Abstract
Exosomes are nano-sized membrane extracellular vesicles which can be released from various types of cells. Exosomes originating from inflammatory or injured cells can have detrimental effects on recipient cells, while exosomes derived from stem cells not only facilitate the repair and regeneration of damaged tissues but also inhibit inflammation and provide protective effects against various diseases, suggesting they may serve as an alternative strategy of stem cells transplantation. Exosomes have a fundamental role in communication between cells, through the transfer of proteins, bioactive lipids and nucleic acids (like miRNAs and mRNAs) between cells. This transfer significantly impacts both the physiological and pathological functions of recipient cells. Nuclear factor erythroid 2–related factor 2 (Nrf2), a transcription factor, is able to mitigate damage caused by oxidative stress and inflammation through various signaling pathways. The positive effects resulting from the activation of the Nrf2 signaling pathway in different disorders have been documented in various types of literature. Studies have confirmed that exosomes derived from stem cells could act as Nrf2 effective agonists. However, limited studies have explored the Nrf2 role in the therapeutic effects of stem cell-derived exosomes. This review provides a comprehensive overview of the existing knowledge concerning the role of Nrf2 signaling pathways in the impact exerted by stem cell exosomes in some common diseases.
Graphical Abstract

Similar content being viewed by others
Introduction
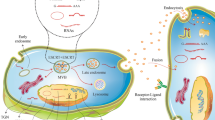
Stem cells are a category of cells that can self-renew and differentiate into various cell types. They are involved in a wide range of physiological and pathological processes including healing of wound, tissue regeneration and tumor formation [1]. Lately, concerns have emerged regarding the safety of using stem cells in the clinical settings. Research indicates that directly transplanting stem cells into specific tissues carries certain risks, such as low survival rates, cell dedifferentiation risk, and the potential for tumorigenesis [2]. Moreover, rejection of transplanted cells by the recipient’s body and ectopic tissue formation further restrict clinical use of stem cells in medical treatments [2]. Over the last ten years, scientists have discovered that extracellular vesicles (EVs) derived from stem cells exhibit therapeutic benefits similar to the parent cells in certain diseases [3]. EV-based therapy, compared to stem cells, provides benefits like immune silence, non-cancerous properties, excellent stability, specific homing to cells and tissues and absence of vascular blockade [4]. EVs are released by different tissues and cells and possess vesicular structures enclosed within a lipid bilayer [5]. These vesicles are categorized into exosomes (30–150 nm, obtained through ultracentrifugation at 100,000×g), microvesicles (100–1000 nm, collected via medium-speed centrifugation at 20,000×g) and apoptotic vesicles (500–5000 nm, isolated using low-speed centrifugation at 2000×g) [6, 7]. As consensus has not yet emerged on specific markers of EV subtypes, it is hard to distinguish exosomes or microvesicles; therefore, MSC exosomes or microvesicles are referred to as MSC-derived small extracellular vesicles (sEVs) [7,8,9]. At present, the main challenge for studying EVs is their isolation. Exosomes and other EVs are isolated using various methods, such as differential ultracentrifugation, ultrafiltration, polyethylene glycol-based precipitation, size-exclusion chromatography, immunoaffinity capture, or by using microfluidics [10].
The exosomes are sEVs that have the smallest average particle size, the highest homogeneity, the most complex composition, and diverse functions among all EVs. Therefore, due to these attributes, they possess the highest practical value and have undergone extensive research and widespread application [11, 12]. Exosomes comprise complex contents, such as nucleic acids including mRNAs, DNA, and noncoding RNAs, lipids, and various proteins that play a vital role in the paracrine mechanisms [13, 14]. Their bilayer lipid membrane enables them to easily penetrate cell membranes, facilitating the information transfer between cells. This ability plays a critical role in modulating the activities of target cells effectively and influencing the development of diseases [15]. Currently, it is widely accepted which the exosomes functional importance relies on their specific contents [16, 17]. These constituents consist of proteins, cytokines, lipids and genetic materials. Notably, research has demonstrated that miRNAs (miRs), transported by exosomes, have substantial impacts on diverse pathological and physiological processes, such as epigenetic modification, regulation of immune system, tumor progression, body development and more [18]. Given that the major molecular components in exosomes are miRs, they play significant regulatory functions in treating diverse diseases by delivering these specific miRs to target cells [19]. Research has highlighted the promising anti-inflammatory and injury repair capabilities of exosomes derived from mesenchymal stem cells (MSCs) [20, 21]. These exosomes are extensively being explored as nanotherapeutic agents for the treatment of stroke [22], diabetes [23], cardiac diseases [24], wound healing [25], liver and kidney diseases [26, 27], autoimmune and neurodegenerative disorders [28], respiratory diseases [29], age-related diseases [30] and