Abstract
Background
A growing number of studies have demonstrated that the polar regions have the potential to be a significant repository of microbial resources and a potential source of active ingredients. Genome mining strategy plays a key role in the discovery of bioactive secondary metabolites (SMs) from microorganisms. This work highlighted deciphering the biosynthetic potential of an Arctic marine-derived strain Aspergillus sydowii MNP-2 by a combination of whole genome analysis and antiSMASH as well as feature-based molecular networking (MN) in the Global Natural Products Social Molecular Networking (GNPS).
Results
In this study, a high-quality whole genome sequence of an Arctic marine strain MNP-2, with a size of 34.9 Mb was successfully obtained. Its total number of genes predicted by BRAKER software was 13,218, and that of non-coding RNAs (rRNA, sRNA, snRNA, and tRNA) predicted by using INFERNAL software was 204. AntiSMASH results indicated that strain MNP-2 harbors 56 biosynthetic gene clusters (BGCs), including 18 NRPS/NRPS-like gene clusters, 10 PKS/PKS-like gene clusters, 8 terpene synthse gene clusters, 5 indole synthase gene clusters, 10 hybrid gene clusters, and 5 fungal-RiPP gene clusters. Metabolic analyses of strain MNP-2 grown on various media using GNPS networking revealed its great potential for the biosynthesis of bioactive SMs containing a variety of heterocyclic and bridge-ring structures. For example, compound G-8 exhibited a potent anti-HIV effect with an IC50 value of 7.2 nM and an EC50 value of 0.9 nM. Compound G-6 had excellent in vitro cytotoxicities against the K562, MCF-7, Hela, DU145, U1975, SGC-7901, A549, MOLT-4, and HL60 cell lines, with IC50 values ranging from 0.10 to 3.3 µM, and showed significant anti-viral (H1N1 and H3N2) activities with IC50 values of 15.9 and 30.0 µM, respectively.
Conclusions
These findings definitely improve our knowledge about the molecular biology of genus A. sydowii and would effectively unveil the biosynthetic potential of strain MNP-2 using genomics and metabolomics techniques.
Similar content being viewed by others
Background
A rich diversity of microorganisms exists in various polar habitats, and these microorganisms have evolved physiological, genetic, and metabolic characteristics adapted to extreme environments under the selection of long-term extreme environmental stresses, and thus have important basic research value and great application potential [1,2,3]. A growing number of studies have demonstrated that the polar regions have the potential to be a significant repository of microbial resources and a potential source of active ingredients. There were 263 new natural products discovered between 2001 and 2020 that were derived from polar organisms, 134 of which were polar microorganisms [4, 5]. These products covered a wide range of structural types, including alkaloids, macrolides, terpenoids, peptides, and polyketides, and they showed promising biological activities like antibacterial [6], antitumor [7], and antiviral [8] effects. The quantity of intriguing secondary metabolites with polar microbial origins has not altered considerably over the past few years, most likely because it is challenging to adapt polar bacteria to some common culture techniques.
Polar marine microbe-derived natural compounds offer a tremendous amount of potential for utilization as sources of therapeutic agent [9]. As fewer drugs become available, researchers are increasingly focusing on special microbial resources, such as habitat-specific microbes, and have begun to shift away from bioactivity-guided fractionation as the gold standard approach for natural product discovery, instead turning to genomics, metabolomics, and other big data approaches to guide isolation efforts towards uncharted chemical space [10, 11]. Even well-studied organisms have untapped biosynthetic potential because they encode a large number of biosynthetic genes that have not yet been connected to metabolite products [12]. Paulus [13] et al. localized the α-pyrone lagunapyrone biosynthetic gene cluster of a marine origin Streptomyces strain by antiSMASH and identified two analogues. Hou [14] et al. successfully identified seven cyclohexadepsipeptides, chrysogeamides A–G, from the coral-derived fungus Penicillium chrysogenum using MN. Liu [15] et al. used MN localization to determine two wealthy polycyclic macrolactam ansamycins from Streptomyces. Sun [16] et al. successfully obtained 11 omicsnin analogues while identifying a biosynthetic gene cluster for antiviral components by integrating antiSMASH, MN, and other techniques. Here, we generated the arctic marine-derived strain Aspergillus sydowii MNP-2 genome using a combination of Nanopore and Illumina sequencing data sets and used Nr, COG, KEGG, CO, Pfam, and some other databases for gene function annotation based on sequence similarity or Motif similarity search. Additionally, to investigate the natural product synthesis ability of strain MNP-2 further, we used the antiSMASH platform to analyze its BGCs and the GNPS platform to establish a molecular network based on LC-MS/MS data to analyze its metabolite profile and associate metabolites with BGCs.
Methods
Genome extraction and next-generation sequencing
The fungal strain MNP-2 of A. sydowii was derived from Arctic marine sediments (73.8° N 168.9° W) and is preserved at the China Center for Type Culture Collection (CCTCC NO: M 2,022,061). The strain MNP-2 grown on potato dextrose agar (PDA) media was inoculated into 250 mL Erlenmeyer flasks containing 100 mL potato dextrose broth (PDB) medium and shaken for 2 days at 200 rpm and 30 ℃. After centrifugation, the supernatant was removed and washed once with phosphate buffered solution (PBS) to obtain mycelium for storage at -80 ℃. The purity and integrity of the genomic DNA were evaluated using 1% agarose gel electrophoresis and densitometry on comparably sized standards.
The yield and purity of the collected DNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and a Qubit 2.0 fluorometer (Thermo Fisher Scientific, USA). After the DNA samples were tested and qualified, the libraries were built, and after they were finished, the libraries were diluted using Qubit 2.0 for initial quantification, and then the insert fragments of the libraries were tested using Agilent 2100. After the insert fragment size exceeded the expectation, the effective concentration of the libraries was correctly measured using the Q-PCR method to guarantee the libraries’ quality. After the libraries passed the test, they were divided into flow cells based on their effective concentrations and downstream data volume requirements. cBOT was formed into clusters and sequenced using Illumina NovaSeq, Illumina’s high-throughput sequencing platform.
