Abstract
Rice endosperm accumulates large amounts of photosynthetic products as insoluble starch within amyloplasts by properly arranging structured, highly branched, large amylopectin molecules, thus avoiding osmotic imbalance. The amount and characteristics of starch directly influence the yield and quality of rice grains, which in turn influence their application and market value. Therefore, understanding how various allelic combinations of starch biosynthetic genes, with different expression levels, affect starch properties is important for the identification of targets for breeding new rice cultivars. Research over the past few decades has revealed the spatiotemporal expression patterns and allelic variants of starch biosynthetic genes, and enhanced our understanding of the specific roles and compensatory functions of individual isozymes of starch biosynthetic enzymes through biochemical analyses of purified enzymes and characterization of japonica rice mutants lacking these enzymes. Furthermore, it has been shown that starch biosynthetic enzymes can mutually and synergistically increase their activities by forming protein complexes. This review focuses on the more recent discoveries made in the last several years. Generation of single and double mutants and/or high-level expression of specific starch synthases (SSs) allowed us to better understand how the starch granule morphology is determined; how the complete absence of SSIIa affects starch structure; why the rice endosperm stores insoluble starch rather than soluble phytoglycogen; how to elevate amylose and resistant starch (RS) content to improve health benefits; and how SS isozymes mutually complement their activities. The introduction of active-type SSIIa and/or high-expression type GBSSI into ss3a ss4b, isa1, be2b, and ss3a be2b japonica rice mutants, with unique starch properties, and analyses of their starch properties are summarized in this review. High-level accumulation of RS is often accompanied by a reduction in grain yield as a trade-off. Backcrossing rice mutants with a high-yielding elite rice cultivar enabled the improvement of agricultural traits, while maintaining high RS levels. Designing starch structures for additional values, breeding and cultivating to increase yield will enable the development of a new type of rice starch that can be used in a wide variety of applications, and that can contribute to food and agricultural industries in the near future.
Similar content being viewed by others
Background
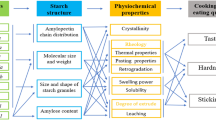
Starch (α-glucan), which is synthesized mainly from photosynthetic products, is deposited in the storage tissues of plants and represents the most important source of carbohydrates in the human diet. Starch is a gigantic polymer of glucose molecules connected by α-1,4- and α-1,6-glucosidc bonds. The major component of starch is amylopectin, a highly branched structure, while that remaining is composed of amylose, which is essentially a linear polymer. The structure of starch, as described above, has been extensively studied at the molecular level; however, the higher order structure of starch has yet to be fully understood. To date, the higher order structure of starch has been postulated by two models and is currently under debate: the Cluster Model (Nikuni 1969; French 1972; Hizukuri 1986) has been proposed by many researchers, and the Backbone Model (Bertoft et al. 2012) is currently being challenged (Crini et al. 2021; Nakamura and Kainuma 2021).
Starch biosynthesis involves at least four classes of enzymes (Smith et al. 1997): ADP-glucose pyrophosphorylase (AGPase), which provides glucose moiety as a substrate; starch synthases (SSs), which use the substrate to elongate α-1,4-linked linear glucose chains; starch branching enzymes (BEs), which create α-1,6-linked branches; and starch debranching enzymes (DBEs), which trim off improper branches (Nakamura 2002). Additional enzymes are also involved in starch biosynthesis: starch phosphorylase, which functions in the initiation step of starch biosynthesis (Satoh et al. 2008; Nakamura et al. 2012); disproportionating enzyme, which transfers α-1,4-linked glucans (Colleoni et al. 1999a, b); and Protein Targeting to Starch (PTST), which guides starch biosynthetic enzymes to the starch granules (Seung et al. 2018). The function of each starch biosynthetic enzyme was elucidated in the 1960s to 1980s using biochemical approaches, and the DNA sequence of the corresponding genes was determined in major crops in the 1990s. Our understanding of the mechanisms of starch biosynthesis rapidly improved after the 2000s because of the isolation, identification, and characterization of mutants lacking starch biosynthetic enzymes in major crops such as maize, rice, wheat, barley, potato, and beans, in addition to model organisms such as Arabidopsis and Chlamydomonas. Our research group revealed the functions of each starch biosynthetic enzyme by exhaustively isolating japonica rice mutants lacking these enzymes and by comparing the characteristics of these mutants with those of the wild-type in detail. The results of our studies served as the basis for the model of starch biosynthesis (Nakamura 2002; Fujita 2014).
The abovementioned rice mutants not only contributed to the discovery of starch biosynthesis mechanisms but also displayed great potential for expanding the use of rice starch in various applications. The starch accumulated in some of these rice mutants exhibited unique physicochemical properties. For example, the texture of high amylose mutant rice ss3a, after cooking, is utterly different from that of wild-type japonica rice. The be2b mutants, which contain high levels of resistant starch (RS), are likely to be utilized as functional rice because the intake of RS is thought to promote human health. Greater variation in the properties of rice starch will likely expand its utilization in various food applications. In this review, the process of generating new rice lines, with unique and novel starch properties, by designing the starch structure, is explained based on accumulating evidence obtained through the analysis of single and double mutants, and by the introduction of high-expressing alleles of starch biosynthetic genes. Please refer to the book “Starch” on rice lines generated before 2015 (Fujita 2015). The present review focuses on rice lines generated after 2015, and describes the breeding of new promising rice cultivars and their potential application in the food industry.
Methods
Mutant rice lines described in this article were isolated from the N-Methyl-N-nitrosourea (MNU)-treated populations of japonica rice cultivars (Kinmaze and Taichung 65) or from the Tos17 insertional mutant panels of japonica rice cultivar, Nipponbare. Mutant lines were identified by western blot analysis of mature seed extracts to confirm the absence of specific proteins. The presence of single nucleotide polymorphisms (SNPs) was confirmed by PCR amplification of genomic DNA isolated from seedlings, followed by genotyping using cleaved amplified polymorphic sequence (CAPS) or derived cleaved amplified polymorphic sequence (dCAPS) markers. SSIIa and/or GBSSI genes derived from indica rice were selected by PCR using SNP-specific primers. Mutant lines lacking starch biosynthetic enzyme(s) were backcrossed with elite rice cultivars, ‘Akita 63’ or ‘Akita Komachi’, and homozygous BC3F2 plants were identified as described above. The selected homozygous plants were grown to maturity, and the seeds were used for extracting starch using the cold-alkaline method. The purified starch was gelatinized, debranched using Pseudomonas isoamylase, and separated by gel filtration chromatography using ToyoPearl HW55s connected in series to three ToyoPearl 50S columns to analyze the apparent amylose content. The amylopectin branch structure was analyzed by capillary electrophoresis, and starch granule morphology was observed by scanning electron microscopy.
SSIIa Alleles
Most of the indica rice cultivars possess active-type SSIIa (wild-type), whereas typical japonica rice cultivars possess mutant SSIIa harboring three amino acid substitutions, thus exhibiting only 10% of SSIIa activity relative to indica rice (Nakamura et al. 2005). Low SSIIa activity in japonica rice leads to a reduction in amylopectin branches with degree of polymerization (DP) 13–24, and an increase in short amylopectin chains with DP ≤ 12 (Umemoto et al. 1999, 2002; Nakamura et al. 2005). These changes in amylopectin branch structure drastically affect the gelatinization temperature of starch (Noda et al. 1998), which is 5–10 °C lower in japonica rice than in indica rice (Nakamura et al. 2002). The effects of SSIIa absence on amylopectin branch structure have also been reported in other plant species such as maize (Zhang et al. 2004; Liu et al. 2012), wheat (Yamamori et al. 2000), barley (Morell et al. 2003), sweet potato (Katayama et al. 2002; Kitahara et al. 2005), and Arabidopsis (Zhang et al. 2008). Loss of SSIIa in maize (Takeda and Preiss 1993), wheat (Yamamori et al. 2000), barley (Morell et al. 2003), and Arabidopsis (Zhang et al. 2008) was accompanied by an increase in amylose content. However, unlike other plant species, accurate evaluation of the association between the increase in amylose content and the loss of SSIIa is not straight forward in rice. This is because the amylose biosynthesis gene granule bound-starch synthase I (GBSSI), which is located close to the SSIIa gene on chromosome 6, carries a SNP at the exon1–intron1 boundary in japonica rice, which reduces the amount of GBSSI protein, thus decreasing the amylose content in japonica rice compared with indica rice (Sano 1984).
