Abstract
Background
This study aimed to investigate the associations between metal exposures and periodontitis among U.S. adults, as well as the mediated effect of biological aging.
Methods
Using data from the National Health and Nutrition Examination Survey (NHANES) 2009–2014, we explored the single and mixed impacts of metal exposures on periodontitis through adjusted weighted logistic regression, robust Poisson regression, restricted cubic spline regression, and Bayesian kernel machine regression models. This study included 2,393 participants, with 46.9% experiencing periodontitis. Concentrations of nine urinary metals, including barium (Ba), cadmium (Cd), cobalt (Co), cesium (Cs), molybdenum (Mo), lead (Pb), thallium (Tl), tungsten (Tu), and uranium (Ur), were measured using inductively coupled plasma-mass spectrometry. In addition, we analyzed the association between metals and periodontitis, stratified by age, body mass index, gender, and smoking status. Mediation models were also applied to investigate the mediated effects of biological aging between metal exposures and periodontitis.
Results
Weighted logistic and robust Poisson regression identified positive associations between Cd, Pb and periodontitis (P < 0.05). BKMR analyses indicated that mixed metal exposures were significantly associated with periodontitis, particularly among smokers, second-hand smokers, and males, with Cd, Pb, Tl, and Ba contributing the most. Furthermore, subgroup analyses observed a modifying effect on the associations between urinary Cd, Pb and periodontitis in stratified gender and BMI subgroups in robust Poisson regression. Phenotype age was found to mediate the association between metals and periodontitis.
Conclusions
This study identified significant positive associations between metal exposures and periodontitis in the U.S. adults. In addition, the association between metal exposures and periodontitis could vary in different gender, BMI and smoking subgroups. These associations were likely partly mediated by biological aging, suggesting that metals may potentially increase the risk of periodontitis by promoting cell senescence and overall aging of the body.
Graphical Abstract

Similar content being viewed by others
Introduction
Periodontitis is a chronic disease caused by bacteria-induced inflammation of tooth-supporting tissues [1]. To date, periodontitis is the most common type of periodontal diseases, affecting one in ten people globally [2]. Among American adults, the prevalence of periodontitis has reached 42.2% with 8% of the population suffering from severe periodontitis [3], making it the major cause of tooth loss [4]. Several epidemiological studies have demonstrated the close association between periodontitis and various systematic diseases such as atherosclerosis, cardiovascular diseases [5, 6], type 2 diabetes [7], and cancer [8].
Notably, the impact of environmental exposures on periodontitis has attracted increasing attention in recent years. Metal exposures is a significant environmental concern, primarily stemming from industries such as battery manufacturing, metal processing, printing and other sources. With rapid industrialization, people are exposed to a mixture of metals, including lead and cadmium, through contaminated food, water, air, cigarettes, and occupational sources [9, 10]. Several studies have reported an increased risk of periodontitis due to excessive exposure to individual metals [11]. For example, a cross-sectional study of 4716 participants demonstrated associations between cadmium, lead and periodontitis [12, 13]. Another repeated-measures study with 391 observations in 98 participants showed a strong positive correlation between urinary cesium (Cs) and pro-inflammatory cytokine endothelin-1 [14], while other studies reported an association between endothelin-1 and the progression of periodontitis [15]. In addition, exposure to uranium (Ur) has been found to inhibit periodontal bone formation in rats [16]. However, to date, the association between mixed metal exposures and periodontitis remains unclear. Furthermore, aging is considered as a potential contributing factor to periodontitis according to previous studies, and we speculate that biological aging might mediate the association between metals and periodontitis. This study employed five statistical methods, including weighted logistic regression, robust Poisson regression, restricted cubic spline regression (RCS), Bayesian kernel machine regression (BKMR), and mediation models, to assess the effects of urinary barium (Ba), cadmium (Cd), cobalt (Co), cesium (Cs), molybdenum (Mo), lead (Pb), thallium (Tl), tungsten (Tu), and uranium (Ur) and biological aging on periodontitis among U.S. adults. The aim of this study is to investigate the associations between mixed metal exposures and periodontitis, as well as the mediating effect of biological aging. Data utilized in this study were acquired from National Health and Nutrition Examination Survey 2009–2014.
Methods
Study population
The participants were sourced from NHANES, a large-scale cross-sectional survey conducted by the Centers for disease control and Prevention (CDC) to assess adults and children’s the physical and psychological health and nutritional status in the United State [17]. The National Center for Health Statistics (NCHS) institutional committee approved the NHANES. All participants signed an informed consent form. For more information on the NHANES method, visit the website: www.cdc.gov/nchs/nhanes/.
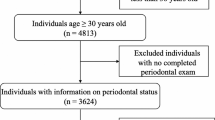
We extracted three consecutive NHANES surveys cycles, spanning 2009–2010, 2011–2012, and 2013–2014. These cycles contained demographic, examination, laboratory, and questionnaire data. Individuals without complete information on urinary metals, periodontal examination, and relevant covariates were excluded from the analysis. Finally, a total of 2,393 participants were included in the study (Fig. S1).