SMRT sequencing
The qualified samples described in 3.1 were randomly interrupted by Megaruptor for genomic DNA, and large fragments of DNA were enriched and purified using magnetic beads. large fragments were cut and recovered using a BluePippin automated nucleic acid recovery instrument, and after purification, end repair and addition of acid was performed at both ends of the DNA fragments, and the SQK-LSK109 kit. Finally, the DNA library was accurately quantified using Qubit. After library construction, a certain concentration and volume of DNA library is added to the flow cell, and the flow cell is transferred to the Oxford Nanopore PromethION sequencer for real-time single-molecule sequencing.
Genome assembly
Because raw data may comprise low-quality sequences, joint sequences, and so on, the raw data must be filtered to obtain legitimate data (clean reads or pass reads) and then stored in FASTQ format to ensure the dependability of the information analysis results. SOAPnuke (v2.1.2, https://github.com/BGI-flexlab/SOAPnuke) is used to filter the raw data from next-generation sequencing. The raw data for third-generation sequencing is fast5 files, which are converted to fastq format after base calling with GUPPY, and then filtered to obtain valid data. K-mer values were automatically selected based on the read length and data type. NECAT (v0.0.1, https://github.com/xiaochuanle/NECAT) software was used to correct and splice the genome to obtain the initial splicing results, then Racon (v1.4.11, https://github.com/isovic/ racon) software was used to perform two rounds of error correction on the splicing results based on third generation sequencing data, and then Pilon (v1.23, https://github.com/broadinstitute/pilon) software was used to perform two rounds of next generation sequencing error correction on the initial assembly results after third generation sequencing error correction. The final assembly results were obtained by deduplicating the corrected genomes using purge_haplotigs (v1.1.2, https://github.com/skingan/purg e_haplotigs_multiBAM).
Gene annotation
Gene structure prediction allows researchers to obtain extensive information about the genome’s gene distribution and structure, as well as vital raw materials for functional annotation and evolutionary study. Gene annotation of the MNP-2 genome was conducted using BRAKER (v2.1.4, https://github.com/Gaius-Augustus/BRAKER) software, which is a combination of GeneMark-ET [17], and AUGUSTUS [18]. The annotation of gene functions and metabolic pathways based on existing databases, containing predictions such as Motif, structural domains, protein activities, and information about the metabolic pathways in which they are placed, is referred to as functional annotation of genes. Gene function annotation was performed on strain MNP-2 using nine databases, including Nr (https://ftp.ncbi.nlm.nih.gov), Pfam (https://pfam.xfam.org/), eggCOG (https://www.ncbi.nlm.nih.gov/COG/), Uniprot (https://www.uniprot.org/), KEGG (https://www.kegg.jp/kegg/), GO (http://geneontology.org/), Pathway (http://www.pathwaycommons.org/), Refseq (https://www.ncbi.nlm.nih.gov/refseq/), Interproscan (https://github.com/ebi-pf-team /interproscan), and so on, in order to acquire comprehensive gene function information.
Non-coding RNA annotation
Non-coding RNAs can all be transcribed from the genome, but rather than being translated into proteins, they can carry out their biological tasks at the RNA level. TRNA and rRNA are two of them that are directly engaged in protein synthesis. Using INFERNAL (v1.1.2, https://github.com/EddyRivasLab/in fernal) software based on the Rfam database (http://rfam.xfam.org/), various forms of ncRNAs were predicted and statistically categorised.
Repetitive sequence annotation
Scattered repeats and tandem repeats are two types of repeated sequences. LTR, LINE, SINE, and DNA transposons are examples of scattered repetitive sequences, also known as transposon elements. They can be characterized as highly repetitive sequences, moderately repetitive sequences, or low repetitive sequences based on the number of repeats. The software RepeatModeler (v1.0.4, https://github.com/Dfam consortiu m/RepeatModeler) was used to create its own repeat library, and RepeatMasker (v4.0.5, https://github.com/rmhubley/RepeatMasker) was used to annotate the genome with repetitive sequences.
Prediction of carbohydrate-active enzymes (CAZymes)
CAZymes are a very important class of enzymes classified as Glycoside Hydrolases (GHs), Glycosyl Transferases (GTs), Polysaccharide Lyases (PLs), Carbohydrate Esterases (CEs), Auxiliary Activities (AAs), Carbohydrate-Binding Modules (CBMs), and so on. The research of carbohydrate-related enzymes can yield a lot of useful biological information. The CAZy database can be used to investigate carbohydrase genomic, structural, and biochemical information. HMMER (v3.2.1, https://github.com/EddyRivasLab/hmmer) was used to annotate protein sequences based on the CAZy database (filtering parameters: E-value < e− 18, coverage > 0.35, http://www.cazy.org/).
Analysis of pathogen-host interaction (PHI)
PHI is a database of pathogen-host interactions with experimentally validated content derived primarily from fungal, oomycete, and bacterial pathogen-infected hosts such as animals, plants, and insects. The target protein sequences were annotated using Diamond blastp (v2.9.0, https://github.com/enormandeau/ ncbi_b last_tutorial) based on the PHI database (http://www.phi-base.org).
Prediction of drug-resistant gene
The CARD framework is built as an Antibiotic Resistance Ontology (ARO) taxonomic unit to correlate information on antibiotic modules and their targets, resistance mechanisms, gene variants, etc. The comparison results display the position of each gene annotated in the CARD database (https://card.mc maste r.ca/), as well as the ARO ID and classification description, which can be used to understand the specific function of each gene related to antibiotic resistance.
Cytochromes P450 (CYP450) annotation
CYP450 is a large protein family that catalyzes the oxidation of a variety of substrates and participates in the metabolism of endogenous and exogenous substances such as drugs and environmental compounds. The target protein sequences were annotated using Diamond blastp based on the FungalP450 database (http://drnelson.utmem.edu/CytochromeP450.html).
Prediction of virulence gene
Database of fungal virulence factors (DFVF, http://sysbio.unl.edu/DFVF/) is a database dedicated to the study of fungal virulence factors. To investigate the virulence-related genes present in strain MNP-2, the predicted protein sequences were compared with DFVF using Diamond blastp.
Other annotations
Classification of membrane transporter proteins using Transporter Classification Database (TCDB, http://www.tcdb.org/). All predicted gene pair protein sequences were analyzed using the software signalP (v5.0, http://www.cbs.dtu.dk/services/SignalP/) to identify proteins containing signal peptides. To identify proteins containing transmembrane helices and secreted proteins, all predicted gene-to-protein sequences were analyzed using the software tmhmm (v2.0, http://www.cbs.dtu.dk/services/TMHMM/).
