WT:
-
Wild-type
- ΔarcA :
-
arcA isogenic deletion mutant strain
- pBAD33-arcA + :
-
Complementation strain of arcA
- LB:
-
Luria-Bertani
- M9:
-
M9 medium which contains only glucose as a carbon source
- PBS:
-
Phosphate-buffered saline
- EMSA:
-
Electrophoretic mobility shift
References
Maciejewska A, Bednarczyk B, Lugowski C, Lukasiewicz J. Structural studies of the lipopolysaccharide isolated from Plesiomonas shigelloides O22:H3 (CNCTC 90/89). Int J Mol Sci. 2020;21(18):6788.
Mandal BK, Whale K, Morson BC. Acute colitis due to Plesiomonas shigelloides. Br Med J (Clin Res Ed). 1982;285(6354):1539–40.
McNeeley D, Ivy P, Craft JC, Cohen I. Plesiomonas: biology of the organism and diseases in children. Pediatr Infect Dis. 1984;3(2):176–81.
Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275–80.
Escobar JC, Bhavnani D, Trueba G, Ponce K, Cevallos W, Eisenberg J. Plesiomonas shigelloides infection, Ecuador, 2004-2008. Emerg Infect Dis. 2012;18(2):322–4.
Billiet J, Kuypers S, Van Lierde S, Verhaegen J. Plesiomonas shigelloides meningitis and septicaemia in a neonate: report of a case and review of the literature. J Inf Secur. 1989;19(3):267–71.
Fischer K, Chakraborty T, Hof H, Kirchner T, Wamsler O. Pseudoappendicitis caused by Plesiomonas shigelloides. J Clin Microbiol. 1988;26(12):2675–7.
Pennycook KM, Pennycook KB, McCready TA, Kazanowski D. Severe cellulitis and bacteremia caused by Plesiomonas shigelloides following a traumatic freshwater injury. IDCases. 2019;19:e00637.
Salerno A, Cižnár I, Krovacek K, Conte M, Dumontet S, González-Rey C, et al. Phenotypic characterization and putative virulence factors of human, animal and environmental isolates of Plesiomonas shigelloides. Folia Microbiol (Praha). 2010;55(6):641–7.
Jandl G, Linke K. Bericht über zwei Fälle von akuter gastroenteritis durch Plesiomonas shigelloides [Report about two cases of gastroenteritis caused by Plesiomonas shigelloides (author’s transl)]. Zentralbl Bakteriol Orig A. 1976;236(1):136–40.
Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303–16.
Iuchi S, Lin EC. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988;85(6):1888–92.
Lynch AS, Lin EC. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J Bacteriol. 1996;178(21):6238–49.
Gunsalus RP, Park SJ. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res Microbiol. 1994;145(5-6):437–50.
Teran-Melo JL, Peña-Sandoval GR, Silva-Jimenez H, Rodriguez C, Alvarez AF, Georgellis D. Routes of phosphoryl group transfer during signal transmission and signal decay in the dimeric sensor histidine kinase ArcB. J Biol Chem. 2018;293(34):13214–23.
Jeon Y, Lee YS, Han JS, Kim JB, Hwang DS. Multimerization of phosphorylated and non-phosphorylated ArcA is necessary for the response regulator function of the Arc two-component signal transduction system. J Biol Chem. 2001;276(44):40873–9.
Salmon KA, Hung SP, Steffen NR, Krupp R, Baldi P, Hatfield GW, et al. Global gene expression profiling in Escherichia coli K12: effects of oxygen availability and ArcA. J Biol Chem. 2005;280(15):15084–96.
Nyström T, Larsson C, Gustafsson L. Bacterial defense against aging: role of the Escherichia coli ArcA regulator in gene expression, readjusted energy flux and survival during stasis. EMBO J. 1996;15(13):3219–28.
Myers KS, Yan H, Ong IM, Chung D, Liang K, Tran F, et al. Genome-scale analysis of Escherichia coli FNR reveals complex features of transcription factor binding. PLoS Genet. 2013;9(6):e1003565.
Jiang F, Huang X, Barbieri NL, Logue CM, Nolan LK, Li G. Citrate utilization under anaerobic environment in Escherichia coli is under direct control of Fnr and indirect control of ArcA and Fnr via CitA-CitB system. Environ Microbiol. 2021;23(3):1496–509.
