Abstract
Background
Rice blast disease is a major restriction in rice production. That is usually managed using chemical pesticides, which are expensive in terms of cost and environment hazards. Use of blast-resistance genes to develop resistant varieties may therefore be a more economical and environmentally friendly method for effective control.
Results
In this study, we improved the blast resistance of four sterile lines, Y58S, GuangZhan63S (GZ63), C815S and HD9802S, by introgression of 9 cloned broad-spectrum blast resistance genes Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, Pikm and Pb1. Through molecular marker-assisted selection and backcross breeding, 31 single-gene derived lines and 20 double-gene combination lines were obtained. When infected naturally, single-gene lines with Pigm or Pid3 showed significantly enhanced resistance during whole growth period relative to their recurrent parent. Single-gene lines with Pi37, Pi5, Pit, Pi36, Pi54 or Pikm showed significantly enhanced resistance in some of the four backgrounds. No obviously enhanced resistance was observed in single-gene line with Pb1 for the whole growth period. Compared with recurrent parents, most of the double-gene lines showed improved resistance. Among these double-gene lines, lines with Pi37/Pid3, Pi5/Pi54, Pi54/Pid3 or Pigm/Pi37, exhibited significantly enhanced resistance and observable additive effects.
Conclusions
Two blast resistance genes, Pigm and Pid3, showed significantly enhanced resistance for the whole rice growth period, and six blast resistance genes Pi37, Pi5, Pit, Pi36, Pi54 or Pikm showed significantly enhanced resistance for some of the four backgrounds. Double-gene lines with Pi37/Pid3, Pi5/Pi54, Pi54/Pid3 and Pigm/Pi37 exhibited significantly enhanced resistance and observable additive effects. These lines could be used in rice hybrid and production.
Similar content being viewed by others
Introduction
Rice (Oryza sativa) is a staple food crop for more than 50% of the world’s population. Rice blast disease is a major restriction on rice production in both tropical and temperate countries, and it is also a major obstacle to hybrid rice production in China due to the relatively narrow genetic base of hybrid rice and the increased use of nitrogen fertilizer (Liu et al. 2010a). The average blast infected area was more than 3.8 million hectares in 1982–1985, with yield losses of several million tons (Sun et al. 1999). In 1993, a yield loss of 1.1 million tons was recorded in Southern China alone. Conventional methods of controlling blast depended on fungicides, which generate additional costs in rice production and chemical contamination of the environment and food. The development and use of resistant varieties with the major resistance genes is therefore one of the most economical and effective ways to control this disease (Koide et al. 2009; Deng et al. 2017).
To date, over 100 blast resistant genes or quantitative trait loci (QTL) have been identified (Su et al. 2015; Vasudevan et al. 2016; Zheng et al. 2016; Xiao et al. 2017). Among them, 35 genes have been cloned (Wang et al. 2017). Many of these resistance genes are clustered on rice chromosomes 6, 11 and 12. Notably, at least 11 resistance genes—including Pi2, Pi9, Piz, Pizt, Pigm, Pi22, Pi25, Pi26, Pi40, Pi42 and Pi50—are concentrated in the short-arm region near the centromere of chromosome 6. Blast resistance gene Pi37 was mapped from rice cultivar St. No. 1, which encoded a nucleotide-binding site leucine-rich repeat (NBS-LRR) protein on rice chromosome 1 (Chen et al. 2005; Lin et al. 2007). The Pit gene was originally identified in cultivar K59 (Hayashi and Yoshida 2009); it was a member of the NBS-LRR family of R genes. The Pid3 gene was identified by genome-wide comparison of paired NBS-LRR genes and their pseudogene alleles between the two sequenced rice genomes 9311 and Nipponbare, and an allelic Pid3 in Digu was identified on chromosome 6 (Shang et al. 2009). The broad-spectrum resistance gene Pigm was identified from the native Chinese variety Gumei 4. PigmR confers broad-spectrum resistance, whereas PigmS competitively attenuates PigmR homodimerization to suppress resistance. The expression of PigmS that triggered PigmR-mediated resistance is subjected to tight epigenetic regulation (Deng et al. 2009; Deng et al. 2017). Pi36 identified in an indica cultivar, Q61, was mapped on chromosome 8 and encoded as an NBS-LRR protein (Liu et al. 2005; Liu et al. 2010b). The Pi5 gene was identified from RIL260, and the resistance of Pi5 to Magnaporthe oryzae requires the presence of the two coiled-coil NBS-LRR genes Pi5–1 and Pi5–2 (Lee et al. 2009). Pi54 was originally identified from the rice variety Tetep and was mapped on chromosome 11 with two tightly linked simple sequence repeat (SSR) markers TRS26, TRS33 and a functional marker Pi54-MAS (Sharma et al. 2005; Ramkumar et al. 2011). Pi54 was ∼2.5 Mb away from the Pik locus on rice chromosome 11 (Sharma et al. 2010). It had been reported that the Pik locus was actually a cluster of genes including Pikm, Pik-h and Pik-p presenting on rice chromosome 11 (Ashikawa et al. 2008; Zhai et al. 2014; Yuan et al. 2011). Pikm-specific rice blast resistance is conferred by a combination of two genes, Pikm1-TS and Pikm2-TS, with an NBS-LRR (Ashikawa et al. 2010). The Pb1 gene derived from an indica cultivar, Modan, and is characterized by durability of resistance and adult/panicle blast resistance (Hayashi et al. 2010; Table 1).