We isolated a null mutant of the SSIIa gene, EM204, from the MNU mutant panel of the japonica rice cultivar Kinmaze (Miura et al. 2018). EM204 possesses a SNP at the end of the 5th intron, resulting in the skipping of the 6th exon during translation, eventually producing a negligible amount of the truncated SSIIa protein lacking 15 amino acids (Miura et al. 2018). EM204 showed no SSIIa activity, higher amounts of short amylopectin chains (DP ≤ 12), and lower amounts of amylopectin branches with DP 13–24 compared with wild-type japonica rice, expressing SSIIa with low activity (Fig. 1; Miura et al. 2018). Comparison of amylose content between lines containing or lacking SSIIa under the same japonica-type gbss1L allele revealed that loss of SSIIa led to a higher amylose content, as in other plant species (Miura et al. 2018).
Analysis of the molecular structure of amylopectin by capillary electrophoresis. A Chain-length distribution patterns of amylopectin in mature rice endosperm. B Differential plots of Kinmaze (japonica rice; ss2aL gbss1L), EM204 (ss2a mutant; ss2a gbss1L), and line #1110-290 (expressing indica-type SSIIa; SS2a gbss1L). Each panel shows a typical representative data set of at least three replications. DP, degree of polymerization. Data were obtained from Miura et al. (2018)
Knocking down SSIIa from wild-type japonica rice, Nipponbare, by RNAi also did not show any increase in amylose content in endosperm starch. Its amylopectin structure, physicochemical properties of starch, and seed phenotype resembled those of EM204 (Butardo et al. 2020). SSIIa locus is known to be the same as ALK (Umemoto et al. 1999, 2002), and there are four known alleles, ALKa, ALKb, ALKc, ALKd, depending on the combinations of amino acid substitutions (Zhang et al. 2020; Chen et al. 2020). ALKc encodes indica-type active SSIIa, whereas ALKa encodes japonica-type SSIIa with Glu88Asp, Gly604Ser, Val737Met substitutions resulting in low activity and an increase in amylopectin short chains with DP < 12 (Nakamura et al. 2005; Zhang et al. 2020). ALKb encodes japonica-type SSIIa with Glu88Asp, Gly604Ser, Leu781Phe substitutions, and analyses of near isogenic lines showed a further increase in amylopectin short chains with DP < 12 and decrease in gelatinization temperature compared to ALKa. In contrast, ALKd with Glu to Asp substitution in Exon 1 of ALKc showed a decrease in short amylopectin chains with DP < 12 and an increase in gelatinization temperature (Zhang et al. 2020). Given abovementioned starch structure characteristics of SSIIa alleles, it is no doubt that the strength of SSIIa activity determines the ratio of short amylopectin chains with DP < 12 and intermediate amylopectin chains with DP 13–24. These greatly influence physicochemical properties such as gelatinization temperature and retrogradation of starch. Particularly, the ss2a mutant or SSIIa knockdown rice lines could be utilized practically as a genetic material for preventing the retrogradation of starch, and for breeding rice that tastes excellent after cooking and that maintains its stickiness and softness after cooling.
Ultra-High RS Content Rice
Rice and maize genomes encode three BE isozymes, BEI, BEIIa, and BEIIb, and single mutants of each BE gene have been isolated in japonica rice. BEI is expressed in both endosperm and vegetative tissues (Yamanouchi and Nakamura 1992). The phenotype of the be1 single mutant generally resembles that of the wild-type, with minor differences in endosperm amylopectin structure; the amount of amylopectin branches with DP 12–21 and DP > 37 is slightly higher in be1, whereas that of DP < 10 is slightly increased, compared with the wild-type (Satoh et al. 2003). BEIIa is highly expressed in vegetative organs, and its absence does not affect the structure of endosperm starch (Nakamura 2002). By contrast, BEIIb is expressed exclusively in the endosperm, and be2b mutants exhibit a drastically different endosperm starch structure compared with the wild-type japonica rice: the amount of amylopectin chains with DP < 14 is much lower, while the amylose content is considerably higher in be2b mutants than in the wild-type (Nishi et al. 2001). Owing to its unique structure, starch in be2b mutants resists degradation by digestive enzymes, resulting in high RS content (Tsuiki et al. 2016; Miura et al. 2021). Knocking out BEIIb in indica rice by CRISPR/Cas9 approach gave similar effects on starch properties, and increased amylopectin branches with DP 6, 7 and > 25, decreased amylopectin chains with DP 9–24, increased gelatinization temperature by 6 °C, and increased amylose content by 7% (Tappiban et al. 2021).
RS is defined as the starch that is not easily digested by human digestive enzymes and therefore reaches the large intestine, where it prevents a sudden increase in postprandial glucose levels and improves the intestinal environment, thus promoting human health (Englyst et al. 1992; Nugent 2005; Matsuki 2010). RS contents were analyzed in various rice cultivars and mutants, including indica rice cultivars with high amylose content, single and double mutants of ss3a with high amylose content, and single and double mutants of be2b with high levels of long amylopectin chains. The results of the survey revealed that the RS contents of be2b mutants were an order of magnitude higher than those of other lines. The RS content of high amylose rice, such as indica rice and the ss3a single mutant, was only several times higher than that of wild-type japonica rice. Previously, amylose was considered to be one of the factors contributing to the RS content; however, our study clearly demonstrated that high levels of long amylopectin branches is the primary factor responsible for the increase in RS content (Tsuiki et al. 2016).
Compared with the be2b single mutant, the be1 be2b double mutant contained fewer amylopectin chains with DP 10–20 and more amylopectin chains with DP ≥ 21, and showed an amylose content of 52%, which was the highest in the entire rice lines (Fig. 2; Miura et al. 2021). Given its extreme starch composition, the be1 be2b double mutant showed an increase in the gelatinization temperature of starch by 7.5 °C, and in the RS content of its cooked rice (76%) by threefold, compared with the be2b single mutant. Further analysis demonstrated that RS extracted from the cooked rice of be1 be2b consisted of partially degraded amylose and long amylopectin branches. Although the be1 be2b double mutant lacked two major BE isozymes, its plant growth and fertility rate were not affected, and it showed rather higher seed weight than the be2b single mutant (Miura et al. 2021). Suppression of BEI and BEIIb expression in japonica rice using the transgenic approach has been reported previously (Zhu et al. 2012; Wang et al. 2017; Pan et al. 2018; Sawada et al. 2018); however, this was the first report of BEI and BEIIb suppression in non-transgenic rice. Further breeding of RS-rich rice lines with improved agricultural traits will provide valuable genetic material for producing functional foods in the near future.