Prediction of biosynthetic gene clusters (BGCs)
The genes responsible for secondary metabolite production are typically organized in BGCs. antiSMASH (v7.1.0, https://docs.antismash.secondarymetabolites.org/) is the most extensively used tool for finding and characterizing BGCs in bacteria and fungi at the moment. antiSMASH employs a rule-based technique to detect a variety of SM-producing biosynthetic pathways. For BGCs encoding NRPSs, type I and type II PKSs, lanthipeptides, lasso peptides, sactipeptides, and thiopeptides, which cluster-specific analyses can provide more information about the biosynthetic steps performed and thus provide more detailed predictions on the compounds produced, more in-depth analyses are performed.
Analysis of molecular networking (MN)
MN has swiftly become an extensively used technology in the field of natural products chemistry, with applications ranging from dereplication to genome mining, metabolomics, and chemical space visualization since the advent of the online open-source Global Natural Products Social (GNPS, https://gnps.ucsd.edu/). The samples were dissolved in methanol (1 mg/mL) and analyzed using a SCIEX X500 QTOF (SCIEX, USA) mass spectrometer to generate LC-MS/MS data, which were pre-processed by Mzmine and analyzed on the GNPS online platform to generate molecular networks, which were visualized using Cytoscape. Separation was done on a chromatographic column (Phenomenex, H19-046789, 1.7 μm C18 100 Å, 150 × 2.1 mm, maintained at 40 °C with a flow rate of 0.3 mL/min). A linear gradient system composed water and acetonitrile was used, starting from 10% (vol/vol) acetonitrile and increasing to 100% in 20 min. Samples were analyzed in positive electrospray scanning, m/z 50 − 1,500.
Fermentation and extraction
The strain MNP-2 grown on potato dextrose agar (PDA) medium was inoculated into 500 mL Erlenmeyer flasks containing 200 mL potato dextrose broth (PDB) medium, and shaken for 3 days at 200 rpm and 30 ℃. The fermentation was performed in Erlenmeyer flasks (2 × 1 L) with sterilized rice (80 g) and tap water (120 mL). After autoclaving at 121 ℃ for 20 min, each flask was inoculated with 5% seed cultures and then incubated at room temperature under static conditions for 30 days. The fermented rice in each flask was extracted with 500 mL EtOAc by an ultrasonic instrument for 20 min three times followed by filtration using gauze. All the filtrate was combined and evaporated under vacuum to dryness, obtaining the sample 1 (approx. 1.56 g). The strain MNP-2 was inoculated in 500 mL flasks containing 200 mL Czapek-Dox or PDB Medium (2 flasks each) and shaken for 15 days at 200 rpm and 30 ℃. After completion of fermentation, the fermentation broth was extracted three times by EtOAc (twice the volume of the fermentation broth) and evaporated under vacuum to dryness, obtaining samples 2 (approx. 0.27 g) and 3 (approx 0.31 g). The culture medium composition is shown in the supporting material.
Antimicrobial assay
Antimicrobial activities were carried out according to the filter paper disc (5 mm in diameter) diffusion technique [19, 20]. Two human pathogenic strains of Staphylococus aureus ATCC 25,923 and Escherichia coli ATCC 25,922 were obtained from Nanjing Medical University (China). The pathogenic bacteria were incubated in Luria-Bertani (LB) medium at 37 °C for 24 h and then spread evenly on LB solid plates. The samples were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/mL for crude extract and 2 mg/mL for ampicillin sodium. Each disc was impregnated with 20 µl of samples and ampicillin sodium. Discs with DMSO (20 µl) served as a control.
The discs were dried at 37 °C for 2 h and introduced to the surface of the medium (Containing the pathogenic bacteria) using sterile tweezers. The plates were incubated at 37 °C for 24 h to obtain zones of inhibition. The experiments were repeated three times.
Results
Morphology, classification and phylogenetic analysis of strain MNP-2
On potato dextrose agar PDA medium, strain MNP-2 grows quickly, starting out as white filamentous, turning green in 2–3 days, and eventually turning dark green and powdery in a few days (Fig. 1a). Mycelium is more branched, septate multinucleate, conidial peduncle apical expansion into a spherical apical capsule, and a small peduncle bearing a string of conidia, according to electron microscope observations (Fig. 1b).
The nuclear ribosomal DNA (nr DNA) internal transcribed spacer region (ITS) of strain MNP-2 was amplified and sequenced, and the ITS sequence was searched for homology in the nucleic acid database genbank. Blast analysis [21] of the ITS gene sequence revealed that strain MNP-2 had the highest similarity with the strain A. sydowii CBS 593.65 (100%, Fig. 2).
Genome feature of strain MNP-2
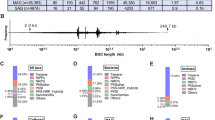
The whole genome of strain MNP-2 contains 10 contigs with an N50 of 4.1 Mb and 50.0% GC content (Table 1), and its size was determined as 34.9 Mb (Fig. 3). N50 is the shortest contig length that needs to be included for covering 50% of the genome. In general, the contig N50 size of the genome is used to assess genome continuity; the larger the contig N50, the better the genome continuity. The overall genomic characteristics are similar to those of the five strains of A. sydowii currently included in the NCBI database (https://www.ncbi.nlm.nih.gov/) (Fig. S2).
Genome diagram of strain MNP-2. From inside to outside, the first line shows in-paralog pairs; The second circle shows GC skew; The third circle shows GC content; The fourth circle shows putative BGCs; The fifth circle shows nc RNA; The sixth shows repeat; The seventh circle and the eighth circle display protein-encoding sequences (CDSs) annotation information, and different colors represent different COG function classification. The seventh circle indicates that CDSs is in a negative chain, and the eighth circle indicates that CDSs is in a positive chain. The outer rim shows the scaffold
Prediction of genetic structure
The prediction and completeness evaluation of coding genes showed that there were 13,218 total genes, with an average mRNA length of 1,610.07 bp, an average CDS length of 1,444.90 bp, a total of 42,982 exons, a total of 29,764 introns, an average number of exons per gene of 3.25, an average exon length of 444.34 bp, and an average intron length of 29,764 bp. The gene had an average exon count of 3.25, an average exon length of 444.34, an average intron length of 73.35, and a single copy BUSCO of 98% [22] (Table S1, Fig. S3). Results of non-coding RNA annotation for rRNA, sRNA, snRNA, and tRNA were 46, 7, 32, and 119, respectively (Table S2). According to the repeat sequence annotation results, there were 19 SINE (Short interspersed element), 414 LINE (Long interspersed nuclear element), 1,728 L (Long terminal repeat), 606 DNA transposons, 40 satellite DNAs, and 91 others (Table S3).
