Abstract
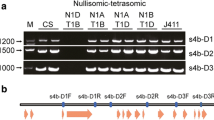
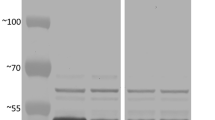
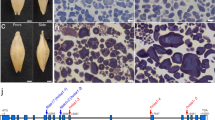
Mutations in the maize gene sugary2 (su2) affect starch structure and its resultant physiochemical properties in useful ways, although the gene has not been characterized previously at the molecular level. This study tested the hypothesis that su2 codes for starch synthase IIa (SSIIa). Two independent mutations of the su2 locus, su2-2279 and su2-5178, were identified in a Mutator-active maize population. The nucleotide sequence of the genomic locus that codes for SSIIa was compared between wild type plants and those homozygous for either novel mutation. Plants bearing su2-2279 invariably contained a Mutator transposon in exon 3 of the SSIIa gene, and su2-5178 mutants always contained a small retrotransposon-like insertion in exon 10. Six allelic su2 − mutations conditioned loss or reduction in abundance of the SSIIa protein detected by immunoblot. These data indicate that su2 codes for SSIIa and that deficiency in this isoform is ultimately responsible for the altered physiochemical properties of su2 − mutant starches. A specific starch synthase isoform among several identified in soluble endosperm extracts was absent in su2-2279 or su2-5178 mutants, indicating that SSIIa is active in the soluble phase during kernel development. The immediate structural effect of the su2 − mutations was shown to be increased abundance of short glucan chains in amylopectin and a proportional decrease in intermediate length chains, similar to the effects of SSII deficiency in other species.
Similar content being viewed by others
References
Ball, S. and Morell, M. 2003. From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 54: 207–233.
Beavis, W., Berlyn, M., Burr, B., Chandler, V., Coe, E., Fauron, C., Nelson, O., Polacco, M., Rodermel, S., Sachs, M. and Wetzel, C. 1995. A standard for maize genetics nomenclature. Maize Genet. Coop. Newsl. 69: 182–184.
Bennetzen, J.L. 1984. Transposable element Mu1 is found in multiple copies only in Robertson's Mutator maize lines. J. Mol. Appl. Genet. 2: 519–524.
Bradford, M.M. 1976. A rapid and sensitive method for the quanitation of microgram quanitities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248–254.
Campbell, M.R., White P.J. and Pollak J.M. 1994. Dosage effect at the Sugary-2 locus on maize starch structure and function. Cereal. Chem. 71: 464–468.
Cao, H., Imparl-Radosevich, J., Guan, H., Keeling, P.L., James, M.G. and Myers, A.M. 1999. Identification of the soluble starch synthase activities of maize endosperm. Plant Physiol. 120: 205–216.
Cao, H., James, M.G. and Myers, A.M. 2000. Purification and characterization of soluble starch synthases from maize endosperm. Arch. Biochem. Biophys. 373(1): 135–146.
Colleoni, C., Myers, A.M. and James, M.G. 2003. One-and two-dimensional native PAGE activity gel analyses of maize endosperm proteins reveal functional interactions between specific starch metabolizing enzymes. J. Appl. Glycosci. 50: 207–212.
Commuri, P.D. and Keeling, P.L. 2001. Chain-length specificities of maize starch synthase I enzyme: studies of glucan affinity and catalytic properties. Plant J. 25: 475–486.
Craig, J., Lloyd, J.R., Tomlinson, K., Barber, L., Edwards, A., Want, T.L., Martin, C., Hedley, C. and Smith, A.M. 1998. Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell 10: 413–426.
Creech, R.G. 1965. Genetic control of carbohydrate synthesis in maize endosperm. Genetics 52: 1175–1186.
Dellaporta, S. 1994. Plant DNA Miniprep and Microprep: Versions 2.1–2.3. In: V. Freeling MaW, (Ed.), The Maize Handbook, Springer-Verlag, New York, pp. 522–525.
Denyer, K., Sidebottom, C., Hylton, C.M. and Smith, A.M. 1993. Soluble isoforms of starch synthase and starchbranching enzyme also occur within starch granules in developing pea embryos. Plant J. 4: 191–198.
Dinges, J.R., Colleoni, C., James, M.G. and Myers, A.M. 2003. Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15: 666–680.