Elution profiles of isoamylase-treated debranched starch and amylopectin analyzed by gel filtration chromatography (ToyoPearl HW55S-50S × 3). Fraction I contains amylose or an extra-long chain of amylopectin. Fraction II contains long amylopectin chains. Fraction III contains short amylopectin chains. Red lines indicate the patterns obtained from starch, and blue lines indicate the patterns obtained from purified amylopectin. *Differences in retention time (RT) in Kinmaze were due to the different lot of the column. Data was obtained from Miura et al. (2021)
Introduction of SS2a from Indica Rice into ss1 and ss3a
Among all starch biosynthetic enzymes, SSs possess the largest numbers of isozymes. Among these isozymes, SSI, SSIIa, and SSIIIa play major roles in amylopectin biosynthesis in the endosperm. As described above, SSIIa found in indica rice is the highly active wild-type enzyme, whereas that found in japonica rice exhibits only 10% of the activity of indica SSIIa (Nakamura et al. 2005). Because of the low-level activity of SSIIa in japonica rice, the genotype of wild-type japonica rice is shown as SS1 ss2aL SS3a. Japonica rice mutants lacking SSI (Fujita et al. 2006) or SSIIIa (Fujita et al. 2007) have been isolated previously, both of which show no activity of the corresponding enzymes and different amylopectin structures relative to wild-type japonica rice. However, the ss1 and ss3a mutants accumulate starch to levels similar to the wild-type, which implies that other SS isozymes complement the functions of missing isozymes. In fact, the ss3a mutant showed increased levels of SSI and GBSSI proteins, and these effects were clearly reflected in the starch structure (Fujita et al. 2007). Homozygous ss1 ss3a double mutant is sterile; however, genotypes heterozygous for one of mutant alleles exhibit starch accumulation (Fujita et al. 2011).
Active-type SSIIa derived from indica rice was introduced into the ss1 and ss3a single mutant lines of japonica rice to generate ss1 SS2a SS3a and SS1 SS2a ss3a mutants, and their amylopectin structures were investigated. The results showed that rice lines with the SS2a allele, such as ss1 SS2a SS3a and SS1 SS2a ss3a, showed a reduction in amylopectin chains with DP ≤ 11 and an increase in amylopectin chains with 12 ≤ DP ≤ 24 compared with lines harboring the ss2aL allele, such as ss1 ss2aL SS3a and SS1 ss2aL ss3a. These results indicate that SSIIa functions in the absence of SSI or SSIIIa (Crofts et al. 2017a).
Analysis of japonica ss1 or ss3a mutant rice revealed that each SS isozyme plays a distinct role in amylopectin biosynthesis. SSI elongates the non-reducing ends of amylopectin chains with DP 6 or 7 to DP 8–12 (Fujita et al. 2006); SSIIa functions to elongate amylopectin chains with DP 6–12 to DP 13–24 (Nakamura et al. 2005); and SSIIIa synthesizes long amylopectin chains with DP > 30 (Fujita et al. 2006, 2007). Generation of new rice lines by introducing indica rice-derived active-type SS2a allele into japonica ss1 or ss3a mutant rice enabled the demonstration of compensatory roles among SSI, SSIIa, and SSIIIa (Crofts et al. 2017a). The chain-length distribution pattern of indica rice (SS1 SS2a SS3a) was similar to that of the ss1 SS2a SS3a line, containing active-type SSIIa in the absence of SSI. The absolute value of the subtraction curve of “ss1 SS2a SS3a (rice containing active-type SSIIa in the absence of SSI)—SS1 SS2a SS3a (wild-type indica rice)” was considerably lower than that of “ss1 ss2aL SS3a (japonica ss1 mutant)—SS1 ss2aL SS3a (wild-type japonica rice)”. These results indicated that the active-type SSIIa enzyme derived from indica rice compensated most of the functions of SSI. The increase in the amount of amylopectin chains with DP 6–7 and the reduction in that of chains with DP 8–12 in the absolute value of the subtraction curve “ss1 SS2a SS3a—SS1 SS2a SS3a” were minimized by 80%; however, the increase of DP 16–19 was minimized by only 50%. This implies that SSIIa derived from indica rice compensates the function of SSI in elongating the non-reducing ends of A-chains with DP 6–7 into those with DP 8–12, but does not perfectly compensate its role in the elongation of the non-reducing ends of B1-chains into those with DP 16–19. By contrast, the SS1 SS2a ss3a line generated by introducing indica rice-derived active-type SS2a allele into the ss3a japonica mutant did not increase the amount of long amylopectin chains with DP > 30. This clearly indicates that SSIIa derived from indica rice does not compensate for the function of SSIIIa. Similarly, SSI could not compensate for the function of SSIIa or SSIIIa, and SSIIIa could not compensate for the function of SSI or SSIIa (Fig. 3; Crofts et al. 2017a).
Summary of the relationships between three starch synthase (SS) isozymes in the rice endosperm. (1) Active SSIIa compensates for most of the function of SSI, particularly elongating amylopectin chains from the degree of polymerization (DP) of 6–7 to DP 8–12 (thick arrow). (2) The function of SSIIa could not be complemented by SSI or SSIIIa (dotted arrows). (3) Neither SSI nor SSIIa could compensate for the function of SSIIIa, which synthesizes amylopectin chains with DP > 35 (dotted arrows). (4) The function of SSI could not be complemented by SSIIIa (dotted arrow). This figure was obtained from Crofts et al. (2017a)
Introduction of SS2a and/or GBSS1 from Indica Rice into ss3a ss4b
SS enzymes possess a total of 11 isozymes: one SSI isozyme, three SSII isozymes, two SSIII isozymes, two SSIV isozymes, one SSV isozyme, and two GBSS isozymes. The function of each SS isozyme, including SSI, SSIIa, SSIIIa, and GBSSI, strongly expressed in the endosperm, became apparent through the characterization of the corresponding mutants and the biochemical analysis of purified enzymes. However, the functions of other SS isozymes have not yet been elucidated because they are expressed in tissues other than the endosperm. Mutants lacking SSIVb, one of the SSIV isozymes, have been isolated; however, their amylopectin structure and seed phenotype were the same as those of the wild-type. In contrast to the ss4b single mutant, double mutants lacking both SSIIIa and SSIVb (ss2aL ss3a ss4b) showed a remarkably different starch granule morphology (spherical) compared with the wild-type (polygonal) (Toyosawa et al. 2016). The amounts of B2 and B3 long amylopectin chains, which connect amylopectin clusters, were further reduced in ss3a ss4b mutant compared with ss3a. This suggests that SSIVb is involved in the synthesis of B2 and B3 chains of amylopectin, and therefore is functionally redundant to SSIIIa. Each amyloplast contains multiple starch granules, which form a compound structure in rice (Matsushima et al. 2013), within which individual starch granules are separated from each other by a septum-like structure (Kawagoe 2013). Immuno-electron microscopy observation using anti-SSIVb antibody revealed that SSIVb localizes to septum-like structures in the amyloplast in the developing endosperm of rice (Toyosawa et al. 2016), while SSIIIa localizes to the starch granules as well as the outer envelope membrane surrounding the amyloplast. Loss of SSIIIa alone results in slightly rounded starch granule morphology compared with the wild-type (Fujita et al. 2007). Confocal laser scanning microscopy analysis of the ss3a ss4b double mutant revealed the presence of compound-type starch granules, although they resembled the spherical simple-type starch granules in shape. Thus, the loss of both SSIIIa and SSIVb made the septum-like structure fragile, reduced starch biosynthesis, and fully expanded the starch granules, resulting in spherical starch granules (Toyosawa et al. 2016).