Annotation of gene functions
The total number of predicted genes was 13,218 of these, the number of genes with annotation information was 12,912 (97.68%), and the total number of functional gene annotations in the databases of Nr, Pfam [23], eggCOG [24], Uniprot [25], KEGG [26], GO [27], Pathway [28], Refseq [29], and Interproscan [30] were 12,894 (97.55%), 10,642 (80.39%), 1,047 (7.92%), 7,772 (58.8%), 2,946 (22.29%), 7,693 (58.20%), 2,764 (20.91%), 6,252 (47.30%), 10,626 (80.39%, Table S4), respectively. According to the analysis of the Nr library comparison annotation results, the species with the most strain MNP-2 comparisons was Aspergillus sp. fungi (Fig. 4a). The COG database, which was created based on the evolutionary connections between bacteria, algae, and eukaryotes, can be used to categorize genes according to their direct homology. Energy generation and conversion, amino acid transport and metabolism, carbohydrate transport and metabolism, and lipid transport and metabolism are the COG group’s more prevalent categories, according to the examination of COG data (Fig. 4b). The sequences’ major classification after KEGG annotation was broken down into cellular processes, environmental information processing, genetic information processing, metabolism, cellular systems, etc. Among them, metabolism (3,823, 56.25%) has the most annotated genes, particularly involved in carbohydrate metabolism and amino acid metabolism, with 742 and 732 annotated genes, respectively. These annotated genes suggest the existence of rich and diverse functions for protein and lipid metabolism, resulting in higher energy conversion efficiency (Fig. 4c). The GOslim classification was obtained by simplifying the GO annotation information, and the top 20 most annotated GOslim secondary classifications under each classification were chosen for mapping (Fig. 4e) after summarizing the gene functions in terms of cellular components, molecular functions, and biological processes. The gene enrichment of each GO secondary function in the context of all genes was used to understand the status of each secondary function. The Pfam database contains information about protein families. The genes annotated in each structural domain were statistically summarized, and the top 20 annotated structural domains were mapped (Fig. 4d), with the number of genes matching on the Major Facilitator Superfamily (MFS) found to be the highest, 620, according to the annotations.
Annotation of gene function. (a) Distribution of species obtained from Non-redundant protein sequence (Nr) database matching. (b) Classification statistics with Cluster of Orthologous Group of proteins (COG) annotations. (c) Functional classification of Kyoto Encyclopedia of Gene and Genome (KEGG) pathway. (d) Classification statistics with Pfam annotations. (e) Classification statistics with Gene Ontology (CO) annotations
Annotations to proprietary databases
In addition to the studies mentioned above, 6 carbohydrate-related enzymes were annotated using the Carbohydrate-active enzymes (CAZy) database [31](Table S5). Based on the Pathogen Host Interactions (PHI) database [32], 6 assay sequences were annotated, and the target sequences and similarity of the database matches were given (Table S6). 10 drug resistance genes were annotated using the Comprehensive Antibiotic Research database (CARD) [33]to learn more about the drug resistance genes present in each genome (Table S7). On the basis of the FungalP450 database [34], a total of 1366 cytochrome P450 (CYP450) protein sequences were annotated. CYP450 is a broad family of proteins with ferroheme as a cofactor (Table S8). The predicted protein sequences were compared with the Database of Fungal Virulence Factors (DFVF) [35], and a total of 6 virulence-related genes were found in the sequenced strains (Table S9). Meanwhile, 2139 membrane transport proteins were annotated in the Transporter Classification Database (TCDB) [36], 1182 protein sequences containing signal peptides were predicted by SignalP software, and 2729 protein sequences containing transmembrane proteins and 926 protein sequences containing secreted proteins were predicted by TMHMM (Table S10). The analysis software and database information used in the study are shown in Table S11.
Prediction of secondary metabolite clusters (BGCs)
SM clusters prediction in A. sydowii was done using an available software packages antiSMASH [37]. A total of 287 putative BGCs were detected in the 6 genomes, corresponding to an average of 47.8 per strain, with the highest number of 56 BGCs being observed in strain MNP-2 (A. sydowii Fsh102 43 SM clusters, A. sydowii AS31 48 SM clusters, A. sydowii AS42 43 SM clusters, A. sydowii BOBA1 50 SM clusters, A. sydowii CBS 593.65 47 SM clusters, Fig. 5). The predicted SM clusters of strains are defined by the “backbone enzymes” that generate the putative SM’s carbon skeleton. The majority of the “backbone enzymes” in strain MNP-2 are polyketide synthase (PKS) or non-ribosomal peptides synthase (NRPS). 18 SM clusters contain sequence coding for a NRPS/NRPS-like enzymes, 10 SM clusters contain sequence coding for a PKS/PKS-like enzymes and 10 SM clusters are hybrid clusters. The remaining SM clusters seem to be required in terpene/terpenoid metabolites production as the “backbone enzyme” is a terpene cyclase (8 SM clusters) or indole synthase (5 SM clusters). These predicted BGCs reveal that strain MNP-2 has a diverse variety of secondary metabolite production potential, and some of them are strikingly similar to BGCs from compounds like neosartorin (A-1) [38], nidulanin A (A-2) [39], asperlactone (A-3) [40], squalestatin (A-4) [41], penicillin (A-5) [42], fellutamide B (A-6) [43], equisetin (A-7) [44], destruxin A (A-8) [45], and others that have been reported in current publications (Fig. 6). These compounds are all secondary metabolites produced by microorgnisms and exhibit a variety of biological functions (Table S12). Some of the substances mentioned above demonstrate the ability of strain MNP-2 to produce these kinds of substances.