Marker-assisted selection (MAS) is a highly efficient breeding approach that could offer an opportunity to select the targeted gene rapidly and precisely (Tanksley et al. 1989). It is a promising method to provide broad-spectrum and durable rice blast resistance through gene or QTL pyramiding (Tabien et al. 2002). Recently, the development of near-isogenic lines or the pyramiding of different resistance genes has been applied in blast resistance breeding programs by marker-assisted selection. Three blast resistance genes (Pi1, Pi2 and D12) were introduced into rice variety Jin23B (Jiang et al. 2012), and Pi9, Pizt and Pi54 were recently introduced into rice variety 07GY31 by marker assisted backcross breeding (Xiao et al. 2017). Evaluation of blast resistance suggested that single or polygene pyramid lines showed significantly enhanced resistance relative to control.
Y58S, GZ63S, C815S and HD9802S are elite rice varieties in rice production in China, but these four varieties and their derived hybrids are highly susceptible to blast. Improvement of blast resistance in Y58S, GZ63S, C815S and HD9802S is therefore critical in utilizing the hybrids in rice production in China.
In this study, 9 cloned blast resistance genes, Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, Pikm and Pb1, were introgressed into male sterile lines including Y58S, GZ63S, C815S and HD9802S. Our objective was to evaluate the natural resistance performance of these cloned blast resistance genes and to improve hybrid rice blast resistance in production.
Materials and methods
Plant materials
Nine rice varieties, Q1333 (Pi37), K59 (Pit), Digu (Pid3), Gumei4 (Pigm), Q61 (Pi36), RIL260 (Pi5), Tsuyuake (Pikm), Tetep (Pi54) and Modan (Pb1), were used as donors of the cloned resistance genes (Table 1). Four male sterile rice lines (Y58S, GZ63S, C815S and HD9802S), which are the main female parent of indica hybrid rice in southern China, were used as the recurrent parents. The F1, BC1F1, BC2F1 and BC3F1 lines were derived from crosses between the recurrent parents and the donor parents. The BC1F2 and BC2F2 populations were developed from the BC1F1 and BC2F1 resistant individuals. These populations were used for genetic and phenotypic analysis. The susceptible variety CO39 was used as a negative control.
DNA extraction and genotyping
For MAS during each generation, DNA was isolated from the leaf tissues of the parent, BCnF1, BCnF2 and F2 plants using the CTAB method. In the MAS system, Pit was detected using SSR marker RM10125 and InDel marker RMLTJ-3; Pi37 was detected using SSR marker RM11726 and InDel marker RMLJ-1; and Pigm was detected using InDel marker Pi2–4 and SSR markers HC26 and HC3. The flanking InDel marker RML3J-1 and SSR marker RM19951 were used to detected Pid3; the SSR marker RM22385 and InDel marker RMLJ-2 were used to detected Pi36; and two SSR markers, RM24019 and RM24034, and one InDel marker, RMLJ-7, were used to detected Pi5. Three tightly linked SSR markers RM27150, RM27181 and RM27189 were used to confirm gene Pi54. Two tightly linked SSR markers RM26998 and RM26964 were used to confirm Pb1. SSR marker RM224 and InDel marker RMLMJ-1 were used to confirm Pikm (Additional file 1: Table S1). The InDel markers RMLTJ-3, RMLJ-1, Pi2–4, RML3J-1, RMLJ-2, RMLJ-7 and RMLMJ-1 were designed based on sequence alignments of the two genome references of Nipponbare and 93–11 (Additional file 1: Table S1). The SSR analysis was carried out essentially according to the procedures described by Wu and Tanksley (1993).