Urinary metals measurement
Urine samples were stored at − 20 °C and transported to the U.S. CDC for testing. The samples were detected using inductively coupled plasma mass spectrometry (ICP-MS) following conjugation treatment by enzymatic hydrolysis of Glucuronidation metabolites [18]. Nine urinary metal elements with detection rates exceeding 80% were selected for analysis: Ba, Cd, Co, Cs, Mo, Pb, Tl, Tu, and Ur [19]. Concentration of metals in urinary specimen of participants in three cycles (NHANES 2009–2014) was tested to evaluate metal exposure levels of individuals. Urinary metal levels were all adjusted for urinary creatinine. More details can be found in the NHANES Laboratory Procedures Manual.
Definition of periodontitis
Periodontal status was assessed through a full-mouth periodontal examination by a licensed and calibrated dental examiner. This comprehensive examination, which included gingival recession assessment and periodontal pocket depth measurement at the mobile examination center, is considered more accurate than a half-mouth examination [20]. Periodontitis was defined based on the criteria established by American Academy of Periodontology (AAP) [1]. Mild periodontitis was defined as the presence of clinical attachment loss (AL) ≥ 3 mm at least two interproximal sites (not on the same tooth), and two interproximal sites with probing depth (PD) ≥ 4 mm (not on the same tooth), or at least one site with PD ≥ 5 mm. Moderate was defined as AL ≥ 4 mm at least two interproximal sites with (not on the same tooth), or at least two interproximal sites with PD ≥ 5 mm (not on the same tooth). Severe periodontitis was defined as AL ≥ 6 mm at least two interproximal sites with (not on the same tooth) and at least one interproximal site with PD ≥ 5 mm [21]. Total periodontitis was defined as the combination of mild, moderate, and severe periodontitis. Due to the limited sample size of severe periodontitis, participants were eventually categorized as having periodontitis and non-periodontitis.
Measurements of biological aging markers
Biological aging has been recognized as a common risk factor for various chronic diseases in previous studies [22]. In vivo experiments have linked periodontitis to aging and senescence [23]. To date, phenotype age is currently widely identified as a marker of biological aging, representing senescence in inflammation, immune system and multiple organic functions [24]. In this study, we used 10 aging-relevant parameters, including albumin, creatinine, CRP, lymphocyte percent, mean cell volume, red cell distribution width, alkaline phosphatase, white blood cell count, chronological age, and glucose, to measure phenotype age [25]:
Covariates
Information on age, gender, race/ethnicity, education level, poverty-to-income ratio (PIR), marital status, recreational activities, cardiovascular diseases, diabetes status, alcohol intake, alveolar bone loss, and dental floss usage were collected through standardized questionnaires. In addition, data on serum vitamin D, body mass index (BMI), total bone mineral density (BMD), renal function status, smoking status, and urinary creatinine was obtained from examination conducted by experienced staffs.
Race/ethnicity was categorized into Non-Hispanic White/Black, Mexican American, other Hispanic, and other races [26]. Education level was categorized as lower than high school, graduate/GED (General educational development) or equivalent, and college or above. Compared to PIR (poverty income ratio) = 1, the population was divided into the low-income and normal-income levels [27]. Marital status was classified as married/cohabited, widowed/divorced/separated, and never married. Vigorous/moderate recreational activities were defined by the questions “Vigorous recreational activities” and “Moderate recreational activities” [28]. Cardiovascular diseases were defined as meeting one of the following situations: (1) Ever told had congestive heart failure; (2) Ever told you had coronary heart disease; (3) Ever told you had angina/angina pectoris; (4) Ever told you had heart attack; and (5) Ever told you had a stroke [6]. Diabetes was determined by the questions “Doctor told you have diabetes” or “Take diabetic pills to lower blood sugar” [29]. Daily alcohol intake was calculated through the drinking frequency in last 12 months multiply average intake divide 365. If males drank ≥ 2 cups or females drank ≥ 1 cup of alcohol per day would be defined as excessive [30]. The answer of “Ever been told of bone loss around teeth” was used to classify the situation of dental bone loss. The floss usage was grouped into 0, 1–2, 3–4, and 5–7 times per week [31].
The body mass index (BMI) was calculated by dividing the participants’ weight by the square of the height (kg/m2) [32]. The serum 25(OH)D concentration was categorized into four groups (< 30, 30–50, 50–75, > 75 nmol/L) [33], while BMD and urinary creatinine was included in the regression analysis as continuous variables. CKD (chronic kidney disease) was defined as eGFR (glomerular filtration rate) < 60 mL/min/1.73 m2 and/or ACR (albumin to creatinine ratio) > 30 mg/g [34]. We combined the questionnaire “Used tobacco/nicotine last 5 days” and blood cotinine concentration (< 0.015, 0.015–3.08, > 3.08 ng/mL) to distinguish non-smokers (without tobacco exposure), second-hand smokers, and smokers [30].
Statistical methods
Continuous variables were presented as mean and standard deviation (Mean ± SD) for normally distributed data, median and interquartile range (IQR) for skewed data, and comparative analysis was performed by t test or Kruskal–Wallis test. The categorical variables were expressed as case sample numbers (n) and percentages (%), and compared by Chi-square test. Spearman correlation analysis was used to examine the correlation between urinary metals, with the concentrations of urinary metals being transformed into logarithmic form.