Basan M, Hui S, Williamson JR. ArcA overexpression induces fermentation and results in enhanced growth rates of E. coli. Sci Rep. 2017;7(1):11866.
Jiang F, An C, Bao Y, Zhao X, Jernigan RL, Lithio A, et al. ArcA controls metabolism, chemotaxis, and motility contributing to the pathogenicity of avian pathogenic Escherichia coli. Infect Immun. 2015;83(9):3545–54.
Kato Y, Sugiura M, Mizuno T, Aiba H. Effect of the arcA mutation on the expression of flagella genes in Escherichia coli. Biosci Biotechnol Biochem. 2007;71(1):77–83.
Evans MR, Fink RC, Vazquez-Torres A, Porwollik S, Jones-Carson J, McClelland M, et al. Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol. 2011;11:58.
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45.
Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180(9):2442–9.
Dorel C, Vidal O, Prigent-Combaret C, Vallet I, Lejeune P. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol Lett. 1999;178(1):169–75.
Hengge R. The two-component network and the general stress sigma factor RpoS (sigma S) in Escherichia coli. Adv Exp Med Biol. 2008;631:40–53.
Xi D, Yang S, Liu Q, Li Y, Li Y, Yan J, et al. The response regulator ArcA enhances biofilm formation in the vpsT manner under the anaerobic condition in Vibrio cholerae. Microb Pathog. 2020;144:104197.
Buettner FF, Maas A, Gerlach GF. An Actinobacillus pleuropneumoniae arcA deletion mutant is attenuated and deficient in biofilm formation. Vet Microbiol. 2008;127(1-2):106–15.
Ding L, Wen X, He L, Yan X, Wen Y, Cao S, et al. The arcA gene contributes to the serum resistance and virulence of Haemophilus parasuis serovar 13 clinical strain EP3. Vet Microbiol. 2016;196:67–71.
Wu J, Xie H. Role of arginine deiminase of Streptococcus cristatus in Porphyromonas gingivalis colonization. Antimicrob Agents Chemother. 2010;54(11):4694–8.
Sun H, Song Y, Chen F, Zhou C, Liu P, Fan Y, et al. An ArcA-modulated small RNA in pathogenic Escherichia coli K1. Front Microbiol. 2020;11:574833.
Pardo-Esté C, Hidalgo AA, Aguirre C, Briones AC, Cabezas CE, Castro-Severyn J, et al. The ArcAB two-component regulatory system promotes resistance to reactive oxygen species and systemic infection by Salmonella typhimurium. PLoS One. 2018;13(9):e0203497.
Sengupta N, Paul K, Chowdhury R. The global regulator ArcA modulates expression of virulence factors in Vibrio cholerae. Infect Immun. 2003;71(10):5583–9.
De Souza-Hart JA, Blackstock W, Di Modugno V, Holland IB, Kok M. Two-component systems in Haemophilus influenzae: a regulatory role for ArcA in serum resistance. Infect Immun. 2003;71(1):163–72.
Wong SM, Alugupalli KR, Ram S, Akerley BJ. The ArcA regulon and oxidative stress resistance in Haemophilus influenzae. Mol Microbiol. 2007;64(5):1375–90.
Buettner FF, Bendallah IM, Bosse JT, Dreckmann K, Nash JH, Langford PR, et al. Analysis of the Actinobacillus pleuropneumoniae ArcA regulon identifies fumarate reductase as a determinant of virulence. Infect Immun. 2008;76(6):2284–95.
Iuchi S, Cameron DC, Lin EC. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J Bacteriol. 1989;171(2):868–73.
Green J, Crack JC, Thomson AJ, LeBrun NE. Bacterial sensors of oxygen. Curr Opin Microbiol. 2009;12(2):145–51.
Iuchi S, Lin EC. Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J Bacteriol. 1992;174(17):5617–23.
Park DM, Akhtar MS, Ansari AZ, Landick R, Kiley PJ. The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genet. 2013;9(10):e1003839.