Synteny plots of investigated clusters made using Easyfig BLASTn. (a) The synteny of the predicted neosartorin A cluster in strain MNP-2 (Location 1,037,103-1,079,796 nt) and the identified candidate cluster in A. novofumigatus IBT 16,806. (b) The synteny of the predicted nidulanin A cluster in strain MNP-2 (Location 4,611,786-4,665,953 nt) and the identified candidate cluster in A. nidulans FGSC A4. (c) The synteny of the predicted asperlactone cluster in strain MNP-2 (Location 449,413–499,803 nt) and the identified candidate cluster in Aspergillus ochraceus. (d) The synteny of the predicted squalestatin cluster in strain MNP-2 (Location 3,433,532-3,454,746 nt) and the identified candidate cluster in Aspergillus sp. Z5. (e) The synteny of the predicted penicillin cluster in strain MNP-2 (Location 219,956 − 272,605 nt) and the identified candidate cluster in Penicillium chrysogenum.(f) The synteny of the predicted fellutamide B cluster in strain MNP-2 (Location 220,650 − 272,412 nt) and the identified candidate cluster in A. nidulans FGSC A4. (g) The synteny of the predicted equisetin cluster in strain MNP-2 (Location 777,609–829,611 nt) and the identified candidate cluster in Fusarium heterosporum.(h) The synteny of the predicted destruxin A cluster in strain MNP-2 (Location 2,262,285-2,306,801 nt) and the identified candidate cluster in Metarhizium robertsii ARSEF 23. The core biosynthetic genes has been highlighted with red, the regulatory genes has been highlighted in green, the additional biosynthetic genes has been highlighted in pink, the transport-related genes has been highlighted in blue and and gray markers represent genes without functional annotation
Analysis of molecular networking (MN)
The metabolite analysis demonstrated that strain MNP-2 has the capacity to create complex molecular skeletons, particularly some heterocyclic or compound skeletons with a bridge-ring (G-1 - G-8, Fig. 7). These compounds come from a wide range of sources and have diverse biological activities (Table S13) [46,47,48,49,50]. Destruxin A (A-8), a cyclic peptide with strong bioactivity, was also found in sample 1 (Fig. 7, G-4), and the antiSMASH analytical platform was used to look into its potential BGC (Fig. 6h). Similarly, neosartorin (A-1), a xanthone analogue, and asperlactone (A-3), which contains a lactone ring, were both found to have similar structures present in the MN (G-6, G-7), greatly facilitating the analysis of biosynthetic pathways for this class of compounds. Furthemore, the majority of the metabolites analyzed were not matched to the corresponding BGCs, indicating a significant research gap that needs to be filled.
Molecular network analysis of strain MNP-2-derived secondary metabolites. Sample 1 is the crude extract of strain MNP-2 grown on rice-solid medium for 30 days (cyan node), with the sterile rice-solid medium crude extract serving as control 1(blue node); Sample 2 is the crude extract of strain MNP-2 cultivated in Czapek-Dox medium for 15 days (yellow node), with the sterile Czapek-Dox medium crude extract serving as control 2 (red node); Sample 3 is the crude extract of strain MNP-2 cultivated PDB for 15 days (purple node), with the sterile PDB medium extract serving as control 3 (orange node)
The analysis revealed that the metabolites of strain MNP-2 were extremely abundant, and their structural features mainly included aromatic polyketides, peptides, alkaloids, terpenoids, and fatty acids. In rice-solid medium, strain MNP-2 had the most abundant metabolites, as shown in Fig. 7. Furthermore, there are more monochromatic block nodes in the molecular network, indicating that strain MNP-2 has significant metabolic differences in different mediums. There were many unknown nodes present in clusters, and some of the marker compounds were closely related to the BGCs of strain MNP-2.
Antimicrobial activity
The antibacterial activity of these samples was evaluated by the filter paper disc diffusion technique, with ampicillin sodium as the positive control (Fig. S1). The results showed that samples 1 and 2 had moderate inhibitory effects on S. aureus ATCC 25923, with inhibition zones of 10.04 ± 0.49 and 10.40 ± 0.36 mm, respectively.
Discussion
Till now, only five complete genome sequences of A. sydowii strains from various sources had been deposited in the NCBI database. Comparative analysis of the total gene features of these strains with those of A. sydowii MNP-2 showed that the strain MNP-2 possesses the middle level of genome size (34.9 Mb) and GC content (50%). However, the contig number of strain MNP-2 was only 10, suggesting the quality of genome assembly is more higher than others.
AntiSMASH study revealed that strain MNP-2 possessed 56 BGCs, including NRPS/NRPS-like (18 SM clusters), PKS/PKS-like (10 SM clusters), terpene cyclase (8 SM clusters), indole synthase (5 SM clusters), heterozygous route (10 SM clusters), and fungal-RiPP (5 SM clusters). Compared to the strains A. sydowii Fsh102, A. sydowii AS31, A. sydowii AS42, and A. sydowii BOBA1, A. sydowii CBS 593.65, the strain A. sydowii MNP-2 had the highest number of BGCs. Some BGCs may manufacture several complicated structurally active compounds (such as A-1 - A-8) [38,39,40,41,42,43,44,45]. However, the majority of the other BGCs do not match similar clusters that might synthesize unique chemical skeleton. Although the types of BGCs can be accurately predicted based on the core SM biosynthetic genes encoding backbone enzymes, it is still impossible to exactly predict the boundaries of BGCs or the functions of some clusters without backbone enzymes. This is due to the fact that a large number of genes around the core SM biosynthetic genes in strain MNP-2 cannot been characterized using open-source bioinformatics tools [51, 52]. Metabolic analyses of strain MNP-2 grown on various media (rice-solid, Czapek-Dox and PDB) using GNPS networking revealed its great potential of biosynthesis of bioactive SMs containing a variety of heterocyclic and bridge-ring structures. For example, compound G-8 exhibited potent anti-HIV effect with an IC50 value of 7.2 nM and an EC50 value of 0.9 nM [50]. Compound G-6 had excellent in vitro cytotoxicities against the K562, MCF-7, Hela, DU145, U1975, SGC-7901, A549, MOLT-4 and HL60 cell lines with IC50 values ranged from 0.10 to 3.3 µM, and showed significant anti-viral (H1N1 and H3N2) activities with IC50 values of 15.9 and 30.0 µM, respectively [48]. These findings indicate that the Arctic marine-derived strain MNP-2 is one of prolific producers of therapeutic agents. To deeply mine the biosynthetic potential of strain MNP-2, leveraging genomic and metabolomic data rapidly facilitates assessing the novelty of metabolites and linking them to their BGCs [53,54,55].