First, three weighted logistic regression models were conducted to estimate the associations between single urinary metal and periodontitis by comparing to tertile1 of each metal’s exposure level. Model 1 was a crude model; Model 2 included the basic demographic information such as age, gender, race/ethnicity, the ratio of family income to poverty, education level, marriage, body mass index, recreational activities, smoking status, drinking status as covariates; Model 3 adjusted for cardiovascular diseases, diabetes, CKD, serum vitamin D level, BMD, dental health problems and urinary creatinine based on Model 2. Considering the high prevalence of periodontitis, we further performed robust Poisson regression to explore the association between metals and periodontitis as sensitivity analyses for weighted logistic regression [35, 36]. All available co-variates were adjusted in the robust Poisson models, where urinary metals were fitted as both continuous and tertiles variables. Besides, we employed the restricted cubic spline (RCS) regression with three knots (10th, 50th, 90th) to explore the non-linear associations of urinary metals and periodontitis, using the median values of urinary metals as references.
We used the Bayesian kernel machine regression (BKMR) model to assess the joint effect of mixed exposure to nine urinary metals on periodontitis, allowing quantification and visualization of the overall exposure–response relationship and the impacts of individual components of the mixture to periodontitis. We modeled the exposure–response function using the Gaussian kernel function and ran it through the Markov chain Monte Carlo (MCMC) algorithm for 20,000 iterations [37]. Posterior inclusion probabilities (PIPs), a variable importance measure ranging from 0 to 1, were calculated to identify the key anions that contribute most to the risk of periodontitis [38]. A PIP threshold of 0.5 was usually used to judge the importance of environmental exposures. Then we investigated the univariate exposure–response function when a single metal was at the 75th and 25th percentiles, while fixing other metals at their median concentrations [39]. We utilized the bivariate exposure–response function to explore potential interactions among different exposures [40].
To examine the modification effect by age, gender, BMI and smoking status of the associations between single and mixed metals and periodontitis, we performed stratified analyses based on total effect robust Poisson regression and BKMR. We used Wald test to investigate the interaction correlation [41].
The potential mediating effects of biological aging markers on the associations of single and mixed metals with periodontitis were estimated by mediation analyses (bootstrap = 1000). Models were adjusted for all available covariates listed above.
The statistical analyses for this study were performed using Stata version 17.0 (Stata Corp LP, College Station, Texas, USA) and R software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). We performed robust Poisson, RCS, BKMR and mediation analysis with the R packages “robustbase” (version 0.99-2), “rms” (version 6.7-0), “bkmr” (version 0.2.2) and “mediation” (version 4.5.0), respectively. A P value < 0.05 was considered statistically significant [42].
Results
Descriptive analysis
2393 participants were included in our study (Fig. S1), with 1123 individuals diagnosed with periodontitis and 1270 subjects without (46.93% vs 53.07%). Table 1 presents the participants’ general information, with an average age of 48.25 ± 12.73 years, and a near-equal distribution between males and females (50.10% vs 49.90%). Compared with the non-periodontitis group, participants in the periodontitis group had higher BMI, lower education level, less recreational activities, higher rates of active smoking and excessive drinking, had more diabetes, CKD and cardiovascular diseases, poorer dental bone health, and lower usage of dental floss (P < 0.05).
Table S1 shows the distribution of urinary metals between periodontitis and non-periodontitis participants. The detection rate of the nine urinary metals were all > 80% (Table S1). Figure S2 presents correlation coefficient matrices, and all metals were positively correlated, with correlation coefficients ranging from 0.29 to 0.77.
Association between urinary metals and periodontitis by weighted logistic, robust Poisson and RCS regression
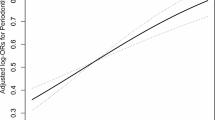
Figure 1 displays OR and 95% CI on the single urinary metal exposures using three logistic regression models. Figure 1C shows the positive associations between exposure to Cd, Cs and Pb and periodontitis at tertile3, after controlling for all available covariates (OR [95%CI] 2.64 [1.13, 6.14], 1.71 [1.03, 2.82], 2.02 [1.15, 3.57], respectively). The robust Poisson regression suggested that the association between Pb, Cd and periodontitis at tertile3 were consistent with weighted logistic regression (prevalence ratio (PR) [95%CI] 1.26 [1.05, 1.51], 1.49 [1.24, 1.80], respectively) (Table S2).
Figure 1A shows a crude model. Figure 1B is adjusted for age, gender, race/ethnicity, the ratio of family income to poverty, education level, marriage, BMI, recreational activities, smoking status, drinking status. Figure 1C is further adjusted for cardiovascular diseases, diabetes, CKD, serum vitamin D level, BMD, dental health problems and urinary creatinine based on Fig. 1B. Note: OR, odds ratio; CI, Confidence interval; P–t, P for trend; *P < 0.05; **P < 0.01; ***P < 0.001.
The adjusted RCS models depict the dose–response relationships between single urinary metal exposures and periodontitis (Fig. 2). We identified significant liner correlations between urinary Cd and Pb and periodontitis (P < 0.001). However, no statistically non-linear association was observed between metals and periodontitis (P > 0.05).