Dinges, J.R., Colleoni, C., Myers, A.M. and James M.G.: 2001. Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects. Plant Physiol. 125: 1406–1418.
Dry, I., Smith, A., Edwards, A., Bhattacharyya, M., Dunn, P. and Martin, C. 1992. Characterization of cDNAs encoding two isoforms of granule-bound starch synthase which show differential expression in developing storage organs of pea and potato. Plant J. 2: 193–202.
Edwards, A., Fulton, D.C., Hylton, C.M., Jobling, S.A., Gidley, M., Rossner, U., Martin, C. and Smith, A.M. 1999. A combined reduction in activity of starch synthases II and III of potato has novel effects on the starch of tubers. Plant J. 17: 251–261.
Edwards, A., Marshall, J., Sidebottom, C., Visser, R.G., Smith, A.M. and Martin, C. 1995. Biochemical and molecular characterization of a novel starch synthase from potato tubers. Plant J. 8: 283–294.
Eryster, W.H. 1934. Genetics of Zea mays. Bibliogr. Genet. 11: 187–392.
Fisher, D.K., Boyer, C.D. and Hannah, L.C. 1993. Starch branching enzyme II from maize endosperm. Plant Physiol. 102: 1045–1046.
Gao, M., Wanat, J., Stinard, P.S., James, M.G., and Myers, A.M. 1998. Characterization of dull1, a maize gene coding for a novel starch synthase. Plant Cell 10: 399–412.
Guan, H.P. and Preiss, J. 1993. Differentiation of the Properties of the Branching Isozymes from Maize (Zea mays). Plant Physiol. 102: 1269–1273.
Harn, C., Knight, M., Ramakrishnan, A., Guan, H., Keeling, P.L. and Wasserman, B.P. 1998. Isolation and characterization of the zSSIIa and zSSIIb starch synthase cDNA clones from maize endosperm. Plant Mol. Biol. 37: 639–649.
Imparl-Radosevich, J.M, Li, P., Zhang, L., McKean, A.L., Keeling, P.L. and Guan, H. 1998. Purification and characterization of maize starch synthase I and its truncated forms. Arch. Biochem. Biophys. 353: 64–72.
Imparl-Radosevich, J.M., Nichols, D.J., Li, P., McKean, A.L., Keeling, P.L. and Guan, H. 1999. Analysis of purified maize starch synthases IIa and IIb: SS isoforms can be distinguished based on their kinetic properties. Arch. Biochem. Biophys. 362: 131–138.
Inouchi, N., Glover, D.V., Sugimoto, Y. and Fuwa, H. 1984. Developmental changes in starch properties of several endosperm mutants of maize. Starch 36: 8.
Inouchi, N., Glover, D.V., Sugimoto, Y. and Fuwa, H. 1991. DSC characteristics of gelatinization of starches of single, double, and triple mutants and their normal counterpart in the inbred Oh43 maize (Zea mays L.) background. Starch/Staerke 43: 468–472.
James, M.G., Denyer, K. and Myers, A.M. 2003. Starch synthesis in the cereal endosperm. Curr. Opin. Plant Biol. 6: 215–222.
James, M.G., Robertson, D.S. and Myers, A.M. 1995. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7: 417–429.
Klösgen, R.B., Gierl, A., Schwarz-Sommer, Z. and Saedler, H. 1986. Molecular analysis of the waxy locus of Zea mays. Mol. Gen. Genet. 203: 237–244.
Knight, M.E., Harn, C., Lilley, C.E., Guan, H., Singletary, G.W., MuForster, C., Wasserman, B.P. and Keeling, P.L. 1988. Molecular cloning of starch synthase I from maize (W64) endosperm and expression in Escherichia coli. Plant J. 14: 613–622.
Kramer, H.H., Pfahler, P.L. and Whistler, R.J. 1958. Gene interaction in maize affecting endosperm properties. Agronomy J. 50: 207.
Li, J. and Corke, H. 1999. Physiochemical properties of maize starches expressing dull and sugary-2 mutants in different genetic backgrounds. J. Agric. Food Chem. 47: 4939–4943.
Li, J. and Glover, D.V. 1997. Characterization of thermal properties during starch gelatinization among ten endosperm mutants in maize. Acta. Agron. Sin. 23: 76–81.