The ss3a ss4b mutants were derived from japonica rice, and therefore showed low SSIIa activity and GBSSI protein levels compared with indica rice (Nakamura et al. 2005; Sano 1984). To determine whether the alteration of starch granule morphology in japonica ss3a ss4b mutants could be complemented by SSIIa and/or GBSSI derived from indica rice, the corresponding genes were introduced into ss2aL ss3a ss4b gbss1L by crossing with indica rice (SS2a SS3a SS4b GBSS1), and SS2a ss3a ss4b gbss1L, ss2aL ss3a ss4b GBSS1, and SS2a ss3a ss4b GBSSI were generated (Crofts et al. 2021a). However, the expression of SS2a and/or GBSS1 in the japonica ss3a ss4b mutant did not recover the starch granule morphology from spherical to polygonal (Fig. 4). This implies that the loss of SSIIIa and SSIVb cannot be complemented by SSIIa or GBSSI (Crofts et al. 2021a). Lines containing active-type SSIIa, such as SS2a ss3a ss4b gbss1L and SS2a ss3a ss4b GBSS1, showed lower levels of amylopectin chains with DP 7–12 and higher levels of those with DP ≥ 13 compared to the lines containing less active SSIIa. Amylose contents of ss2aL ss3a ss4b GBSS1, SS2a ss3a ss4b GBSS1, and SS2a ss3a ss4b gbss1L were 40%, 36%, and 31%, respectively. The ADP-glucose content of the crude extract prepared from the developing endosperm of lines carrying the gbss1L allele but lacking SSIIIa and SSIVb (such as ss2aL ss3a ss4b gbss1L and SS2a ss3a ss4b gbss1L) was high, whereas that of lines with the GBSS1 allele was low, perhaps because ADP-glucose was used for amylose synthesis (Crofts et al. 2021a).
Scanning electron micrographs of the cross-sections of mature seeds. Scale bar = 5 μm. Data were obtained from Crofts et al. (2021b)
Introduction of SS2a and/or GBSS1 from Indica Rice into High RS Mutant Rice
As a potential target locus for increasing RS content in indica rice, a loss of function mutation in SSIIIa in the presence of GBSS1 (Wxa) allele was identified by map-based cloning (Zhou et al. 2016). The rice grains of ss3a GBSS1 had high amylose content (approximately 35%) and more amylose–lipid complex, which increased the RS content to 6% compared to that of the control line (approximately 1%) (Zhou et al. 2016). In comparison to the loss of SSIIIa, the loss of BEIIb drastically increases the RS content of endosperm starch (Tsuiki et al. 2016), as described above. This is because a considerable decline in the amount of short amylopectin branches proportionally increases the amount of long amylopectin chains, and a reduction in amylopectin biosynthesis elevates the amylose content; both of these factors contribute to the increase in RS content (Tsuiki et al. 2016; Zhou et al. 2016; Chen et al. 2017). To determine whether RS content can be further increased, SS2a and/or GBSS1 alleles from indica rice were introduced into japonica be2b single mutant or ss3a be2b double mutant (Itoh et al. 2017; Miura et al. submitted). To practically utilize high RS rice lines, it is important to increase their yield. However, elevation of the RS content of the endosperm and increase in seed weight and yield are often a trade-off. To solve this problem, RS-rich rice lines were backcrossed three times with the high-yielding and large grain bearing rice cultivar, ‘Akita 63’ (Makino et al. 2020).
Compared with mutants lacking SS isozymes such as SSI, SSIIIa (Crofts et al. 2017a), or SSIIIa and SSIVb (Crofts et al. 2021a) described above, japonica be2b single mutant or ss3a be2b double mutant showed no significant change in starch structure upon the introduction of the indica SS2a allele (Itoh et al. 2017; Luo et al. 2020). This is because the number of short amylopectin branches (DP < 12), which serve as primers for SSIIa, was greatly reduced in the absence of BEIIb. On the other hand, introduction of the GBSS1 allele into the japonica be2b single mutant increased the apparent amylose content to approximately 38% (equivalent to a 1.75-fold increase relative to the original japonica be2b mutant; Miura et al. submitted). Analyses of the recombinant inbred lines, which possess SS2a and GBSS1 but lack BEIIb, also showed high amylose content (31–34%) but had some short branches on amylose (Zhang et al. 2022). Loss of SSIIIa is known to increase the apparent amylose content of japonica rice (ss2aL ss3a be2b) compared with wild-type and be2b single mutant japonica rice, because of a concomitant increase in GBSSI and AGPase protein levels (Asai et al. 2014). However, introduction of the GBSS1 allele into the japonica ss3a be2b double mutant did not cause any further increase in the apparent amylose content (Miura et al. submitted), presumably because the ADP-glucose content reached its upper limit and was insufficient to meet the demand of increased GBSSI levels. The RS content of rice lines lacking BEIIb was higher than that of lines lacking SSIIIa and BEIIb, regardless of the presence or absence of SS2a and/or GBSS1 alleles. This is because the loss of SSIIIa increases the expression levels of SS1, thus increasing the number of short amylopectin branches and decreasing the number of long amylopectin chains connecting amylopectin clusters (Fujita et al. 2007). Introduction of SS2a and/or GBSS1 elevated the RS content, and backcrossing the resultant RS-rich lines increased their seed weight by up to 1.9-fold. This is likely because flowering time was advanced, and temperature during the seed development period became more suitable for efficient starch biosynthesis. The ss2aL be2b GBSS1 rice line showed high RS content and outstanding seed weight (29 mg per grain) compared with typical japonica rice (20 mg per grain), indicating its potential for practical applications.
Introduction of SS2a and/or GBSS1 from Indica Rice into isa1
In addition to elongation of glucans by SS and generation of branches by BE, the trimming of inappropriate glucan branches by one of the DBEs, called isoamylase 1 (ISA1), is essential for formulating properly structured insoluble starch and for generating plump seeds. Loss of ISA1 leads to the accumulation of water-soluble phytoglycogen and the production of shriveled seeds in many plant species, including maize (James et al. 1995) and rice (Nakamura et al. 1996, 1997; Kubo et al. 1999), as shown by the analyses of isa1 (sugary-1, sug-1) mutants (Nakamura 2002). Japonica isa1 mutants can be roughly divided into two groups: severe-type, with phytoglycogen accumulation in the entire endosperm; and mild-type, showing phytoglycogen accumulation in the seed core but starch accumulation in the seed periphery, which can be stained by iodine (Nakamura et al. 1997; Kubo et al. 1999). These differences are dependent on the site of mutation within the ISA1 gene, but their exact cause remains unknown. Introduction of the SS2a allele from indica rice into severe-type japonica isa1 mutant (EM914) using the transgenic approach resulted in the accumulation of iodine-stainable insoluble α-glucans in the endosperm (Fujita et al. 2012). This insoluble glucan contained longer short amylopectin branches than phytoglycogen, but showed no increase in the amount of long amylopectin chains (DP > 30). X-ray diffraction analyses revealed that this glucan showed weak B-type crystallinity, unlike phytoglycogen (no crystallinity) and wild-type rice (clear A-type crystallinity).
When SS2a and/or GBSS1 alleles were introduced into the mild-type japonica isa1 mutant (EM653) by conventional crossing, the resultant genotypes (SS2a gbss1L isa1 and SS2a GBSS1 isa1) accumulated starch instead of phytoglycogen and produced plump seeds (Crofts et al. 2021b). While introduction of the GBSS1 allele alone into the mild-type japonica isa1 mutant (EM653) was phenotypically similar to EM653 and accumulated phytoglycogen in the central region of the shriveled seeds (Fig. 5). Amylopectin branches in rice lines harboring the SS2a allele (SS2a gbss1L isa1 and SS2a GBSSI isa1) were elongated, and their chain-length distribution patterns (DP < 24) were shifted toward longer amylopectin chains, as in the severe-type isa1 japonica mutant transformed with the SS2a allele. The mild- and severe-type isa1 mutants carrying the SS2a allele differed from each other in the amounts of long amylopectin chains (DP 30–60), which were higher in mild-type than in severe-type isa1 mutant background, although the amounts of these amylopectin chains in SS2a gbss1L isa1 and SS2a GBSS1 isa1 lines were less than those in the wild-type. In addition, introduction of the SS2a allele into the mild-type japonica isa1 mutant resulted in displaying clear A-type crystallinity and polygonal starch granules, similar to the wild-type; however, this result was not obtained by the introduction of SS2a into the severe-type japonica isa1 mutant (Fujita et al. 2012).