Conclusions
As one of underexploited organisms on earth, polar marine-derived microbes harbor more diversified genes for the biosynthesis of functional natural products1. In this study, a high-quality whole genome sequence of an Arctic marine strain MNP-2 with a size of 34.9 Mb was successfully obtained. Its total number of genes predicted by BRAKER software was 13,218, and that of non-coding RNAs (rRNA, sRNA, snRNA, tRNA) predicted by using INFERNAL software was 204. The number of annotated genes was found to be 12,912, accounting for 97.68% of all genes using the Nr, Pfam, eggCOG, KEGG and GO databases. The results of these analyses are significant for gene resource mining and polar microbial genome investigations. Additionally, antiSMASH results indicated that strain MNP-2 harbors 56 BGCs, which can produce SMs with various structure motifs. This work effectively unveiled the biosynthetic potential of strain MNP-2 using genomics and metabolomics techniques. Various genome mining strategies should be further employed to awaken most cryptic BGCs in this strain to produce novel and/or valuable SMs [56], such as ribosome engineering [57], metabolic engineering [58], global regulators [59], protein modification genes [60], heterologous expression [61], promoter exchange [62], BGC refactoring [63], BGC-specific regulators [64].
Data availability
The complete genome sequence data reported in this study are available within NCBI GCA_034192605.1.
Abbreviations
- SMs:
-
secondary metabolites
- MN:
-
molecular networking
- GNPS:
-
Global Natural Products Social Molecular Networking
- BGCs:
-
biosynthetic gene clusters
- PDA:
-
potato dextrose agar
- PDB:
-
potato dextrose broth
- PBS:
-
phosphate buffered solution
- CAZymes:
-
carbohydrate-active enzymes
- GHs:
-
Glycoside Hydrolases
- GTs:
-
Glycosyl Transferases
- PLs:
-
Polysaccharide Lyases
- CEs:
-
Carbohydrate Esterases
- AAs:
-
Auxiliary Activities
- CBMs:
-
Carbohydrate-Binding Modules
- PHI:
-
pathogen-host interaction
- ARO:
-
Antibiotic Resistance Ontology
- CYP450:
-
Cytochromes P450
- DFVF:
-
Database of fungal virulence factors
- TCDB:
-
Transporter Classification Database
- ITS:
-
internal transcribed spacer region
- CDSs:
-
protein-encoding sequences
- SINE:
-
Short interspersed element
- LINE:
-
Long interspersed nuclear element
- LTR:
-
Long terminal repeat
- MFS:
-
Major Facilitator Superfamily
- Nr:
-
Non-redundant protein sequence
- COG:
-
Cluster of Orthologous Group of proteins
- KEGG:
-
Kyoto Encyclopedia of Gene and Genome
- CO:
-
Gene Ontology
- CARD:
-
Comprehensive Antibiotic Research database
- TCDB:
-
Transporter Classification Database
- PKS:
-
polyketide synthase
- NRPS:
-
non-ribosomal peptides synthase
References
Santiago IF, Soares MA, Rosa CA, Rosa LH, Lichensphere. A protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles. 2015;19(6):1087–97. https://doi.org/10.1007/s00792-015-0781-y.
Makhalanyane TP, Van Goethem MW, Cowan DA. Microbial diversity and functional capacity in polar soils. Curr Opin Biotech. 2016;38:159–66. https://doi.org/10.1016/j.copbio.2016.01.011.
Liu JT, Lu XL, Liu XY, Gao Y, Hu B, Jiao BH, et al. Bioactive natural products from the Antarctic and arctic organisms. Mini-Rev Med Chem. 2013;13(4):617–26. https://doi.org/10.2174/1389557511313040013.
Tian Y, Taglialatela-Scafati O, Zhao F. Secondary metabolites from polar organisms. Mar Drugs. 2017;15(3):28. https://doi.org/10.3390/md15030028.
dos Santos GS, Teixeira TR, Colepicolo P, Debonsi HM. Natural products from the poles: structural diversity and biological activities. Rev Bras Farmacogn. 2021;31:531–60. https://doi.org/10.1007/s43450-021-00203-z.
Asthana RK, Deepali, Tripathi MK, Srivastava A, Singh AP, Singh SP, et al. Isolation and identification of a new antibacterial entity from the antarctic cyanobacterium Nostoc CCC 537. J Appl Phycol. 2009;21:81–8. https://doi.org/10.1007/s10811-008-9328-2.
Lin A, Wu G, Gu Q, Zhu T, Li D. New eremophilane-type sesquiterpenes from an antarctic deep-sea derived fungus, Penicillium sp. PR19 N-1. Arch Pharm Res. 2014;37(7):839–44. https://doi.org/10.1007/s12272-013-0246-8.
Yang A, Si L, Shi Z, Tian L, Liu D, Zhou D, et al. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the arctic Streptomyces nitrosporeus. Org Lett. 2013;15(20):5366–9. https://doi.org/10.1021/ol4026809.
Tripathi VC, Satish S, Horam S, Raj S, Lal A, Arockiaraj J, et al. Natural products from polar organisms: Structur-Al diversity, bioactivities and potential pharmaceutical applications. Polar Sci. 2018;18:147–66. https://doi.org/10.1016/j.polar.2018.04.006.
Kellogg JJ, Todd DA, Egan JM, Raja HA, Oberlies NH, Kvalheim OM, et al. J Nat Prod. 2016;79(2):376–86. https://doi.org/10.1021/acs.jnatprod.5b01014. Biochemometrics for natural products research: comparison of data analysis approaches and application to identification of bioactive compounds.
Bachmann BO, Lanen SG, Baltz RH. Microbial genome mining for accelerated natural products discovery: is a renaissance in the making? J Ind Microbiol Biot. 2014;41(2):175–84. https://doi.org/10.1007/s10295-013-1389-9.
Caesar LK, Montaser R, Keller NP, Kelleher NL. Metabolomics and genomics in natural products research: complementary tools for targeting new chemical entities. Nat Prod Rep. 2021;38(11):2041–65. https://doi.org/10.1039/D1NP00036E.
Paulus C, Rebets Y, Tokovenko B, Nadmid S, Terekhova LP, Myronovskyi M, et al. New natural products identified by combined genomics-metabolomics profiling of marine Streptomyces sp. MP131-18. Sci Rep. 2017;7:42382. https://doi.org/10.1038/srep42382.