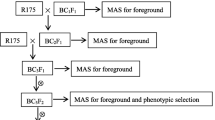
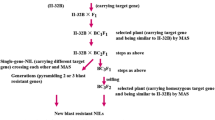
Crossing and selection scheme
Q1333 (Pi37), K59 (Pit), Digu (Pid3), Gumei4 (Pigm), Q61 (Pi36), RIL260 (Pi5), Tsuyuake (Pikm), Tetep (Pi54) and Modan (Pb1) were crossed separately with Y58S, GZ63S, C815S and HD9802S, and their F1 hybrids were backcrossed with Y58S, GZ63S, C815S and HD9802S to obtain the BC1F1 populations (Fig. 1). Markers closely linked with the blast resistance genes were used to check the target genes among the above BC1F1 populations (Fig. 1; Fig. 2a). Twenty plants with the target genes from each BC1F1 population were selected to backcross with the corresponding parents up to BC3F1. From each generation, plants carrying single gene Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, Pikm and Pb1 in the background of Y58S, GZ63S, C815S and HD9802S were obtained. After selfing, the BC1F2 and BC2F2 populations were obtained, which were then used to evaluate the effects of individual genes in different backgrounds. In the BC3F1 populations, two genes in the same background were crossed with each other, and then the F1 hybrids and the corresponding F2 population were obtained (Fig. 1).
Identification of improved blast resistant lines with MAS. a PCR amplification of the marker LJ3–1 for blast resistance gene Pid3 in the BC2F2 population with the background of GZ63S. Lines 1, 2 and 3 are Digu (resistance homozygous), GZ63S (infected homozygous) and heterozygous (Digu/GZ63S), respectively. b Red arrows indicate susceptible parents GZ63S, yellow arrows show the resistant lines carrying target gene Pid3
Scoring rice blast
The BC1F2, BC2F2 and F2 families of the blast resistance genes Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, Pikm and Pb1 in the background of Y58S, GZ63S, C815S and HD9802S were planted in a randomized complete block design in 2014, 2015 and 2016 in Xianfeng, Hubei Province, China. Xianfeng is a mountainous area at an altitude of 600 m, with an average temperature of approximately 25 °C, showing high humidity and heavy fog annually. The tests were performed in three replications. In each replication, each of the plots consisted of 8 rows with 12 plants per row at a planting density of 16 cm between plants and 26.5 cm between rows. To adequately induce blast disease infection, the diseased straws collected the previous year were sown evenly in each plot and the highly susceptible variety, CO39, was planted at both sides of each row and around the population. Field management essentially followed normal agricultural practices with the exception of not using bactericides.
All the plants were scored for leaf blast at the tillering stage and were recorded for neck blast severity at maturity. The most seriously infected leaf among the top two or three new leaves was scored for each plant at the tillering stage, as determined using the 0–9 scale rating system from IRRI (2002). Neck blast severity was recorded as a percentage of the infection on the neck of the rice panicle at physiological maturity. The number of panicles showing symptoms of neck blast was expressed as percent infection.
Statistical analysis
The data obtained from the experiments were statistically analyzed using analysis of variance (ANOVA) of respective experimental designs. Phenotypic and genotypic data were collected for each individual plant in the BC1F2 and BC2F2 populations. The additive effect (A), dominant effect (D) and phenotypic variation explained (PVE) of the resistance genes were analyzed in a segregated population between different genotypes at P = 0.05 and 0.01 significance. The analyses of these statistical parameters were carried out using the statistical software SPSS 20.0.
Results
Improved blast resistance materials obtained through MAS
Seven blast resistance genes, Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, were introgressed into Y58S, GZ63S, C815S and HD9802S separately following a recurrent backcrossing procedure, combined with MAS as described in Fig. 1. In addition, Pikm for blast resistance was introgressed into C815S and HD9802S, and Pb1 for blast resistance was introgressed into GZ63S, using the marker-assisted backcross breeding method (Fig. 1). Thirty-one BC1F2, BC2F2 and BC3F1 lines with single resistance genes were obtained.
The improved lines containing a single resistance gene in BC3F1 progenies were intercrossed with each other to pyramid two resistance genes. The F1 plants containing two resistance genes were selected through the linked DNA markers of each target gene. Two gene pyramiding lines were also obtained, including Y58S (Pi37/Pi54), Y58S (Pi5/Pi37), Y58S (Pi5/Pigm), Y58S (Pigm/Pi54), Y58S (Pi37/Pid3), Y58S (Pi37/Pigm) in Y58S background, GZ63S (Pit/Pigm), GZ63S (Pit/Pb1), GZ63S (Pi5/Pit), GZ63S (Pi5/Pi37), GZ63S (Pi36/Pb1), GZ63S (Pi5/Pigm), GZ63S (Pi36/Pid3), GZ63S (Pb1/Pigm), GZ63S (Pi5/Pi54), GZ63S (Pid3/Pi54) in GZ63S background, C815S (Pit/Pigm), C815S (Pi5/Pit), C815S (Pi5/Pigm) and C815S (Pi37/Pigm) in C815S background (Fig. 1).