Association between urinary metals and periodontitis by BKMR model
BKMR model shows the positive association between mixed urinary metal exposures and periodontitis compared to the 25th and the 50th percentiles (Fig. 3A). Table S3 shows the posterior inclusion probability (PIP) for each metal exposure, where urinary Cd and Pb played the most important roles (PIP = 1.000). We estimated the univariate effect between exposure and periodontitis by fixing other urinary metals at the 50th percentile exposure level (Fig. 3B). Urinary Cd and Pb are positively associated with periodontitis, while urinary Cs and Tl are negatively correlated. In addition, bivariate exposure–response functions for single urinary metal at the 25th, 50th, and 75th percentiles were applied to reveal the effect of individual metal on periodontitis when other metals were fixed at the 50th percentiles (Fig. S3). Finally, we examined the potential pairwise interactions among urinary metals and found that the exposure–response curves were roughly parallel, suggesting no clues of interaction (Fig. S4).
Subgroup analyses
Based on the total effect robust Poisson models, we further conducted subgroup analyses to explore the modifying effects of stratified age, gender, BMI and smoking status in the association between metal exposures and periodontitis (Tables S4–S7). Potential interaction effects were observed in the association between Cd, Pb exposure and periodontitis in gender and BMI subgroups. A stronger association between metal exposures and periodontitis was found in males and BMI lower than 25 kg/m2 subgroups, as evidenced by robust Poisson regression (Tables S4, S6). In addition, the BKMR model indicated a positive association between metals and periodontitis in males, while no statistically significant association was observed in females (Fig. 4B). Furthermore, a more significant association of mixed metals exposure with periodontitis was observed among smokers and second-hand smokers than non-smokers (Fig. 5).
Mediation analyses
Mediation analyses were performed to examine the potential mediating effect of phenotype age on the association between metals and periodontitis after adjusting for all available covariates listed above. A significant mediation effects of phenotype age was found on the association between Cd, Pb and periodontitis, with proportion of mediation of 31.06% and 38.25%, respectively (P < 0.05) (Fig. 6).
Discussion
This study identified that exposure to Pb and Cd was associated with periodontitis in the total population. Subgroup analyses showed that the effects were stronger in males, normal weight participants than females, overweight or obese participants, respectively. BKMR model suggested the positive association between the mixed metals exposure and periodontitis, in which urinary Cd and Pb contributed the most (PIP = 1.000). Furthermore, mediation analysis showed that biological aging could be a potential mediator for the association between Pb, Cd exposures and periodontitis risks. This is an important finding that adds to our understanding of how these exposures may impact periodontal health.
To date, the evidence from epidemiological studies on the effects of metal exposures on periodontitis was sparse and inconsistent. Previous studies have reported a correlation between alveolar bone loss and blood Pb levels in US adults using linear regression models [12]. Other epidemiological studies suggested an association between urinary Cd and periodontal disease [11, 13]. In addition, a cross-sectional study by Li et al. suggested that single and mixed metal exposure were associated with periodontitis. Besides, some studies have shown that Ur is an inhibitor of alveolar bone formation by histomorphometry in rats [16]. However, it has been reported that high concentrations of Ur in bone microenvironment inhibited osteoclastic bone resorption [43], which could reduce the risk of periodontitis. Our study shows that mixed metals exposure was strongly associated with periodontitis, which supported the previous studies regarding cadmium exposures as a risk for periodontitis [13]. Furthermore, biological aging probably partly mediated the association between metal exposures and periodontitis.
Our study suggested that biological aging potentially mediated the association between Pb and Cd exposures and periodontitis, one of the plausible mechanisms is that the generation of ROS under metal exposures [44] promoted the aging process in periodontal tissues. ROS are oxidants mainly produced by mitochondrion [45], which is considered as an important cause for cellular sequence and whole-body aging [46]. In Vitro studies shows that redox cycling of metals such as Pb and Cd may deplete antioxidants [47], disrupting the balance between antioxidants and ROS. Pb inhibit antioxidant defense enzymes, such as superoxide dismutase and glutathione reductase [48], leading to excessive production of ROS. In vitro experiments, oxidative stress induced by ROS can cause lipid peroxidation [49], damage DNA by destroying the deoxyribose backbone structure, lead to protein degradation by modifying polypeptides [50], which may result in telomere shortening [25], thus exacerbating cellular sequence and biological aging in periodontal tissues. Besides, Cd cause mitochondrial malfunction and induced mitochondrial DNA mutations [51], which induce excessive production of ROS [52] in mitochondria, causing oxidative phosphorylation dysfunction and cellular apoptosis through MAPK pathway [53], which exacerbate biological aging in periodontal tissues [54]. Furthermore, previous animal experiments have shown that excessive ROS is strongly associated with alveolar bone resorption in rats [55]. We speculate that metal exposures could increase the risk of periodontitis due to overproduction of ROS and reduction of antioxidants, in which biological aging mediated the oxidative stress process [54].