Hou XM, Li YY, Shi YW, Fang YW, Chao R, Gu YC, et al. Integrating molecular networking and H-1 NMR to target the isolation of chrysogeamides from a library of marine-derived Penicillium fungi. J Org Chem. 2019;84(3):1228–37. https://doi.org/10.1021/acs.joc.8b02614.
Liu LL, Chen ZF, Liu Y, Tang D, Gao HH, Zhang Q, et al. Molecular networking-based for the target discovery of potent antiproliferative polycyclic macrolactam ansamycins from Streptomyces cacaoi subsp. Asoensis. Org Chem Front. 2020;7(24):4008–18. https://doi.org/10.1039/D0QO00557F.
Sun HM, Li X, Chen M, Zhong M, Li Y, Wang K, et al. Multi-omics-guided discovery of omicsynins produced by Streptomyces sp. 1647: pseudo-tetrapeptides active against influenza a viruses and coronavirus HCoV-229E. Engineering. 2022;16:176–86. https://doi.org/10.1016/j.eng.2021.05.010.
Hoff KJ, Lange S, Lomsadze A, Borodovsky M, Stanke M. BRAKER1: unsupervised RNA-seq-based genome annotation with GeneMark-ET and AUGUSTUS. Bioinformatics. 2016;32(5):767–9. https://doi.org/10.1093/bioinformatics/btv661.
Stanke M, Keller O, Gunduz I, Hayes A, Waack S, Morgenstern B, et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–9. https://doi.org/10.1093/nar/gkl200.
Baba H, Onanuga A. Preliminary phytochemical screening and antimicrobial evaluation of three medicinal plants used in Nigeria. Afr J Tradit Complement Altern Med. 2011;8(4):387–90. https://doi.org/10.4314/ajtcam.v8i4.7.
Uchegbu RI, Ahuchaogu AA, Mbadiugha CN, Amanze KO, Igara C, Iwu I, et al. Antioxidant, anti-inflammatory and antibacterial activities of the seeds of Mucuna pruriens (UTILIS). Am Chem Sci J. 2016;13:1–8. https://doi.org/10.9734/ACSJ/2016/24043.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. https://doi.org/10.1093/molbev/msw054.
Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2. https://doi.org/10.1093/bioinformatics/btv351.
Finn RD, Mistry J, Schuster-Böckler B, Griffiths-Jones S, Hollich V, Lassmann T, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–51. https://doi.org/10.1093/nar/gkj149.
Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28(1):33–6. https://doi.org/10.1093/nar/28.1.33.
The UniProt Consortium. Reorganizing the protein space at the universal protein resource (UniProt). Nucleic Acids Res. 2012;40(D1):D71–5. https://doi.org/10.1093/nar/gkr981.
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–61. https://doi.org/10.1093/nar/gkw1092.
The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2018;47(D1). https://doi.org/10.1093/nar/gky1055. D330-D338.
Karp PD, Latendresse M, Paley SM, Krummenacker M, Ong QD, Billington R, et al. Pathway tools version 19.0 update: Software for pathway/genome informatics and systems biology. Brief Bioinform. 2015;17(5):877–90. https://doi.org/10.1093/bib/bbv079.
O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44(D1):D733–45. https://doi.org/10.1093/nar/gkv1189.
Quevillon NA, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, et al. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33:W116–20. https://doi.org/10.1093/nar/gki442.
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(D1):D490–5. https://doi.org/10.1093/nar/gkt1178.
Urban M, Cuzick A, Seager J, Wood V, Rutherford K, Venkatesh SY, et al. PHI-base: the pathogen–host interactions database. Nucleic Acids Res. 2019;48(D1):D613–20. https://doi.org/10.1093/nar/gkz904.
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2019;48(D1):D517–25. https://doi.org/10.1093/nar/gkz935.
Jongsun P, Lee S, Choi J, Ahn K, Park B, Park J, et al. Fungal cytochrome P450 database. BMC Genom. 2008;9(1):402. https://doi.org/10.1186/1471-2164-9-402.
Lu T, Yao B, Zhang C. Database of fungal virulence factors. Database. 2012;bas032. https://doi.org/10.1093/database/bas032.
Saier MH, Reddy VS, Tsu BV, Ahmed MS, Li C, Moreno-Hagelsieb G. The transporter classification database (TCDB): recent advances. Nucleic Acids Res. 2016;44(D1):D372–9. https://doi.org/10.1093/nar/gkv1103.
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–7. https://doi.org/10.1093/nar/gkz310.
Matsuda Y, Gotfredsen CH, Larsen TO. Genetic characterization of neosartorin biosynthesis provides insight into heterodimeric natural product generation. Org Lett. 2018;20(22):7197–200. https://doi.org/10.1021/acs.orglett.8b03123.
Andersen MR, Nielsen JB, Klitgaard A, Petersen LM, Zachariasen M, Hansen TJ, et al. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc Natl Acad Sci U S A. 2012;110(1):E99–107. https://doi.org/10.1073/pnas.1205532110.
Bacha N, Dao HP, Atoui A, Mathieu F, O’Callaghan J, Puel O, et al. Cloning and characterization of novel methylsalic-ylic acid synthase gene involved in the biosynthesis of isoasperlactone and asperlactone in aspergillus westerdijkiae. Fungal Genet Biol. 2009;46(10):742–9. https://doi.org/10.1016/j.fgb.2009.07.002.
Bonsch B, Belt V, Bartel C, Duensing N, Koziol M, Lazarus CM, et al. Identification of genes encoding squalestatin S1 biosynthesis and in vitro production of new squalestatin analogues. Chem Commun. 2016;52(41):6777–80. https://doi.org/10.1039/C6CC02130A.
Fierro F, García-Estrada C, Castillo NI, Rodríguez R, Velasco-Conde T, Martín JF, et al. Transcriptional and bioinformatic analysis of the 56.8 kb DNA region amplified in tandem repeats containing the penicillin gene cluster in Penicillium Chrysogenum. Fungal Genet Biol. 2006;43(9):618–29. https://doi.org/10.1016/j.fgb.2006.03.001.
Yeh HH, Ahuja M, Chiang YM, Oakley CE, Moore S, Yoon O, et al. Resistance gene-guided genome mining: serial promoter exchanges in aspergillus nidulans reveal the biosynthetic pathway for fellutamide B, a proteasome inhibitor. ACS Chem Biol. 2016;11(8):2275–84. https://doi.org/10.1021/acschembio.6b00213.