Blast resistance of the donor parents and recurrent parents
Four recurrent parents and nine donor parents were planted in Xianfeng in 2014, 2015 and 2016. Each variety was planted in three replications with 12 plants per plot. The resistance scores for leaf blast for Y58S were 6.99, 6.57 and 6.53, and the percentages of infection for neck blast were 98.71%, 95.28% and 100% in 2014, 2015 and 2016, respectively. The resistance scores for leaf blast for GZ63 were 7.42, 7.75 and 7.02 and the percentages of infection for neck blast were 98.83%, 100% and 100% in 2014, 2015 and 2016, respectively. At the disease nursery of Xianfeng, the resistance scores for leaf blast for C815S and HD9802S were 6.90, 6.38 and 6.98 and 6.50, 6.68 and 6.45, and the percentages of infection for neck blast were 99.08%, 93.15% and 100% and 98.17%, 100% and 100%, in 2014, 2015 and 2016, respectively. Four recurrent parents, Y58S, GZ63S, C815S and HD9802S, showed medium or high susceptibility to leaf blast at the tillering stage and high susceptibility to neck blast at maturation in Xianfeng in 2014, 2015 and 2016 (Table 2). These results illustrated a serious loss of blast resistance in the main hybrid rice, and it is imperative to improve the blast resistance in hybrid rice in China.
The resistance scores for leaf blast at the tillering stage and the percentage of infection for neck blast at maturation were 5.70 and 97.93% for K59; 4.40 and 98.85% for Tsuyuake; 4.60 and 98.93% for Modan; 5.70 and 97.10% for RIL260; 8.50 and 100.00% for Q61; 3.80 and 21.11% for Q1333; 2.98 and 3.00% for Gumei4; 3.10 and 3.04% for Digu; 3.02 and 2.05% for Tetep, respectively, in 2014 (Table 2). The resistance scores for leaf blast at tillering stage for these nigh varieties were 2.33, 2.67, 3.30, 1.75, 7.67, 3.01, 1.33, 3.08 and 1.00, respectively, in 2015, and 3.92, 4.08, 3.17, 4.83, 6.75, 1.99, 1.92, 1.17 and 2.02, respectively, in 2016 (Table 2). The percentages of infection for neck blast at maturity stage for the nine variety were 100.00%, 100.00%, 100.00%, 79.08%, 40.17%, 35.95%, 36.99%, 7.97% and 1.00%, respectively in 2015 and 100.00%, 100.00%, 100.00%, 78.06%, 62.03%, 5.99%, 5.04%, 7.97% and 1.00%, respectively in 2016 (Table 2). These results showed that Gumei4, Digu, Tetep and Q1333 had high resistance to leaf blast and neck blast during these 3 years. K59, Tsuyuake, Modan and RIL260 had high resistance to leaf blast in 2015 and medium resistance to leaf blast in 2014 and 2016, but were susceptible to neck blast for all 3 years. The resistance scores of Q61 to leaf blast and percentage of infection for neck blast showed that the variety was susceptible to both leaf blast and neck blast.
Blast resistance of the genes in different backgrounds
Seven blast resistance genes (Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54) were introduced into four recurrent parents Y58S, GZ63S, C815S and HD9802S, one blast resistance gene (Pikm) was introduced into C815S and HD9802S, and one blast resistance gene (Pb1) was introduced into GZ63S. The resistance to leaf blast and neck blast of single gene introduced lines was tested in 2014 and 2015 in Xianfeng. Pid3 showed greater resistance to leaf blast and neck blast in the background of Y58S, GZ63S, C815S and HD9802S than the control in 2014 and 2015 (Table 3; Fig. 2). Pigm showed significantly enhanced resistance to leaf blast in the background of Y58S, C815S and HD9802S in 2015, and it showed significantly enhanced resistance to neck blast in the background of Y58S, GZ63S, C815S and HD9802S in 2014 and 2015 (Table 3). Pi5 showed significantly enhanced resistance to leaf blast in the background of C815S in 2014 and in the background of GZ63S and HD9802S in 2015 and significantly enhanced resistance to neck blast in the background of GZ63S in 2015 (Table 3). Pi37 showed significantly enhanced resistance to leaf blast in the background of GZ63S in 2014 and in the background of Y58S and C815S in 2015 and significantly enhanced resistance to neck blast in the background of Y58S and GZ63S in 2015. Pi54 showed significantly enhanced resistance to leaf and neck blast in the background of Y58S, GZ63S and HD9802S in 2015. Except for Pb1, all of the blast resistance genes showed enhanced resistance to leaf or neck blast in at least one background from a recurrent parent. However, the blast resistance of Pi5, Pi36 and Pit was not stable in most recurrent parents (Table 3).