In addition, metal exposure has been linked to immune aging according to previous studies [56]. By influencing DNA methylation, metals could impair the cellular immune system, which featured with senescence-associated secretory phenotype (SASP) [23]. SASP includes pro-inflammation cytokines such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ) [57, 58], which are involved in periodontitis progression. In vitro cell experiments, Cd induces IL-6 by activating the p65 NF-κB through phosphorylation of p38 MAPK [59]. IL-6 could enhance osteoclast function through the RANKL cytokine pathway [60] and cause pathological alveolar bone resorption [61, 62]. In mice, Cd can destroy alveolar bone [63], thus increasing periodontitis risk. In cell experiments, Pb exposure can promote the production of TNF-α [58], leading to abnormal host immune response [64, 65], activating osteoclasts [61], and exacerbating alveolar bone destruction. Accumulation of senescent osteocytes could damage periodontal tissues by promoting inflammation and inducing deterioration in periodontal tissues [23]. More in vivo/vitro studies are needed to explore whether metal exposure-induced periodontitis is immune aging-mediated mechanism.
Besides, the fact that the strength of the associations varied between metal exposures and periodontitis in different subgroups are noteworthy. Subgroup analyses suggested that the impact of single and mixed metals exposure on periodontitis appear to vary by gender, BMI and smoking status (Figs. 4, 5) (Tables S4–S7). Notably, subgroup analyses revealed that the association between mixed metals exposure and periodontitis were more significant in males, while the influence of metal exposure on periodontitis in females was relatively limited. One of the plausible explanations was that males might be more vulnerable when exposed to metals than females. Previous researches suggested that females owned better metals resistance, since higher estrogen levels provided females more protection against metal toxicity [66]. By comparison, males are more vulnerable to the toxicity of metals, which partly owe to hormone levels and lower density of estrogen receptors [66, 67]. Some studies pointed out that pathways of metal toxicity differentiate in different genders, due to gender difference in metals absorption rates and metabolism [67, 68]. Besides, harmful effects of metals on periodontitis diverse among different BMI subgroups, which is consistent with previous studies [69, 70]. At the same time, we also observed that there is a difference in the effect of metal exposure on periodontitis in different smoking status subgroups in BKMR model, after controlling all available covariates (Fig. 5). We speculated that the modifying effects of smoking status could be partly due to the distinct immune microenvironment between smokers, second-hand smokers and non-smokers [71]. However, it should be noted that despite our efforts to adjust for available co-variates in the models, the existence of residual confounding could not be totally eliminated in the subgroup analyses. For example, the occupation as well as behavioral factors could vary between males and females and thus influence metal exposure, while these data were inadequate in NHANES study. Besides, smoking is a strong established risk factor of periodontitis, which could cause residual confounding in subgroup analyses [30]. Therefore, we should interpretate the results of subgroup analyses more cautiously. Besides, further mechanism researches and cohort studies are needed to verify our hypotheses.
This is the first study focused on the mediated effects of biological aging in association between metal exposures with periodontitis among U.S. adults in different subgroups. We identified the robust results that metal exposures associated with periodontitis by five statistical methods. Still, there are several limitations in the current study. First, we cannot determine the causality of metal exposures on periodontitis as well as metal exposures duration due to the cross-sectional study design. Second, metals have short half-lives, and a one-timepoint exposure measurement of nine urinary metals might skew the results. Since RCS and BKMR models do not yet support weighted calculation, we only adjusted the sample weighted in the logistic model. In addition, medications, oral hygiene practice, the presence of dental plaque, and frequency of dental check-ups, are all known to be significant in the development of periodontitis, however, detailed information on these factors was not collected in the NHANES survey data. Though we have considered almost every available confounding variate in our study, there might still be some residual confounding factors beyond our control. Therefore, further longitudinal studies, cohort researches as well as molecular experiments are needed to detail on the role and mechanism of metal exposures in periodontitis, which is significant for control and prevention of periodontitis (Fig. 7).
Conclusion
Using the data from NHANES 2009–2014 of the U.S. adults, our study revealed that biological aging might potentially mediate the impacts of metals on periodontitis, and mixed metals exposures were positively associated with periodontitis. In addition, our subgroup analyses suggested that the association between metal exposures and periodontitis could vary in different gender, BMI and smoking subgroups. These findings shed light on the complex relationships between metal exposure, biological aging and periodontal health, and offer valuable insights for future research and public health initiatives.
Availability of data and materials
Not applicable.