Kakule TB, Sardar D, Lin Z, Schmidt EW. Two related pyrrolidinedione synthetase loci in fusarium heterosporum ATCC 74349 produce divergent metabolites. ACS Chem Biol. 2013;8(7):1549–57. https://doi.org/10.1021/cb400159f.
Wang B, Kang Q, Lu Y, Bai L, Wang C. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc Natl Acad Sci U S A. 2012;109(4):1287–92. https://doi.org/10.1073/pnas.1115983109.
Shen SY, Xiong W, Li SS, Liu XS, Li YK, Miao D, et al. Chromones from the Tobacco derived fungus aspergillus versicolor and their antiviral activity. Chem Nat Compd+. 2023;59(3):462–6. https://doi.org/10.1007/s10600-023-04024-5.
Truman P, Stirling DJ, Northcote P, Lake RJ, Hannah DJ. Determination of brevetoxins in shellfish by the neuroblastoma assay. J AOAC Int. 2002;85(2002):1057–63. https://doi.org/10.1093/jaoac/85.5.1057.
Wang JF, He WJ, Zhang XX, Zhao BQ, Liu YH, Zhou XJ, et al. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg Med Chem Lett. 2015;25(19):4082–4. https://doi.org/10.1016/j.bmcl.2015.08.034.
Ma TT, Shan WG, Ying YM, Ma LF, Liu WH, Zhan ZJ. Xanthones with α-glucosidase inhibitory activities from Aspergillus Versicolor, a fungal endophyte of Huperzia serrata. Helv Chim Acta. 2015;98(1):148–52. https://doi.org/10.1002/hlca.201400165.
Sato M, Motomura T, Aramaki H, Matsuda T, Yamashita M, Ito Y, et al. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J Med Chem. 2006;49(5):1506–8. https://doi.org/10.1021/jm0600139.
Li X, Xu JZ, Wang WJ, Chen YW, Zheng DQ, Di YN. Genome sequencing and evolutionary analysis of marine gut fungus aspergillus sp. Z5 from ligia oceanica. EBO. 2016;12(Suppl 1):1–4. https://doi.org/10.4137/EBO.S37532.
Yaegashi J, Oakley BR, Wang CC. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in aspergillus nidulans. J Ind Microbiol Biotechnol. 2014;41(2):433–42. https://doi.org/10.1007/s10295-013-1386-z.
Louwen JJR, Medema MH, van der Hooft JJJ. Enhanced correlation-based linking of biosynthetic gene clusters to their metabolic products through chemical class matching. Microbiome. 2023;13. https://doi.org/10.1186/s40168-022-01444-3.
van der Hooft JJJ, Mohimani H, Bauermeister A, Dorrestein PC, Duncan KR, Medema MH. Linking genomics and metabolomics to chart specialized metabolic diversity. Chem Soc Rev. 2020;49:3297–314. https://doi.org/10.1039/D0CS00162G.
Louwen JJ, Van Der Hooft JJJ. Msystems. 2021;6(4). https://doi.org/10.1128/mSystems.00726-21. Comprehensive large-scale integrative analysis of omics data to accelerate specialized metabolite discovery.
Kalkreuter E, Pan G, Cepeda AJ, Shen B. Targeting bacterial genomes for natural product discovery. Trends Pharmacoll Sci. 2019;41(1):13–26. https://doi.org/10.1016/j.tips.2019.11.002.
Liu L, Pan J, Wang Z, Yan X, Yang D, Zhu X, et al. Ribosome engineering and fermentation optimization leads to overproduction of tiancimycin A, a new enediyne natural product from Streptomyces sp. CB03234. J Ind Microbiol Biot. 2018;45(3):141–51. https://doi.org/10.1007/s10295-018-2014-8.
Xu F, Wu Y, Zhang C, Davis KM, Moon K, Bushin LB, et al. A genetics-free method for high-throughput discovery of cryptic microbial metabolites. Nat Chem Biol. 2019;15:161–8. https://doi.org/10.1038/s41589-018-0193-2.
Peng Q, Gao G, Lü J, Long Q, Chen X, Zhang F, et al. Engineered Streptomyces lividans strains for optimal identification and expression of cryptic biosynthetic gene clusters. Front Microbiol. 2018;9. https://doi.org/10.3389/fmicb.2018.03042.
Zhang B, Tian W, Wang S, Yan X, Jia X, Pierens GK, et al. Activation of natural products biosynthetic pathways via a protein modification level regulation. ACS Chem Biol. 2017;12(7):1732–6. https://doi.org/10.1021/acschembio.7b00225.
Alberti F, Khairudin K, Venegas ER, Davies JA, Hayes PM, Willis CL, et al. Heterologous expression reveals the biosynthesis of the antibiotic pleuromutilin and generates bioactive semi-synthetic derivatives. Nat Commun. 2017;8:1831. https://doi.org/10.1038/s41467-017-01659-1.
Liu Y, Ren CY, Wei WP, You D, Yin BC, Ye BC. A CRISPR-Cas9 strategy for activating the Saccharopolyspora erythraea erythromycin biosynthetic gene cluster with knock-in bidirectional promoters. ACS Synth Biol. 2019;8(5):1134–43. https://doi.org/10.1021/acssynbio.9b00024.
Ren H, Biswas S, Ho S, van der Donk WA, Zhao H. Rapid discovery of glycocins through pathway refactoring in Escherichia coli. ACS Chem Biol. 2018;13(10):2966–72. https://doi.org/10.1021/acschembio.8b00599.
Chen Y, Yin M, Horsman GP, Shen B. Improvement of the enediyne antitumor antibiotic C-1027 production by manipulating its biosynthetic pathway regulation in Streptomyces globisporus. J Nat Prod. 2011;74(3):420–4. https://doi.org/10.1021/np100825y.
Funding
This work was financially supported by the National Key Research and Development Program of China (2022YFC2804203).
Author information
Authors and Affiliations
Contributions
Huawei Zhang designed the research; Zhiyang Fu performed the research; Xiangzhou Gong, Zhe Hu and Bin Wei modified the figures; Zhiyang Fu wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, Z., Gong, X., Hu, Z. et al. Unveiling biosynthetic potential of an Arctic marine-derived strain Aspergillus sydowii MNP-2. BMC Genomics 25, 603 (2024). https://doi.org/10.1186/s12864-024-10501-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10501-0