Blast resistance of two gene pyramiding lines
Through MAS, 6, 10 and 4 lines with two-gene pyramiding in Y58S, GZ63S and C815S backgrounds were obtained. The blast resistance of two-gene pyramiding lines was scored for leaf blast at the tillering stage and neck blast at maturation in 2016. The resistance scores for leaf blast were 3.56 for Y58S (Pi37/Pi54), 4.00 for Y58S (Pi5/Pi37), 1.75 for Y58S (Pi5/Pigm), 2.00 for Y58S (Pigm/Pi54), 1.60 for Y58S (Pi37/Pid3), and 2.50 for Y58S (Pi37/Pigm), while the percentage of infection for neck blast was 3.88%, 32.34%, 2.08%, 10.00%, 6.80% and 3.05%, respectively (Fig. 3). At the tillering stage, the resistance scores for leaf blast for pyramiding lines GZ63S (Pit/Pigm), GZ63S (Pit/Pb1), GZ63S (Pi5/Pit), GZ63S (Pi5/Pi37), GZ63S (Pi36/Pb1), GZ63S (Pi5/Pigm), GZ63S (Pi36/Pid3), GZ63S (Pb1/Pigm), GZ63S (Pi5/Pi54), GZ63S (Pid3/Pi54) were 5.42, 5.84, 6.00, 4.58, 6.33, 6.00, 5.18, 5.50, 2.80 and 2.25, respectively. At maturation, the percentage of infection for neck blast was 55.02%, 50.29%, 47.30%, 11.40%, 23.83%, 15.20%, 16.69%, 16.34%, 12.67% and 0%, respectively for the 10 pyramiding lines in GZ63S background (Fig. 3). The resistance scores for the two-gene pyramiding lines C815S (Pit/Pigm), C815S (Pi5/Pit), C815S (Pi5/Pigm), C815S (Pi37/Pigm) were 3.50, 4.50, 3.62 and 2.29, respectively, for leaf blast and 4.76%, 22.50%, 22.50% and 0%, respectively, for neck blast (Fig. 3). By contrast, the resistance scores of the recurrent parents Y58S, GZ63S and C815S were 6.53, 7.02 and 6.98, respectively, for leaf blast and the percentage of infection for neck blast was 100.00%, 100.00% and 100.00%, respectively. These results indicate that the pyramiding lines were more strongly resistant to blast than the control.
Blast resistance scores of homozygous plants of pyramiding populations under different backgrounds. Shown are the average values for leaf blast (LB) in different pyramiding populations in the backgrounds Y58S (a) GZ63S (c) and C815S (e) and the average value of neck blast (NB) of different pyramiding populations in the backgrounds Y58S (b), GZ63S (d) and C815S (f)
In the background of Y58S, the leaf blast sores for Pi37, Pid3 and Pi37/Pid3 were 3.90, 1.83 and 1.60, respectively, which explained the phenotype variation of 13.89%, 11.39% and 24.46%, respectively. The percentage of infection for neck blast for Pi37, Pid3 and Pi37/Pid3 was 12.98%, 5.15% and 3.05%, respectively, which explained the phenotype variation of 11.91%, 16.79% and 32.38% (Table 4; Fig. 4). In the background of Y58S, the two-gene pyramiding line Pi37/Pid3 showed enhanced resistance to blast compared with single gene lines for leaf and neck blast. In the background of GZ63S, the resistance to leaf blast of the two-gene pyramiding lines of Pi5/Pi54 and Pid3/Pi54 were 2.80 and 2.25, while the resistance to neck blast was 12.67% and 0%, which showed significant difference from the single gene lines (Table 4; Fig. 3; Fig. 4). In the background of C815S, the leaf and neck blast of two-gene pyramiding line Pi37/Pigm were 2.29 and 0%, which showed significant difference from the single gene lines (Table 4; Fig. 3). From the results above, all of the pyramiding lines showed greater resistance to blast than the single gene lines, especially for neck blast.
Phonotypes of improved lines and controls for leaf blast under natural infection conditions. a, b Phenotype of recurrent parent Y58S for leaf blast under natural infection conditions. Phenotypes of improved plants carrying resistance gene under Y58S background to leaf blast under natural infection condition: c, d Y58S (Pi54); e, f Y58S (Pid3); g, h Y58S (Pi54 + Pid3). i, j phenotype of recurrent parent GZ63S to leaf blast under natural infection condition. Phenotype of improved plants carrying resistance gene under GZ63S background to leaf blast under natural infection condition; k, l GZ63S (Pi54); m, n GZ63S (Pi5); o, p GZ63S (Pi54 + Pid3). q, r phenotype of recurrent parent C815SS to leaf blast under natural infection condition. Phenotype of improved plants carrying resistance gene under C815SS background to leaf blast under natural infection condition. s, t C815SS (Pigm); u, v C815SS (Pi37); w, x C815SS (Pigm + Pi37)
Discussion
Since the 1980s, several blast resistance genes have been identified and transferred into elite susceptible varieties, producing a series of improved cultivars with blast resistance. In recent years, MAS has been employed for transferring blast resistance genes to new varieties. Among the 35 cloned genes, the breeding application of blast genes Pi1 and Pi2 has often been reported (Jiang et al. 2012; Liu et al. 2012; Jiang et al. 2015; Ni et al. 2015; Tian et al. 2016), but reports of other cloned blast genes in rice were rare. Here, we introduced 9 cloned blast resistance genes-Pi37, Pit, Pid3, Pigm, Pi36, Pi5, Pi54, Pikm and Pb1-into 4 rice varieties and evaluated their applications in breeding.