Abbreviations
- Ba:
-
Barium
- Co:
-
Cobalt
- Cs:
-
Cesium
- Mo:
-
Molybdenum
- Tl:
-
Thallium
- Tu:
-
Tungsten
- Ur:
-
Uranium
- NHANES:
-
National Health and Nutrition Examination Survey
- RCS:
-
Restricted cubic spline regression
- BKMR:
-
Bayesian kernel machine regression
- ICP-MS:
-
Inductively coupled plasma-mass spectrometry
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- PIP:
-
Prior inclusion probability
References
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ (2012) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83(12):1449–1454. https://doi.org/10.1902/jop.2012.110664
Genco RJ, Sanz M (2020) Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000 83(1):7–13. https://doi.org/10.1111/prd.12344
Eke PI, Borgnakke WS, Genco RJ (2020) Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000 82(1):257–267. https://doi.org/10.1111/prd.12323
Ramseier CA, Anerud A, Dulac M, Lulic M, Cullinan MP, Seymour GJ et al (2017) Natural history of periodontitis: Disease progression and tooth loss over 40 years. J Clin Periodontol 44(12):1182–1191. https://doi.org/10.1111/jcpe.12782
Herrera D, Molina A, Buhlin K, Klinge B (2020) Periodontal diseases and association with atherosclerotic disease. Periodontol 2000 83(1):66–89. https://doi.org/10.1111/prd.12302
Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P et al (2020) Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol 47(3):268–288. https://doi.org/10.1111/jcpe.13189
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F et al (2018) Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 45(2):138–149. https://doi.org/10.1111/jcpe.12808
Nwizu N, Wactawski-Wende J, Genco RJ (2020) Periodontal disease and cancer: epidemiologic studies and possible mechanisms. Periodontol 2000 83(1):213–233. https://doi.org/10.1111/prd.12329
Han DH, Lee HJ, Lim S (2013) Smoking induced heavy metals and periodontitis: findings from the Korea National Health and Nutrition Examination Surveys 2008–2010. J Clin Periodontol 40(9):850–858. https://doi.org/10.1111/jcpe.12133
Bonberg N, Pesch B, Ulrich N, Moebus S, Eisele L, Marr A et al (2017) The distribution of blood concentrations of lead (Pb), cadmium (Cd), chromium (Cr) and manganese (Mn) in residents of the German Ruhr area and its potential association with occupational exposure in metal industry and/or other risk factors. Int J Hyg Environ Health 220(6):998–1005. https://doi.org/10.1016/j.ijheh.2017.05.009
Won YS, Kim JH, Kim YS, Bae KH (2013) Association of internal exposure of cadmium and lead with periodontal disease: a study of the Fourth Korean National Health and Nutrition Examination Survey. J Clin Periodontol 40(2):118–124. https://doi.org/10.1111/jcpe.12033
Dye BAHR, Brody DJ (2002) The relationship between blood lead levels and periodontal bone loss in the United States, 1988–1994. Environ Health Perspect 110(10):997–1002. https://doi.org/10.1289/ehp.02110997
Arora M, Weuve J, Schwartz J, Wright RO (2009) Association of environmental cadmium exposure with periodontal disease in U.S. adults. Environ Health Perspect 117(5):739–744. https://doi.org/10.1289/ehp.0800312
Li A, Mei Y, Zhao M, Xu J, Zhao J, Zhou Q et al (2022) Do urinary metals associate with the homeostasis of inflammatory mediators? Results from the perspective of inflammatory signaling in middle-aged and older adults. Environ Int 163:107237
Ansai TYE, Awano S, Yu W, Turner AJ, Takehara T (2002) Effects of periodontopathic bacteria on the expression of endothelin-1 in gingival epithelial cells in adult periodontitis. Clin Sci. https://doi.org/10.1042/CS103S327S
Ubios AMGM, Steimetz T, Cabrini RL (1991) Uranium inhibits bone formation in physiologic alveolar bone modeling and remodeling. Environ Res 54:17–23
Wu Y, Song J, Zhang Q, Yan S, Sun X, Yi W et al (2023) Association between organophosphorus pesticide exposure and depression risk in adults: a cross-sectional study with NHANES data. Environ Pollut 316:120445
Yuan C-G, Shi J-B, He B, Liu J-F, Liang L-N, Jiang G-B (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ Int 30(6):769–783
Bulka CM, Persky VW, Daviglus ML, Durazo-Arvizu RA, Argos M (2019) Multiple metal exposures and metabolic syndrome: a cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ Res 168:397–405. https://doi.org/10.1016/j.envres.2018.10.022
Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA (2010) Accuracy of NHANES periodontal examination protocols. J Dent Res 89(11):1208–1213. https://doi.org/10.1177/0022034510377793
Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ (2018) Periodontitis in US Adults. J Am Dent Assoc 149(7):576–88.e6. https://doi.org/10.1016/j.adaj.2018.04.023
LA Levine ME, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10:573
Aquino-Martinez REB, Rowsey JL, Fraser DG, Khosla S, Farr JN, Monroe DG (2021) Senescent cells exacerbate chronic inflammation and contribute to periodontal disease progression in old mice. J Periodontol. https://doi.org/10.1002/JPER.20-0529
Klemera PDS (2006) A new approach to the concept and computation of biological age. Mech Ageing Dev. https://doi.org/10.1016/j.mad.2005.10.004
Chen L, Zhao Y, Liu F, Chen H, Tan T, Yao P et al (2022) Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. https://doi.org/10.1186/s12916-022-02403-3
Borrell LN, Crawford ND (2011) Social disparities in periodontitis among US adults: the effect of allostatic load. J Epidemiol Community Health 65(2):144–149. https://doi.org/10.1136/jech.2009.098269