Analysis of rice seed morphology. Images show the morphologies of dehulled mature rice seeds (top), stereo-micrographs of iodine-stained cross-section of mature seeds (middle), and scanning electron micrographs of the central region of mature seeds (bottom). Data were obtained from Crofts et al. (2021a)
Taken together, these findings suggest that SSIIa partially complements the loss of ISA1 by avoiding generation of phytoglycogen and by accumulating amylopectin. SSIIa could also sufficiently extend the branches of phytoglycogen. On the other hand, while the introduction of the GBSS1 allele alone into the japonica isa1 mutant could elevate the amylose content by 10%, it could not convert phytoglycogen into amylopectin, and the seeds remained shriveled.
Practical Applications of Mutant Lines
The absence of enzymes required for starch biosynthesis partially inhibits the production of starch in the developing endosperm, leading to smaller grains and poor yield; the loss of BEIIb is a classic example. In addition, loss of more than one enzyme, such as both SSI and SSIIIa (Fujita et al. 2011) or both SSI and BEIIb (Abe et al. 2014), results in sterility. Unless the plant is completely sterile, the reduction in grain yield can be overcome by the introduction of loci responsible for high yield. As an attempt to improve agricultural traits, such as early flowering, large grain size, and high grain yield, we crossed rice mutants accumulating structurally unique starch with a high-yielding and elite rice cultivar ‘Akita 63’ (Makino et al. 2020).
The ss3a mutant accumulates a large amount of amylose, with almost no reduction in seed weight, compared with the wild-type; however, its late-flowering phenotype, as observed in our research paddy filed at high latitude in Akita prefecture, suggests that ss3a is not suitable for commercial cultivation. The ss3a mutant exhibited delayed flowering because its genes controlling flowering time were derived from the late-flowering parental rice cultivar ‘Nipponbare’. We backcrossed the ss3a mutant three times either with a high-yielding elite rice cultivar ‘Akita 63’ or with an excellent tasting elite rice cultivar ‘Akita Komachi’, resulting in the production of ‘Akita Sarari’ or ‘Akita Parari’, respectively (Fujita 2020). Cultivars ‘Akita 63’ and ‘Akita Komachi’ were selected as recurrent parents for backcrossing, since both these cultivars are suitable for cultivation in high latitude regions such as the Akita prefecture. In theory, genes derived from an elite parental rice cultivar account for 93.75% of the genetic background of the BC3 progeny. The flowering time of newly generated rice cultivars ‘Akita Sarari’ and ‘Akita Parari’ was similar to that of the elite rice cultivars (early August) and approximately 1 month earlier than that of the original ss3a mutant. The seed weight and yield of ‘Akita Sarari’ and ‘Akita Parari’ were also similar to those of ‘Akita 63’ and ‘Akita Komachi’, respectively. ‘Akita Sarari’ presented high yield and high amylose content, and its flour could be used for the production of noodles and bread. ‘Akita Parari’ exhibited a less sticky texture relative to the typical japonica rice cultivars and could be used for making pilaf, fried rice, and risotto. Both ‘Akita Sarari’ and ‘Akita Parari’ are now commercially available (https://starchtec.com/).
As described earlier, RS content and seed weight are often in a trade-off relationship in the absence of BEIIb. We tried to overcome this problem by crossing the high RS lines with a highly yielding rice cultivar. The high RS rice cultivar ‘Manpuku Surari’ was generated by backcrossing the ss3a be2b mutant (Asai et al. 2014) with ‘Akita 63’ three times. The resultant rice line ‘Manpuku Surari’ exhibited 1.5-fold higher seed weight compared with the original ss3a be2b mutant, and tenfold higher RS content in cooked rice and rice flour relative to the typical japonica rice, in addition to the ability to suppress postprandial increase in glucose levels (Saito et al. 2020). Thus, ‘Manpuku Surari’ is anticipated to be sold as “Foods with Function Claims”, as designated by the Consumer Affairs Agency.
Another effective way to overcome the low seed weight in high RS lines is to combine the absence of BEIIb with an absence of ISA1. ‘Chikushi-kona 85’ is a high RS japonica-rice mutant lacking BEIIb activity, but generated relatively plump seeds unlike shrunken seeds often seen in be2b mutants (Wada et al. 2018). The detailed genetic analyses of ‘Chikushi-kona 85’ and its parental mutant EM129 revealed that they were the double mutants lacking both BEIIb and ISA1 (Nagamatsu et al. 2022). BEIIb and ISA1 are the enzymes responsible for the generation of short amylopectin branches (Nishi et al. 2001; Tanaka et al. 2004) and removal of improper amylopectin branches (Nakamura 2002), respectively. The absence of these enzymes gives opposite effects on amylopectin structure. Loss of BEIIb drastically decreases short amylopectin chains with DP < 14 and increases long amylopectin chains (Nishi et al. 2001), while loss of ISA1 greatly increases short amylopectin chains with DP < 11 and decreases intermediate amylopectin chains (Nakamura et al. 1997). Both of these single mutant lines display shrunken or wrinkled seed phenotype with low seed weight (Nishi et al. 2001; Nakamura et al. 1997). Endosperm starch of ‘Chikushi-kona 85’ and EM129, both of which lack BEIIb and ISA1, showed milder starch phenotypes compared with the be2b single mutant, and accumulated a very small amount of phytoglycogen at the center of the seeds (Nagamatsu et al. 2022). In other words, the deficiency of BEIIb and ISA1 enzymes canceled each other's effects, and their double mutant resulted in high RS content and high seed weight (Nagamatsu et al. 2022). ‘Chikushi-kona 85’, generated by crossing EM129 with a high yield elite rice cultivar, was registered as a rice variety that suppresses fluctuation of postprandial blood glucose levels (Wada et al. 2018).
Conclusions
Our results showed that the structure and properties of starch in the rice endosperm could be manipulated by abolishing and/or increasing the expression levels of various starch biosynthetic enzymes; however, the degree and type of change relative to wild-type rice varied widely. The longer the length between the non-reducing ends to the branch point of amylopectin branches within a cluster, the higher the temperature required for starch gelatinization. Moreover, as the gelatinization temperature increases, the retrogradation of starch becomes easier, while its degradation by digestive enzymes becomes more difficult. Conversely, the shorter the amylopectin branches, the easier the gelatinization and degradation, and the more difficult the retrogradation. Among the dozens of isozymes of starch biosynthetic enzymes, ISA1, SSIIa, and BEIIb had the greatest influence on the structure and properties of amylopectin. The length of amylopectin branches decreased in the absence of ISA1 and SSIIa, and increased in the absence of BEIIb, thus demonstrating that ISA1, SSIIa, and BEIIb directly influence the physicochemical properties of starch, including gelatinization temperature, retrogradation, and enzymatic degradation.
Research has also elucidated the order of starch biosynthesis. A recent study showed that BEIIb generates amylopectin branches near the amorphous region of the crystalline lamella (Nakamura et al. 2020). Amylopectin branches generated by BEIIb are first elongated by SSI by the addition of two glucose residues, and then further elongated by active-type SSIIa in indica rice (Abe et al. 2014; Crofts et al. 2017a). This is supported by the drastic reduction in SSI activity upon the loss of BEIIb (Nishi et al. 2001). Furthermore, SSI, SSIIa, and BEIIb are thought to form a trimeric complex, which facilitates efficient starch biosynthesis during seed development (Liu et al. 2012; Crofts et al. 2017b).