Inoue K, Kosako Y, Suzuki K, Shimada T. Peritrichous flagellation in Plesiomonas shigelloides strains. Jpn J Med Sci Biol. 1991;44(3):141–6.
Merino S, Aquilini E, Fulton KM, Twine SM, Tomás JM. The polar and lateral flagella from Plesiomonas shigelloides are glycosylated with legionaminic acid. Front Microbiol. 2015;6:649.
De Maayer P, Pillay T, Coutinho TA. Flagella by numbers: comparative genomic analysis of the supernumerary flagellar systems among the Enterobacterales. BMC Genomics. 2020;21(1):670.
Canals R, Altarriba M, Vilches S, Horsburgh G, Shaw JG, Tomás JM, et al. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J Bacteriol. 2006;188(3):852–62.
Merino S, Shaw JG, Tomás JM. Bacterial lateral flagella: an inducible flagella system. FEMS Microbiol Lett. 2006;263(2):127–35.
Be'er A, Ariel G. A statistical physics view of swarming bacteria. Mov Ecol. 2019;7:9.
Murray TS, Kazmierczak BI. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella. J Bacteriol. 2008;190(8):2700–8.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Tsugawa H, Ito H, Ohshima M, Okawa Y. Cell adherence-promoted activity of Plesiomonas shigelloides groEL. J Med Microbiol. 2007;56(Pt 1):23–9.
Edwards MS, McLaughlin RW, Li J, Wan X, Liu Y, Xie H, et al. Putative virulence factors of Plesiomonas shigelloides. Antonie Van Leeuwenhoek. 2019;112(12):1815–26.
Hathroubi S, Hu S, Ottemann KM. Genetic requirements and transcriptomics of Helicobacter pylori biofilm formation on abiotic and biotic surfaces. NPJ Biofilms Microbiomes. 2020;6(1):56.
Laganenka L, López ME, Colin R, Sourjik V. Flagellum-mediated mechanosensing and RflP control motility state of pathogenic Escherichia coli. mBio. 2020;11(2):e02269–19.
Khan F, Tabassum N, Anand R, Kim YM. Motility of Vibrio spp.: regulation and controlling strategies. Appl Microbiol Biotechnol. 2020;104(19):8187–208.
Andreozzi E, Uhlich GA. PchE regulation of Escherichia coli O157:H7 flagella, controlling the transition to host cell attachment. Int J Mol Sci. 2020;21(13):4592.
Zhou M, Duan Q, Yang Y, Zhu G. Use of fimbrial rod for F18ab fimbriae+ STEC colonization to host cells. J Vis Exp. 2020;163:10.3791/61761.
Zhang M, Shi H, Zhang X, Zhang X, Huang Y. Cryo-EM structure of the nonameric CsgG-CsgF complex and its implications for controlling curli biogenesis in Enterobacteriaceae. PLoS Biol. 2020;18(6):e3000748.
Jain N, Chapman MR. Bacterial functional amyloids: order from disorder. Biochim Biophys Acta Proteins Proteomics. 2019;1867(10):954–60.
Dueholm MS, Albertsen M, Otzen D, Nielsen PH. Curli functional amyloid systems are phylogenetically widespread and display large diversity in operon and protein structure. PLoS One. 2012;7(12):e51274.
Klein RD, Shu Q, Cusumano ZT, Nagamatsu K, Gualberto NC, Lynch AJL, et al. Structure-function analysis of the curli accessory protein CsgE defines surfaces essential for coordinating amyloid fiber formation. mBio. 2018;9(4):e01349–18.
de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172(11):6568–72.
Edwards RA, Keller LH, Schifferli DM. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207(2):149–57.
Vettiger A, Basler M. Type VI secretion system substrates are transferred and reused among sister cells. Cell. 2016;167(1):99–110.e12.
Xi D, Jing F, Liu Q, Cao B. Plesiomonas shigelloides sipD mutant, generated by an efficient gene transfer system, is less invasive. J Microbiol Methods. 2019;159:75–80.
Wilhelms M, Fulton KM, Twine SM, Tomás JM, Merino S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J Biol Chem. 2012;287(33):27851–62.
Hung DT, Zhu J, Sturtevant D, Mekalanos JJ. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol Microbiol. 2006;59(1):193–201.