In our study, the blast resistance of 9 donor parents was evaluated under natural infection conditions in 2014, 2015 and 2016. Four donor parents—Gumei4 (Pigm), Digu (Pid3), Tetep (Pi54) and Q1333 (Pi37)—have high resistance to leaf and neck blast, four donor parents K59 (Pit), Tsuyuake (Pikm), Modan (Pb1) and RIL260 (Pi5) have medium or high resistance to leaf blast and susceptible to neck blast, while Q61 (Pi36) was susceptible to leaf and neck blast. From the results, we can see that the resistance of donor parents could differ even under the same infection conditions.
We used BC1F2 and BC2F2 populations to evaluate the effects of individual genes in different backgrounds. Additive and dominant effects were used to evaluate gene effects in F2 population. Gene effects differed in the four rice backgrounds. The gene Pigm and Pid3 showed significantly enhanced resistance to blast in all four rice backgrounds, but the gene Pb1 showed no significant difference in rice blast resistance than the control. The same gene might also perform differently in different years in the same background. The blast resistance of gene Pi54 was better in 2015 than in 2014 in the background of Y58S. The reason for this is mainly due to the different gene effects influenced by different climate or physiological factors.
From the blast resistance performance of the 9 genes in 3 years in Xianfeng, we can see that Pid3 and Pigm have better resistance than the other genes, so these two genes are valuable for application in the Wuling mountain area. Pigm have a broad-spectrum and durable resistance to rice blast that confers durable resistance to the fungus Magnaporthe oryzae without yield penalty through epigenetic regulation of paired antagonistic NBS-LRR (Deng et al. 2017). The genes Pi54, Pi37 and Pi5 should be used according to background. Genes such as Pb1 and Pit have little resistance to blast, mainly because the fungus Magnaporthe oryzae in Xianfeng can overcome them, so these genes are not suitable for the Xianfeng area, although they may be suitable in other areas.
Two blast resistance genes Pi54 and Pikm on rice chromosome 11 were used to improve blast resistance of the sterile lines through MAS with different markers with the distance of ∼2.5 Mb. Genes Pikm, Pik-h and Pik-p were mapped on the Pik locus, and these genes comprised a pair of NBS-LRR genes, but gene Pi54 encodes one NBS-LRR protein. The genes Pi54, Pikm, Pik-h and Pik-p were clone from rice variety Tetep, Tsuyuauke, K3 and K60, respectively. Donor parents Tetep and Tsuyuauke were high or medium resistant to rice blast in three years, and K3 and K60 may have a potentially value in rice blast resistance breeding.
Broad spectrum and durable resistance are the major objectives of rice blast resistance breeding. Most efforts in breeding for blast resistance have been directed towards incorporating single genes. Rice varieties containing only one major resistance gene have a tendency to break down as unpredictable changes occur in the composition of pathogen populations, so pyramiding more blast resistance genes in a rice cultivar may solve this problem (Ahn and Ou 1982; Kiyosawa 1982). Gene pyramiding can overlap different resistance genes, which seems promisingly to provide broad spectrum and durable resistance (Tabien et al. 2002). In this study, we developed lines containing two blast resistance genes Y58S (Pi37/Pid3), GZ63S (Pi5/Pi54), GZ63S (Pi54/Pid3) and C815S (Pigm/Pid37) that were highly resistant to leaf and neck blast under natural infection conditions, and the phenotypes showed that the effect on neck blast was better than on leaf blast in the Y58S, GZ63S and C815S backgrounds.
Most rice varieties lose their resistance after a few years planted in the same area because many varieties of single resistance genes must cope with new M. oryzae races. Studies have indicated that the genetic control of blast resistance is complex and involves both major and minor resistance genes with complementary or additive effects, as well as environmental interactions. Mapping new blast resistance genes and developing durable resistance varieties are, therefore, of high value. To pursue durable resistance to blast, we should exploit genetic diversity, which is an ecological approach to disease control that can be highly effective over a large area and contribute to the sustainability of crop production (Zhu et al. 2000). In this study, we introduced 9 cloned blast resistance genes into 4 recurrent parents. The effects of the genes on blast resistance were evaluated in the natural environment, and the results provide an important theoretical basis for the utilization of these rice blast resistance genes in China.