High-level expression of GBSSI in japonica rice mutants, achieved by the introduction of the GBSS1 allele from indica rice, generally increases their amylose content. However, introduction of the GBSS1 allele into the ss3a be2b double mutant, in which the expression level of the endogenous GBSSI gene is also high, does not cause any further increase in amylose content, most likely because of the depletion of its substrate (ADP-glucose) (Miura et al. submitted).
The generation of new allelic combinations of starch biosynthetic genes exhibiting no and/or high expression levels in rice, and analysis of their starch properties are starting to allow us to freely design the starch structure according to the application-based requirements. We have isolated numerous japonica rice mutant lines lacking starch biosynthetic enzymes, analyzed their starch phenotypes, and revealed the function of individual enzymes (Fujita 2014). However, a considerable proportion of the world’s population consumes indica rice as their staples. Comparing with the rich source of japonica rice mutant panels, the number of mutants isolated from indica rice and their reports are currently limited. However, presence of unique allele(s) that affect starch properties in indica rice is expected, such as ALKd described in the present review article. In fact, six starch biosynthetic enzymes, SSI, SSIIa, GBSSI, BEI, BEIIa, and BEIIb, are known to have different alleles between japonica and indica rice cultivars. Their recombinant inbred lines showed that not only SSIIa but also SSI, EBI, and BEIIa gave a minor effect on starch structure (Luo et al. 2015). These suggest that the presence of unique alleles derived from indica rice that are different from the japonica rice. Accumulating the knowledge of different alleles in indica rice variety and combining with the alleles from japonica rice will enable to expand the variation of starch further. If these allelic combinations inhibit starch biosynthesis and deteriorate certain agricultural traits such as seed size, then the lines carrying these allelic combinations could be backcrossed with high-yielding elite varieties. Designing starch structures, breeding and cultivating to suit their growth environment and increase yield, and popularizing them will enable the development of a new type of rice starch, which can be used in a wide range of applications, and can contribute to the food industry and agricultural sector in the near future.
Availability of data and materials
Not applicable.
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- BE:
-
Starch branching enzymes
- DBE:
-
Starch debranching enzymes
- DP:
-
Degree of polymerization
- GBSS:
-
Granule-bound starch synthase
- ISA:
-
Isoamylase
- RS:
-
Resistant starch
- SNP:
-
Single nucleotide polymorphism
- SS:
-
Starch synthases
References
Abe N, Asai H, Yago H, Oitome NF, Itoh R, Crofts N, Nakamura Y, Fujita N (2014) Relationships between starch synthase I and branching enzyme isozymes determined using double mutant rice lines. BMC Plant Biol 14:80. https://doi.org/10.1186/1471-2229-14-80
Asai H, Abe N, Matsushima R, Crofts N, Oitome NF, Nakamura Y, Fujita N (2014) Deficiencies in both starch synthase (SS) IIIa and branching enzyme IIb lead to a significant increase in amylose in SSIIa inactive japonica rice seeds. J Exp Bot 65:5497–5507. https://doi.org/10.1093/jxb/eru310
Bertoft E, Koch K, Aman P (2012) Building block organization of clusters in amylopectin from different structural types. Int J Biol Macromol 50:1212–1223. https://doi.org/10.1016/j.ijbiomac.2012.03.004
Butardo VM Jr, Luo J, Li Z, Gidley MJ, Bird AR, Tetlow IJ, Fitzgerald M, Jobling SA, Rahman S (2020) Functional genomic validation of the roles of soluble starch synthase IIa in japonica rice endosperm. Front Genet 11:289. https://doi.org/10.3389/fgene.2020.00289
Chen MH, Bergman CJ, McClung AM, Everette JD, Tabien RE (2017) Resistant starch: variation among high amylose rice varieties and its relationship with apparent amylose content, pasting properties and cooking methods. Food Chem 234:180–189. https://doi.org/10.1016/j.foodchem.2017.04.170
Chen Z, Lu Y, Feng L, Hao W, Li C, Yang Y, Fan X, Li Q, Zhang C, Liu Q (2020) Genetic dissection and functional differentiation of ALKa and ALKb, two natural alleles of the ALK/SSIIa gene, responding to low gelatinization temperature in rice. Rice 13:39. https://doi.org/10.1186/s12284-020-00393-5
Colleoni C, Dauvillée D, Mouille G, Buléon A, Gallant D, Bouchet B, Morell M, Samuel M, Delrue B, d’Hulst C, Bliard C, Nuzillard JM, Ball S (1999a) Genetic and biochemical evidence for the involvement of a-1,4 glucanotransferases in amylopectin synthesis. Plant Physiol 120:993–1003. https://doi.org/10.1104/pp.120.4.993
Colleoni C, Dauvillée D, Mouille G, Morell M, Samuel M, Slomiany MC, Liénard L, Wattebled F, d’Hulst C, Ball S (1999b) Biochemical characterization of the Chlamydomonas reinhardtii a-1,4 glucanotransferase supports a direct function in amylopectin biosynthesis. Plant Physiol 120:1005–1014. https://doi.org/10.1104/pp.120.4.1005
Crini G, French AD, Kainuma K, Jane JL, Szente L (2021) Contributions of Dexter French (1918–1981) to cycloamylose/cyclodextrin and starch science. Carbohydr Polym 257:117620. https://doi.org/10.1016/j.carbpol.2021.117620
Crofts N, Nakamura Y, Fujita N (2017a) Critical and speculative review of the roles of multi-protein complexes in starch biosynthesis in cereals. Plant Sci 262:1–8. https://doi.org/10.1016/j.plantsci.2017.05.007
Crofts N, Sugimoto K, Oitome NF, Nakamura Y, Fujita N (2017b) Differences in specificity and compensatory functions among three major starch synthases determine the structure of amylopectin in rice endosperm. Plant Mol Biol 94:399–417. https://doi.org/10.1007/s11103-017-0614-8
Crofts N, Domon A, Miura S, Hosaka Y, Oitome NF, Itoh A, Noge K, Fujita N (2021a) Starch synthases SSIIa and GBSSI control starch structure but do not determine starch granule morphology in the absence of SSIIIa and SSIVb. Plant Mol Biol 237:116118. https://doi.org/10.1016/j.carbpol.2020.116118
Crofts N, Satoh Y, Miura S, Hosaka Y, Abe M, Fujita N (2021b) Active-type starch synthase (SS) IIa from indica rice partially complements the sugary-1 phenotype in japonica rice endosperm. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01161-9
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:33–50
French D (1972) Fine structure of starch and its relationship to the organization of starch granules. J Jpn Soc Starch Sci 19:8–25. https://doi.org/10.5458/jag1972.19.8
Fujita N (2014) Starch biosynthesis in rice endosperm. Agri-Biosci Monogr 4:1–18
Fujita N (2015) Manipulation of rice starch properties for application. In: Nakamura Y (ed) Starch. Springer, Japan. https://doi.org/10.1007/978-4-431-55495-0_10
Fujita N (2020) Establishment of “Starchmics”: cataloguing of structure, physicochemical properties and special use of various rice starch. Impact. https://doi.org/10.21820/23987073.2020.9.74
Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140:1070–1084. https://doi.org/10.1104/pp.105.071845