Borrell LNCN (2012) Socioeconomic position indicators and periodontitis: examining the evidence. Periodontol 2000 58(1):69–83. https://doi.org/10.1111/j.1600-0757.2011.00416.x
Almohamad MKKE, Mofleh D, Spartano NL (2022) The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J Clin Periodontol 49(8):758–767. https://doi.org/10.1111/jcpe.13669
Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K et al (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55(1):21–31. https://doi.org/10.1007/s00125-011-2342-y
Baumeister SE, Freuer D, Nolde M, Kocher T, Baurecht H, Khazaei Y et al (2021) Testing the association between tobacco smoking, alcohol consumption, and risk of periodontitis: a Mendelian randomization study. J Clin Periodontol 48(11):1414–1420. https://doi.org/10.1111/jcpe.13544
Han SJ (2022) The use of interdental care products in korean adults aged 30 years and older and factors affecting their use: 4th to 7th Korean National Health and Nutrition Examination Survey. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19148639
Dong J, Gong Y, Chu T, Wu L, Li S, Deng H et al (2022) Mendelian randomization highlights the causal association of obesity with periodontal diseases. J Clin Periodontol 49(7):662–671. https://doi.org/10.1111/jcpe.13640
Machado V, Lobo S, Proenca L, Mendes JJ, Botelho J (2020) Vitamin D and periodontitis: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu12082177
Serni L, Caroti L, Barbato L, Nieri M, Serni S, Cirami CL et al (2023) Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral Dis 29(1):40–50. https://doi.org/10.1111/odi.14062
Talbot DMM, Chiu Y, Simard M, Sirois C (2023) An alternative perspective on the robust poisson method for estimating risk or prevalence ratios. Epidemiology 34(1):1–7. https://doi.org/10.48550/arXiv.2112.00547
Ibáñez-Pinilla M, Villalba-Niño S, Olaya-Galán NN (2023) Negative log-binomial model with optimal robust variance to estimate the prevalence ratio, in cross-sectional population studies. BMC Med Res Methodol. https://doi.org/10.1186/s12874-023-01999-1
Baele G, Lemey P, Rambaut A, Suchard MA (2017) Adaptive MCMC in Bayesian phylogenetics: an application to analyzing partitioned data in BEAST. Bioinformatics 33(12):1798–1805. https://doi.org/10.1093/bioinformatics/btx088
Linero AR (2018) Bayesian regression trees for high-dimensional prediction and variable selection. J Am Stat Assoc 113(522):626–636. https://doi.org/10.1080/01621459.2016.1264957
Zhang F, Wang H, Cui Y, Zhao L, Song R, Han M et al (2022) Association between mixed dioxin exposure and hyperuricemia in U.S. adults: a comparison of three statistical models. Chemosphere 303(3):135134. https://doi.org/10.1016/j.chemosphere.2022.135134
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M et al (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508. https://doi.org/10.1093/biostatistics/kxu058
Yu Z, Demetriou M, Gillen DL (2015) Genome-wide analysis of gene-gene and gene-environment interactions using closed-form wald tests. Genet Epidemiol 39(6):446–455. https://doi.org/10.1002/gepi.21907
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67. https://doi.org/10.1186/s12940-018-0413-y
Gritsaenko T, Pierrefite-Carle V, Creff G, Simoneau B, Hagege A, Farlay D et al (2021) Low doses of uranium and osteoclastic bone resorption: key reciprocal effects evidenced using new in vitro biomimetic models of bone matrix. Arch Toxicol 95(3):1023–1037. https://doi.org/10.1007/s00204-020-02966-1
Lopes AC, Peixe TS, Mesas AE, Paoliello MM (2016) Lead exposure and oxidative stress: a systematic review. Rev Environ Contam Toxicol 236:193–238. https://doi.org/10.1007/978-3-319-20013-2_3
Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE et al (2022) Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 23(7):499–515. https://doi.org/10.1038/s41580-022-00456-z
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS et al (2022) Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. https://doi.org/10.3390/cells11030552
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350. https://doi.org/10.1016/j.chemosphere.2020.128350
Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM et al (2005) Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res 40(5):378–384. https://doi.org/10.1111/j.1600-0765.2005.00818.x
Su H, Gornitsky M, Velly AM, Yu H, Benarroch M, Schipper HM (2009) Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic Biol Med 46(7):914–921. https://doi.org/10.1016/j.freeradbiomed.2009.01.008
Kauppila TESKJ, Larsson NG (2017) Mammalian mitochondria and aging: an update. Cell Press Cell Metab. https://doi.org/10.1016/j.cmet.2016.09.017
Jiang WWY, Cao Z, Chen Y, Si C, Sun X, Huang S (2023) The role of mitochondrial dysfunction in periodontitis: from mechanisms to therapeutic strategy. J Periodontal Res. https://doi.org/10.1111/jre.13152
Cao XFM, Bi R, Zheng X, Fu B, Tian S, Liu C, Li Q, Liu J (2021) Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128346
Wang Y, Andrukhov O, Rausch-Fan X (2017) Oxidative stress and antioxidant system in periodontitis. Front Physiol 8:910. https://doi.org/10.3389/fphys.2017.00910
Saita M, Kaneko J, Sato T, Takahashi SS, Wada-Takahashi S, Kawamata R et al (2016) Novel antioxidative nanotherapeutics in a rat periodontitis model: reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials 76:292–301. https://doi.org/10.1016/j.biomaterials.2015.10.077