Li, Z., Chu, X., Mouille, G., Yan, L., Kosar-Hashemi, B., Hey, S., Napier, J., Shewry, P., Clarke, B., Appels, R., Morell, M.K. and Rahman, S. 1999. The localization and expression of the class II starch synthases of wheat. Plant Physiol. 120: 1147–1156.
Li, Z., Sun, F., Xu, S., Chu, X., Mukai, Y., Yamamoto, M., Ali, S., Rampling, L., Kosar-Hashemi, B., Rahman, S. and Morell, M.K. 2003. The structural organisation of the gene encoding class II starch synthase of wheat and barley and the evolution of the genes encoding starch synthases in plants. Funct. Integr. Genomics 3: 76–85.
Lloyd, J.R., Landschutze, V. and Kossmann, J. 1999. Simultaneous antisense inhibition of two starch-synthase isoforms in potato tubers leads to accumulation of grossly modified amylopectin. Biochem. J. 338: 515–521.
Morell, M.K., Kosar-Hashemi, B., Cmiel, M., Samuel, M.S., Chandler, P., Rahman, S., Buleon, A., Batey, I.L. and Li, Z. 2003. Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J. 34: 173–185.
Mu-Forster, C., Huang, R., Powers, J.R., Harriman, R.W., Knight, M., Singletary, G.W., Keeling, P.L. and Wasserman, B.P. 1996. Physical association of starch biosynthetic enzymes with starch granules of maize endosperm. Granule-associated forms of starch synthase I and starch branching enzyme II. Plant Physiol. 111: 821–829.
Neuffer, M.G., Coe, E.H. and Wessler, S.R. 1997. Mutants of Maize. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
O'shea, M.G., Samuel, M.S., Konik, C.M. and Morell, M.K. 1998. Fluorophore-assisted carbohydrate electrophoresis (FACE) of oligosaccharides: efficiency of labelling and high-resolution separation. Carbohydr. Res. 307: 1–12.
Pfahler, P.L., Kramer, H.H. and Whistler, R.J. 1957. Effect of genes on birefringence end-point temperature of starch grains in maize. Science 125: 441.
Robertson, D.S. 1967. A new opaque gene located on chromosome 7. Maize Genet. Coop. Newsl. 41: 94–95.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1984. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Sandstedt, R.M., Strahan, D., Ueda, S. and Abbot, R.C. 1962. The digestibility of high-amylose corn starches compared to that of other starches: the apparent effect of the ae gene on the susceptibility to amylase action. Cereal Chem. 39: 123.
Scanlon, M.J., Stinard, P.S., James, M.G., Myers, A.M. and Robertson, D.S. 1994. Genetic analysis of 63 mutations affecting maize kernel development isolated from Mutator stocks. Genetics 136: 281–294.
Shannon, J.C. and Garwood, D.L. 1984. Genetics and physiology of starch development. In: R.L. Whistler, J.N. Bemiller, E.F. Paschall (Eds.), Starch: Chemistry and Technology, Academic Press, Orlando, FL, pp. 25–86.
Shure, M., Wessler, S. and Federoff, N. 1983. Molecular identification and isolation of the Waxy locus in maize. Cell 35: 225–233.
Stinard, P.S., Robertson, D.S. and Schnable, P.S. 1993. Genetic isolation, cloning, and analysis of a Mutator-induced, dominant antimorph of the maize amylose extender1 locus. Plant Cell 5: 1555–1566.
Takeda, Y. and Preiss, J. 1993. Structures of B90 (sugary) and W64A (normal) maize starches. Carbohydr Res. 240: 265–275.
Umemoto, T., Yano, M., Satoh, H., Shomura, A. and Nakamura, Y. 2002. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 104: 1–8.
White, P., Abbas, I., Pollak, L. and Johnson, L. 1990. Intraand interpopulation variablility of thermal properties of maize starch. Cereal Chem. 67: 70–73.
Yamamori, M., Fujita, S., Hayakawa, K., Matsuki, J. and Yasui, Y. 2000. Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor. Appl. Genet. 101: 21–29.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, X., Colleoni, C., Ratushna, V. et al. Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol Biol 54, 865–879 (2004). https://doi.org/10.1007/s11103-004-0312-1
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-0312-1