Fujita N, Yoshida M, Kondo T, Saito K, Utsumi T, Tokunaga T, Nishi A, Satoh H, Park JH, Jane JL, Miyao A, Hirochika H, Nakamura Y (2007) Characterization of SSIIIa-deficient mutants of rice (Oryza sativa L.); the fucntion of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol 144:2009–2023. https://doi.org/10.1104/pp.107.102533
Fujita N, Satoh R, Hayashi A, Kodama M, Itoh R, Aihara S, Nakamura Y (2011) Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIA. J Exp Bot 62:4819–4831. https://doi.org/10.1093/jxb/err125
Fujita N, Hanashiro I, Suzuki S, Higuchi T, Toyosawa Y, Utsumi Y, Itoh R, Aihara S, Nakamura Y (2012) Elongated phytoglycogen chain-length in transgenic rice endosperm expressing active starch synthase IIa affects the altered solubility and crystallinity of the storage α-glucan. J Exp Bot 63:5859–5872. https://doi.org/10.1093/jxb/ers235
Hizukuri S (1986) Polymodal distribution of the chain-lengths of amylopectins, and its significance. Carbohydr Res 147:342–347. https://doi.org/10.1016/S0008-6215(00)90643-8
Itoh Y, Crofts N, Abe M, Hosaka Y, Fujita N (2017) Characterization of the endosperm starch and the pleiotropic effects of biosynthetic enzymes on their properties in novel mutant rice lines with high resistant starch and amylose content. Plant Sci 258:52–60. https://doi.org/10.1016/j.plantsci.2017.02.002
James MG, Robertson DS, Myers AM (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7:417–429. https://doi.org/10.1105/tpc.7.4.417
Katayama K, Komae K, Kohyama K, Kato T, Tamiya S, Komaki K (2002) New sweet potato line having low gelatinization temperature and altered starch structure. Starch/stärke 54:51–57. https://doi.org/10.1002/1521-379X(200202)54:23.0.CO;2-6
Kawagoe Y (2013) The characteristic polyhedral, sharp-edged shape of compound-type starch granules in rice endosperm is achieved via the septum-like structure of the amyloplast. J Appl Glycosci 60:29–36. https://doi.org/10.5458/jag.jag.JAG-2012_013
Kitahara K, Fukunaga S, Katayama K, Takahata Y, Nakazawa Y, Yoshinaga M, Saganuma T (2005) Physicochemical properties of sweetpotato starches with different gelatinization temperatures. Starch/stärke 57:473–479. https://doi.org/10.1002/star.200400349
Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y (1999) The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol 121:399–409. https://doi.org/10.1104/pp.121.2.399
Liu F, Romanova N, Lee EA, Ahmed R, Evans M, Gilbert EP, Morell MK, Emes MJ, Tetlow IJ (2012) Glucan affinity of starch synthase IIa determines binding of starch synthase I and starch-branching enzyme IIb to starch granules. Biochem J 448:373–387. https://doi.org/10.1042/BJ20120573
Luo J, Jobling SA, Millar A, Morell MK, Li Z (2015) Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice 8:15. https://doi.org/10.1186/s12284-015-0046-5
Luo J, Butardo VM Jr, Yang Q, Konik-Rose C, Colgrave ML, Millar A, Jobling SA, Li Z (2020) The impact of the indica rice SSIIa allele on the apparent high amylose starch from rice grain with downregulated japonica SBEIIb. Theor Appl Genet 133:2961–2974. https://doi.org/10.1007/s00122-020-03649-2
Makino A, Kaneta T, Obara M, Ishiyama K, Kanno K, Kondo E, Suzuki Y, Mae T (2020) High yielding ability of a large-grain rice cultivar, Akita 63. Sci Rep 10:12231. https://doi.org/10.1038/s41598-020-69289-0
Matsuki J (2010) Resistant starch. J Jpn Soc Food Sci 57:224. https://doi.org/10.3136/nskkk.57.224
Matsushima R, Yamashita J, Kariyama S, Enomoto T, Sakamoto T (2013) A phylogenetic re-evaluation of morphological variations of starch grains among poaceae species. J Appl Glycosci 60:37–44. https://doi.org/10.5458/jag.jag.JAG-2012_006
Miura S, Crofts N, Saito Y, Hosaka Y, Oitome NF, Watanabe T, Kumamaru T, Fujita N (2018) Starch synthase IIa-deficient mutant rice line produces endosperm starch with lower gelatinization temperature than japonica rice cultivars. Front Plant Sci 9:645. https://doi.org/10.3389/fpls.2018.00645.eCollection
Miura S, Koyama N, Crofts N, Hosaka Y, Abe M, Fujita N (2021) Generation and starch characterization of non-transgenic BEI and BEIIb double mutant rice (Oryza sativa) with ultra-high level of resistant starch. Rice 14:3. https://doi.org/10.1186/s12284-020-00441-0
Morell MK, Kosar-Hashemi B, Cmiel M, Samuel MS, Chandler P, Rahman S, Buleon A, Batey IL, Li Z (2003) Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J 34:173–185. https://doi.org/10.1046/j.1365-313x.2003.01712.x
Nagamatsu S, Wada T, Matsushima R, Fujita N, Miura S, Crofts N, Hosaka Y, Yamaguchi O, Kumamaru T (2022) Mutation in BEIIb mitigates the negative effect of the mutation in ISA1 on grain filling and amyloplast formation in rice. Plant Mol Biol. https://doi.org/10.1007/s11103-022-01242-3
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43:718–725. https://doi.org/10.1093/pcp/pcf091
Nakamura Y, Kainuma K (2021) On the cluster structure of amylopectin. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01183-3
Nakamura Y, Umemoto T, Takahata Y, Komae K, Amano E, Satoh H (1996) Changes in structure of starch and enzyme activities affected by sugary mutations in developing rice endosperm. Possible role of starch debranching enzyme (R-enzyme) in amylopectin biosynthesis. Physiol Plant 97:491–7498. https://doi.org/10.1111/j.1399-3054.1996.tb00508.x
Nakamura Y, Kubo A, Shimamune T, Matsuda T, Harada K, Satoh H (1997) Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J 12:143–153. https://doi.org/10.1046/j.1365-313X.1997.12010143.x
Nakamura Y, Sakurai A, Inaba Y, Kimura K, Iwasawa N, Nagamine T (2002) The fine structure of amylopectin in endosperm from Asian cultivated rice can be largely classified into two classes. Starch/stärke 54:117–131. https://doi.org/10.1002/1521-379X(200204)54:3/4%3c117::AID-STAR117%3e3.0.CO;2-2
Nakamura Y, Francisco BP Jr, Hosaka Y, Sato A, Sawada T, Kubo A, Fujita N (2005) Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice cultivars. Plant Mol Biol 58:213–227. https://doi.org/10.1007/s11103-005-6507-2
Nakamura Y, Ono M, Utsumi Y, Steup M (2012) Functional interaction between plastidial starch phosphorylase and starch branching enzymes from rice during the synthesis of branched maltodextrins. Plant Cell Physiol 53:869–878. https://doi.org/10.1093/pcp/pcs030
Nakamura Y, Ono M, Hatta T, Kainuma K, Yashiro K, Matsuba G, Matsubara A, Miyazato A, Mizutani G (2020) Effects of BEIIb-deficiency on the cluster structure of amylopectin and the internal structure of starch granules in endosperm and culm of japonica-type rice. Front Plant Sci 11:571346. https://doi.org/10.3389/fpls.2020.571346.eCollection
Nikuni Z (1969) Starch and cooking. J Cook Sci Jpn 2:6–14
Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127:459–472. https://doi.org/10.1104/pp.010127
Noda T, Takahata Y, Sato T, Morishita T, Ishiguro K, Yamakawa O (1998) Relationships between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweet potato and buckwheat. Carbohydr Polym 37:153–158. https://doi.org/10.1016/S0144-8617(98)00047-2
Nugent P (2005) Health properties of resistant starch. Nutr Bull 30:27–54. https://doi.org/10.1111/j.1467-3010.2005.00481.x