References
Ahn SW, Ou SH (1982) Quantitative resistance of rice to blast disease. Phytopathology 72:279–282
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu JZ, Matsumoto T, Ono K, Yano M (2008) Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180(4):2267
Ashikawa I, Wu JZ, Matsumoto T, Ishikawa R (2010) Haplotype diversity and molecular evolution of the rice Pikm locus for blast resistance. J Gen Plant Pathol 76(1):37–42
Chen S, Wang L, Que ZQ, Pan RQ, Pan QH (2005) Genetic and physical mapping of Pi37(t), a new gene conferring resistance to rice blast in the famous cultivar St. no. 1. Theor Appl Genet 111(8):1563–1570
Deng YW, Zhai KR, Xie Z, Yang DY, Zhu XD, Liu JZ, Wang X, Qin P, Yang YZ, Zhang GM, Li Q, Zhang JF, Wu SQ, Milazzo J, Mao BZ, Wang E, Xie H, Tharreau D, He ZH (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355(6328):962
Deng YW, Zhu XD, Xu J, Chen HQ, He ZH (2009) Map-based cloning and breeding application of a broad-spectrum resistance gene Pigm to rice blast. In: Wang X, Valent B (eds) Advances in genetics, genomics and control of Rice blast Disease. Springer, New York
Hayashi K, Yoshida H (2009) Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J 57(3):413–425
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takasuji H (2010) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J 64(3):498–510
IRRI (2002) Standard Evaluation System for Rice. IRRI, Manila
Jiang HC, Feng YT, Bao L, Li X, Gao GJ, Zhang QL, Xiao JH, Xu CG, He YQ (2012) Improving blast resistance of Jin 23B and its hybrid rice by marker-assisted gene pyramiding. Mol Breed 30:1679–1688
Jiang JF, Mou TM, Yu HH, Zhou FS (2015) Molecular breeding of thermo-sensitive genic male sterile (TGMS) lines of rice for blast resistance using Pi2 gene. Rice 8(1):11
Kiyosawa S (1982) Genetics and epidemical modeling of breakdown of plant disease resistance. Annu Rev Phytopathol 20:93–117
Koide Y, Kobayashi N, Xu DH, Fukuta Y (2009) Resistance genes and selection DNA markers for blast disease in rice (Oryza sativa L.). JARQ-Jap Agr Res Q 43:255–280
Lee SK, Song MY, Seo YS, Kim HK, Ko S, Cao PJ, Suh JP, Yi G, Roh JH, Lee S, An G, Hahn TR, Wang GL, Ronald P, Jeon JS (2009) Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 181(4):1627
Lin F, Chen S, Que ZQ, Wang L, Liu XQ, Pan QH (2007) The blast resistance gene Pi37 encodes a nucleotide binding site leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177(3):1871
Liu JL, Wang XJ, Mitchell T, Hu YJ, Liu XL, Dai LY, Wang GL (2010a) Recent progress and understanding of the molecular mechanisms of the rice and Magnaporthe oryzae interaction. Mol Plant Pathol 11:419–427
Liu WG, Wang F, Liu ZR, Zhu XY, Li JH, Huang HJ, Liao YL, Zhu MS, Fu CY, Chen JW (2012) Improvement of rice blast resistance in CMS line Rongfeng a by pyramiding Pi-1 and Pi-2 with molecular marker techniques. Mol Plant Breed 10(5):575–582
Liu XQ, Ling W, Liu XD, Liu XQ, Wang DB, Wang CT, Lin F, Pang QH (2010b) The molecular evolution of the rice blast resistance gene Pi36. Int J Plant Sci 171(3):235–243
Liu XQ, Wang L, Chen S, Lin F, Pan QH (2005) Genetic and physical mapping of Pi36(t), a novel rice blast resistance gene located on rice chromosome 8. Mol Gen Genomics 274(4):394–401
Ni DH, Song FS, Ni JL, Zhang AF, Wang CL, Zhao KJ, Yang YC, Wei PC, Yang JB, Li L (2015) Marker-assisted selection of two-line hybrid rice for disease resistance to rice blast and bacterial blight. Field Crops Res 184:1–8
Ramkumar G, Srinivasarao K, Mohan KM, Sudarshan I, Sivaranjani AKP, Gopalakrishna K, Neeraja CN, Balachandran SM, Sundaram RM, Prasad MS, Rani NS, Prasad AMR, Viraktamath BC, Madhav MS (2011) Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pik h). Mol Breed 27(1):129–135
Shang JJ, Tao Y, Chen XW, Zou Y, Lei CL, Wang J, Li XB, Zhao XF, Zhang MJ, Lu ZK, Xu JC, Cheng ZK, Wan JM, Zhu LH (2009) Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site-leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182(4):1303–1311
Sharma TR, Rai AK, Gupta SK, Singh NK (2010) Broad-spectrum blast resistance gene Pi-k h cloned from rice line Tetep designated Pi54. J Plant Biochem Biotechnol 19(1):87–89