Jennings ME, Quick LN, Ubol N, Shrom S, Dollahon N, Wilson JW. Characterization of Salmonella type III secretion hyper-activity which results in biofilm-like cell aggregation. PLoS One. 2012;7(3):e33080.
Schubert RH, Holz-Bremer A. Cell adhesion of Plesiomonas shigelloides. Zentralbl Hyg Umweltmed. 1999;202(5):383–8.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Programs for Infectious Diseases of China (grant numbers 2017ZX10303405-001, 2017ZX10104002-001-006, 2018ZX1 0712001-017). The funding bodies had no role in the design of the study, collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Junxiang Yan: Investigation, Conceptualization, Project administration, Methodology, Writing - original draft. Yuehua Li: Project administration, Methodology, Writing - original draft. Xueqian Guo: Data curation, Formal analysis. Xiaochen Wang: Methodology, Formal analysis. Fenxia Liu: Software, Visualization. Ang Li: TEM, negative staining. Boyang Cao: Investigation, Conceptualization, Writing - original draft, Funding acquisition, Supervision, Writing - review & editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
A. Putative ArcA binding sites at the flaK promoter region. B. Putative ArcA binding sites at the rpoN promoter region. C. Putative ArcA binding sites at the cheV promoter region. D. The purity of the purified ArcA-His6 fusion protein was analyzed by 10% sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis.
Additional file 2: Figure S2.
Confirmation of the deletion of arcA in P. shigelloides. 1, DL2000 DNA marker (The bands shown in the electrophoretic gel are as follows: 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp and 100 bp); 2, PCR fragment of SX (800 bp); 3, PCR amplicon of S-arcA-X (1284 bp) from the WT genomic DNA; 4, PCR amplificon of SX from the ΔarcA genome DNA; 5, PCR amplification of arcA from the ΔarcA genome DNA; 6, PCR amplification of arcA (717 bp) from the WT genome DNA. Notice: SX,the upstream and downstream homologous fragments of arcA; S-arcA-X, PCR amplicon of the upstream and downstream of arcA and arcA. Moreover, Fig. 2B in manuscript was cropped from Figure S2. Figure S3. Confirmation of the complementation of arcA in P. shigelloides. 1, DL2000 DNA marker; 2, PCR amplification of pBAD33-UD (529 bp) from the pBAD33 plasmid; 3, PCR amplification of pBAD33-U-arcA-D (1246 bp) from the arcA+ complementation strain; 4, PCR amplification of arcA from the genomic DNA of the complementation strain. Notice: pBAD33- UD, The fragment obtained by PCR amplification of pBAD33 plasmid using identification primers; pBAD33-U-arcA-D. The fragment obtained by PCR amplification of pBAD33-arcA+ strain using identification primers. Figure 2C in manuscript was cropped from Figure S3. Figure S4. The EMSA between phosphorylated ArcA protein and the flaK promoter.The concentration of phosphorylated ArcA protein (ArcA-P) increased gradually (0 to 2.0 μg), the non-phosphorylated ArcA was used as a negative control (ArcA (−)) and the amount of promoter DNA used in each reaction was 50 ng. Figure 5A in manuscript was cropped from Figure S4. Figure S5. The EMSA between phosphorylated ArcA protein and the rpoN promoter. The concentration of phosphorylated ArcA protein (ArcA-P) increased gradually (0 to 2.0 μg), the non-phosphorylated ArcA was used as a negative control (ArcA (−)) and the amount of promoter DNA used in each reaction was 50 ng. Figure 5B in manuscript was cropped from Figure S5. Figure S6. The EMSA between phosphorylated ArcA protein and the cheV promoter.The concentration of phosphorylated ArcA protein (ArcA-P) increased gradually (0 to 2.0 μg), the non-phosphorylated ArcA was used as a negative control (ArcA (−)) and the amount of promoter DNA used in each reaction was 50 ng. Figure 5C in manuscript was cropped from Figure S6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, J., Li, Y., Guo, X. et al. The effect of ArcA on the growth, motility, biofilm formation, and virulence of Plesiomonas shigelloides. BMC Microbiol 21, 266 (2021). https://doi.org/10.1186/s12866-021-02322-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02322-y