Pan T, Lin L, Wang J, Liu Q, Wei C (2018) Long branch-chains of amylopectin with B-type crystallinity in rice seed with inhibition of starch branching enzyme I and IIb resist in situ degradation and inhibit plant growth during seedling development: Degradation of rice starch with inhibition of SBEI/IIb during seedling development. BMC Plant Biol 18:9. https://doi.org/10.1186/s12870-017-1219-8
Saito Y, Watanabe T, Sasaki T, Watanabe K, Hirayama M, Fujita N (2020) Effects of single ingestion of rice cracker and cooked rice with high resistant starch on postprandial glucose and insulin responses in healthy adults: two randomized, single-blind, cross-over trials. Biosci Biotechnol Biochem 84:365–371. https://doi.org/10.1080/09168451.2019.1687282
Sano Y (1984) Differential regulation of waxy gene expression in rice endosperm. Theor Appl Genet 68:467–473. https://doi.org/10.1007/BF00254822
Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y (2003) Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol 133:1111–1121. https://doi.org/10.1104/pp.103.021527
Satoh H, Shibahara K, Tokunaga T, Nishi A, Tasaki M, Hwang SK, Okita TW, Kaneko N, Fujita N, Yoshida M, Hosaka Y, Sato A, Utsumi Y, Ohdan T, Nakamura Y (2008) Mutation of the plastidial α-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 20:1833–1849. https://doi.org/10.1105/tpc.107.054007
Sawada T, Itoh M, Nakamura Y (2018) Contributions of three starch branching enzyme Isozymes to the fine structure of amylopectin in rice endosperm. Front Plant Sci 9:1536. https://doi.org/10.3389/fpls.2018.01536
Seung D, Schreier TB, Bürgy L, Eicke S, Zeeman SC (2018) Two plastidial coiled-coil proteins are essential for normal starch granule initiation in Arabidopsis. Plant Cell 30:1523–1542. https://doi.org/10.1105/tpc.18.00219
Smith AM, Denyer K, Martin C (1997) The synthesis of the starch granules. Annu Rev Plant Mol Biol 48:67–87. https://doi.org/10.1146/annurev.arplant.48.1.67
Takeda Y, Preiss J (1993) Structure of B90 (sugary) and W64A (normal) maize starches. Carbohydr Res 240:265–275. https://doi.org/10.1016/0008-6215(93)84189-D
Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, Kawasaki S, Nakamura Y (2004) The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotech J 2:507–516. https://doi.org/10.1111/j.1467-7652.2004.00097.x
Tappiban P, Hu Y, Deng J, Zhao J, Ying Y, Zhang Z, Xu F, Bao J (2021) Relative importance of branching enzyme isoforms in determining starch fine structure and physicochemical properties of indica rice. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01207-y
Toyosawa Y, Kawagoe Y, Matsushima R, Crofts N, Ogawa M, Fukuda M, Kumamaru T, Okazaki Y, Kusano M, Saito K, Toyooka K, Sato M, Ai Y, Jane JL, Nakamura Y, Fujita N (2016) Deficiency of starch synthase IIIa and IVb alters starch granule morphology from polyhedral to spherical in rice endosperm. Plant Physiol 170:1255–1270. https://doi.org/10.1104/pp.15.01232
Tsuiki K, Fujisawa H, Itoh A, Sato M, Fujita N (2016) Alterations of starch structure lead to increased resistant starch of steamed rice: Identification of high resistant starch rice line. J Cereal Sci 68:88–92. https://doi.org/10.1016/j.jcs.2016.01.002
Umemoto T, Nakamura Y, Satoh H, Terashima K (1999) Differences in amylopectin structure between two rice varieties in relation to the effects of temperature during grain-filling. Starch/Stärke 51:58–62. https://doi.org/10.1002/(SICI)1521-379X(199903)51:2%3c58::AID-STAR58%3e3.0.CO;2-J
Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y (2002) Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor Appl Genet 104:1–8. https://doi.org/10.1007/s001220200000
Wada T, Yamaguchi O, Miyazaki M, Miyahara K, Ishibashi M, Aihara T, Shibuta T, Inoue T, Tsubone M (2018) Development and characterization of a new rice cultivar, “Chikushi-kona 85”, derived from a starch-branching enzyme IIb-deficient mutant line. Breed Sci 68:278–283. https://doi.org/10.1270/jsbbs.17069
Wang J, Hu P, Chen Z, Liu Q, Wei C (2017) Progress in high-amylose cereal crops through Inactivation of starch branching enzymes. Front Plant Sci 8:469. https://doi.org/10.3389/fpls.2017.00469
Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T (2000) Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor Appl Genet 101:21–29. https://doi.org/10.1007/s001220051444
Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-Enzyme) in rice. Plant Cell Physiol 33:985–991. https://doi.org/10.1111/j.1744-7909.2008.00714.x
Zhang X, Colleoni C, Ratushna V, Sirghie-Colleoni M, James MG, Myers AM (2004) Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol Biol 54:865–879. https://doi.org/10.1007/s11103-004-0312-1
Zhang X, Szydlowski N, Delvallé D, D’Hulst C, James MG, Myers AM (2008) Overlapping functions of the starch synthase SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol 8:96–113. https://doi.org/10.1186/1471-2229-8-96
Zhang C, Yang Y, Chen Z, Chen F, Pan L, Lu Y, Li Q, Fan X, Sun Z, Liu Q (2020) Characteristics of grain physicochemical properties and the starch structure in rice carrying a mutated ALK/SSIIa gene. J Agric Food Chem 68:13950–13959. https://doi.org/10.1021/acs.jafc.0c01471
Zhang Z, Hu Y, Zhao J, Zhang Y, Ying Y, Xu F, Bao J (2022) The role of different Wx and BEIIb allele combinations on fine structures and functional properties of indica rice starches. Carbohydr Polym 278:118972. https://doi.org/10.1016/j.carbpol.2021.118972
Zhou H, Wang L, Liu G, Meng X, Jing Y, Shu X, Kong X, Sun J, Yu H, Smith SM, Wu D, Li J (2016) Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. PNAS 113:12844–12849. https://doi.org/10.1073/pnas.1615104113
Zhu L, Gu M, Meng X, Cheung SC, Yu H, Huang J, Sun Y, Shi Y, Liu Q (2012) High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol J 10:353–362. https://doi.org/10.1111/j.1467-7652.2011.00667.x
Acknowledgements
The authors thank Ms. Yuko Hosaka, Naoko F. Oitome, Misato Abe, Yuko Nakaizumi and Rika Takahashi (Akita Prefectural University) for technical supports and growing the rice plants and Bio-Edit for English language review. The authors also thank Prof. Hikaru Satoh for providing parental mutant lines (be1 (EM557) and be2b (EM10). Pseudomonas isoamylase used for debranching amylopectin was a kind gift from Hayashibara Co., Ltd.
Funding
This work was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry and Fisheries and Food Industry (25033AB and 28029C; NF), the President’s Funds of Akita Prefectural University (N.F. and N.C.), Grant-in Aid for JSPS fellows from Japan Society for the Promotion of Science (#15J40176 and JP18J40020; NC), and the Japan Society for the Promotion of Science (#16K18571, JP18K14438, and 20K05961; N.C. and 19H01608; N.F.).
Author information
Authors and Affiliations
Contributions
NF designed research, performed experiments and wrote the paper. SM isolated a double mutant and performed experiments, analyzed data. NC isolated a double mutant and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, N., Miura, S. & Crofts, N. Effects of Various Allelic Combinations of Starch Biosynthetic Genes on the Properties of Endosperm Starch in Rice. Rice 15, 24 (2022). https://doi.org/10.1186/s12284-022-00570-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-022-00570-8