Wang C, Hong S, Guan X, Xiao Y, Fu M, Meng H et al (2023) Associations between multiple metals exposure and biological aging: evidence from the Dongfeng-Tongji cohort. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.160596
Jayawardena UA, Ratnasooriya WD, Wickramasinghe DD, Udagama PV (2016) Heavy metal mediated innate immune responses of the Indian green frog, Euphlyctis hexadactylus (Anura: Ranidae): cellular profiles and associated Th1 skewed cytokine response. Sci Total Environ 566–567:1194–1204. https://doi.org/10.1016/j.scitotenv.2016.05.171
Khan MI, Islam N, Sahasrabuddhe AA, Mahdi AA, Siddiqui H, Ashquin M et al (2011) Ubiquitous hazardous metal lead induces TNF-alpha in human phagocytic THP-1 cells: primary role of ERK 1/2. J Hazard Mater 189(1–2):255–264. https://doi.org/10.1016/j.jhazmat.2011.02.027
Phuagkhaopong S, Ospondpant D, Kasemsuk T, Sibmooh N, Soodvilai S, Power C et al (2017) Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-kappaB pathways. Neurotoxicology 60:82–91. https://doi.org/10.1016/j.neuro.2017.03.001
Braun T, Zwerina J (2011) Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthrit Res Ther 13:1–11
Kitaura H, Marahleh A, Ohori F, Noguchi T, Shen WR, Qi J et al (2020) Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. https://doi.org/10.3390/ijms21145169
Huang X, Xie M, Xie Y, Mei F, Lu X, Li X et al (2020) The roles of osteocytes in alveolar bone destruction in periodontitis. J Transl Med 18(1):479. https://doi.org/10.1186/s12967-020-02664-7
Browar AWKE, Wei Y, Leavitt LL, Prozialeck WC, Edwards JR (2018) Cadmium exposure disrupts periodontal bone in experimental animals: implications for periodontal disease in humans. Toxics 6(2):32. https://doi.org/10.3390/toxics6020032
Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35(1):3–11. https://doi.org/10.1016/j.it.2013.09.001
Trombone AP, Ferreira SB Jr, Raimundo FM, de Moura KC, Avila-Campos MJ, Silva JS et al (2009) Experimental periodontitis in mice selected for maximal or minimal inflammatory reactions: increased inflammatory immune responsiveness drives increased alveolar bone loss without enhancing the control of periodontal infection. J Periodontal Res 44(4):443–451. https://doi.org/10.1111/j.1600-0765.2008.01133.x
Qin XSL, Fan G, Liu Q, Wu M, Bi J, Fang Q, Wan Z, Lv Y, Wang Y (2023) Sex-specific associations of single metal and metal mixture with handgrip strength: a cross-sectional study among Chinese adults. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-023-26926-1
Gade MCN, Re DB (2021) Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res. https://doi.org/10.1016/j.envres.2021.111558
Sobolewski M, Varma G, Adams B, Anderson DW, Schneider JS, Cory-Slechta DA (2018) Developmental lead exposure and prenatal stress result in sex-specific reprograming of adult stress physiology and epigenetic profiles in brain. Toxicol Sci 163(2):478–489. https://doi.org/10.1093/toxsci/kfy046
Chen H, Wang M, Zhang C, Li J (2023) A methodological study of exposome based on an open database: association analysis between exposure to metal mixtures and hyperuricemia. Chemosphere. https://doi.org/10.1016/j.chemosphere.2023.140318
Wu WJS, Zhao Q, Zhang K, Wei X, Zhou T, Liu D, Zhou H, Zhong R, Zeng Q, Cheng L, Miao X, Lu Q (2018) Associations of environmental exposure to metals with the risk of hypertension in China. Sci Total Environ 622–623:184–191. https://doi.org/10.1016/j.scitotenv.2017.11.343
Luo W, Zeng Z, Jin Y, Yang L, Fan T, Wang Z et al (2023) Distinct immune microenvironment of lung adenocarcinoma in never-smokers from smokers. Cell Rep Med. https://doi.org/10.1016/j.xcrm.2023.101078
Acknowledgements
This study was analyzed using the data provided by the National Health and Nutrition Examination Survey 2009–2014. Data from this survey will be used in epidemiological studies and health sciences research, which helps develop sound public health policy, direct and design health programs and services, and expand the health knowledge for the Nation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DZ: investigation, methodology, formal analysis, data curation, writing—original draft. FY: validation, data curation, formal analysis, writing—original draft. TY: writing—review and editing. YX: investigation, validation. CY: review and editing. XY: data curation and editing. JC: conceptualization, writing—review and editing, supervision, project administration. ZC: writing—review and editing, supervision, project administration. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The NHANES agreement has been reviewed and approved by the NCHS Research Ethics Committee. All participants provided written informed consent before participating.
Consent for publication
All participants provided written informed consent before participation.
Competing interests
The authors have declared no actual or potential conflict of interest that could inappropriately influence, or be perceived to influence our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dai, Z., Fu, Y., Tan, Y. et al. Association between metal exposures and periodontitis among U.S. adults: the potential mediating role of biological aging. Environ Sci Eur 36, 123 (2024). https://doi.org/10.1186/s12302-024-00949-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00949-y