Sharma TR, Shanker P, Singh BK, Jana TK, Madhav MS, Gaikwad K, Singh NK, Plaha P, Rathour R (2005) Molecular mapping of rice blast resistance gene Pi-k h in the rice variety Tetep. J Plant Biochem Biotechnol 14(2):127–133
Su J, Wang WJ, Han JL, Chen S, Wang CY, Zeng LX, Feng AQ, Yang JY, Zhou B, Zhu XY (2015) Functional divergence of duplicated genes results in a novel blast resistance gene Pi50 at the Pi2/9 locus. Theor Appl Genet 128(11):2213–2225
Sun GC, Cai RY, Du XF, Tao RX, Sun SY (1999) The directional selection and stabilizing selection from the interaction between rice variety and blast fungus Magnaporthe grisea. Acta Phytopathol Sinica 29:45–49
Tabien RE, Li Z, Paterson AH, Marchetti MA, Stansel JW, Pinson SRM (2002) Mapping QTLs for field resistance to the rice blast pathogen and evaluating their individual and combined utility in improved varieties. Theor Appl Genet 105:313–324
Tanksley SD, Young ND, Paterson AH, Bonierbale MW (1989) RFLP mapping in plant breeding: new tools for old science. Biotechnol 7:257–264
Tian HG, Chen HQ, Hu J, Zhu XD, Qian Q (2016) Transmitted three genes by marker-assisted selection to improve rice blast resistance of Kongyu131. J Nucl Agric Biol 30(11):2096–2103
Vasudevan K, Gruissem W, Bhullar NK (2016) Corrigendum: identification of novel alleles of the rice blast resistance gene Pi54. Sci Rep 6(15678):17920
Wang BH, Daniel JE, Wang ZH (2017) The arms race between Magnaporthe oryzae and rice: diversity and interaction of Avr and R genes. J Integr Agric 16(12):2746–2760
Wu KS, Tanksley SD (1993) PFGE analysis of the rice genome: estimation of fragment sizes, organization of repetitive sequences and relationships between genetic and physical distances. Plant Mol Biol 23(2):243–254
Xiao N, Wu YY, Pan CH, Yu L, Chen Y, Liu GQ, Li YH, Zhang XX, Wang ZP, Dai ZY, Liang CZ, Li AH (2017) Improving of rice blast resistances in japonica by pyramiding major R Genes. Front Plant Sci 7:1918
Yuan B, Zhai C, Wang WJ, Zeng XS, Xu XK, Hu HQ, Lin F, Wang L, Pan QH (2011) The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor Appl Genet 122(5):1017–1028
Zhai C, Zhang Y, Yao N, Lin F, Liu Z, Dong ZQ, Wang L, Pan QH (2014) Function and interaction of the coupled genes responsible for Pik-h encoded rice blast resistance. PLoS One 9(6):e98067
Zheng WJ, Wang Y, Wang LL, Ma ZB, Zhao JM, Wang P, Zhang LX, Liu ZH, Lu XC (2016) Genetic mapping and molecular marker development for Pi65(t), a novel broad-spectrum resistance gene to rice blast using next-generation sequencing. Theor Appl Genet 129(5):1035–1044
Zhu YY, Chen HR, Fan JH, Wang YY, Li Y, Chen JB, Fan JX, Yang SS, Hu LP, Leung H, Mew TW, Teng PS, Wang ZH, Mundt CC (2000) Genetic diversity and disease control in rice. Nature 406(6797):718–722
Acknowledgements
We are very grateful to Professor Jong-Seong Jeon in Kyung Hee University, Professor Qinghua Pan in South China Agricultural University and the International Rice Genebank in IRRI for providing seeds for the donor parents of the blast resistance cultivars.
Funding
This work was supported by grants from the National Program on R&D of Transgenic Plants (2016ZX08001002–002), the National Natural Science Foundation (31801438) and the earmarked fund for the China Agriculture Research System (CARS-01-03) of China.
Availability of data and materials
The data sets supporting the results of this article are included within the article and its supporting files.
Author information
Authors and Affiliations
Contributions
YH designed the experiments. HJ, ZL, JL and ZS performed the experiments. GG and QZ helped with field management. HJ and ZL analyzed the data. HJ and YH wrote the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. SSR or InDel markers used for selection of blast resistance genes. (DOCX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jiang, H., Li, Z., Liu, J. et al. Development and evaluation of improved lines with broad-spectrum resistance to rice blast using nine resistance genes. Rice 12, 29 (2019). https://doi.org/10.1186/s12284-019-0292-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-019-0292-z