other diseases. While past research showed that both MSCs and MSC-derived EVs could potentially counteract oxidative damage [31], their effectiveness in inhibiting oxidative dysfunction and structural injury is not yet fully understood. In recent years, nuclear factor erythroid 2–related factor 2 (Nrf2) has received lots of attention as a promising treatment target for a wide range of disorders [32], including neurodegenerative disorders [33, 34], cancer [35], respiratory disorders [36] and cardiocerebral vascular diseases [37]. The kelch-like ECH-associated protein 1(KEAP1)- Nrf2 pathway serves as a main defense mechanism in response to oxidative stress. It controls the transcription of antioxidant genes to eliminate the possible injury resulting from oxidation and the presence of carcinogenic substances [38]. KEAP1 regulates the activity of Nrf2 by targeting it for ubiquitination and subsequent proteasomal degradation, which is a vital process for regulating responses to injury mediated by oxidative stress. In the absence of external stimuli, Nrf2 remains in an inactive state [39]. Under stress conditions, KEAP1 becomes inactive and prevents Nrf2 ubiquitination. As a result, Nrf2 accumulates in large amounts within the cell, translocates to the nucleus, and promotes secondary antioxidative responses [40]. The KEAP1 overexpression suppresses Nrf2’s transcriptional activity; Conversely, the KEAP1 absence triggers Nrf2 activation, enabling its response to oxidative stress. In other words, alterations in the interaction between Nrf2 and KEAP1 can activate the Nrf2 pathway, leading to the production of various factors like glutamate-cysteine ligase (GCL) and heme oxygenase-1 (HO-1) [39]. Thus, specific targeting of the Nrf2/HO-1 axis might offer a new therapeutic approach for managing various human diseases, such as Alzheimer’s, diabetes, hepatotoxicity and more [41,42,43,44]. Research has confirmed that exosomes derived from MSCs could act as Nrf2 effective agonists [45, 46]. In contrast to other Nrf2 agonists, MSC- exosomes provide therapeutic benefits without toxic side effects associated with drugs and they potentially possess the capability to reach cells directly [42]. Recent studies have revealed that MSCs-derived exosomes have the ability to mitigate injuries caused by oxidative stress by modulating the Nrf2 pathway and its downstream antioxidative genes [47, 48]. It also has been reported that exosomes could serve as anti-inflammatory and antioxidants agents in conditions like skin oxidation, neurological disorders and macrophage polarization through regulation of the Keap1/Nrf2 axis [20, 49]. This review aimed to assess the significance of the Nrf2 signaling pathway in the therapeutic effects of exosomes derived from stem cells for various common diseases.
Diabetes mellitus
Diabetes is a heterogeneous medical condition marked by high blood sugar (glucose) levels or hyperglycemia due to impairment of insulin secretion or defects in the action of insulin or a combination of both factors [50]. Diabetic nephropathy stands out as a prevalent complication of diabetes and is considered as the main factor leading to end-stage kidney disease [51]. Approximately one-third of individuals with diabetes experience kidney dysfunction, resulting in a poor prognosis and substantial long-term social and financial burden [52]. The primary approach for treating diabetic nephropathy involves managing symptoms. This includes methods like regulating blood sugar levels, managing blood pressure and decreasing proteinuria [53]. At present, effective therapeutic medications designed specifically for diabetic nephropathy are still lacking [52]. Consequently, exosomes have the potential to function as biomarkers and as therapeutic agents for a range of kidney disorders [54]. Growing interest has been shown in the therapeutic potential of adipose-derived stem cells (ADSCs)-exosomes for diabetic nephropathy treatment in recent years [55]. These exosomes have the ability to improve podocyte injury induced by high glucose and slow down diabetic nephropathy progression [56]. Furthermore, it has been demonstrated that Nrf2 has therapeutic effects on diabetic nephropathy [57]. Liu et al. reported that Nrf2-/- mice exhibited more severe diabetic kidney disease than wild type [58]. Moreover, individuals with Nrf2 genetic mutations are at an increased risk of complications related to diabetes, such as nephropathy, retinopathy, peripheral neuropathy, foot ulcers and microangiopathy [59]. It has been revealed that ADSCs-exosomes could attenuate inflammation and oxidative stress caused by high glucose in podocytes. This effect occurs through the upregulation of FAM129B and reactivation of the Nrf2-HO-1 pathway, offering a novel approach for clinical treatment [60]. FAM129B is a protein known for its antioxidant properties and has the ability to prevent apoptosis in tumors. It competes with Nrf2 and binds to Keap1, leading to reduced Nrf2 ubiquitination and activation of the Nrf2 pathway (Fig. 1) [61, 62].
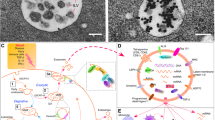
Schematic representation of effects of stem cells-derived exosomes on the Nrf2 pathway. miR-24-3p, miR-141- 3p, miR-125b-5p and miR-200a are transferred by stem cell-exosomes into recipient cells where they target Keap-1, thereby promoting Nrf2 activation. circHIPK3 released from exosomes acts as a ceRNA to bind to miR-20b-5p, which directly inhibits miR-20b-5p and upregulates Nrf2 or/and VEGFA expression, promoting angiogenesis. miR-200a-3p leads to the downregulation of Keap1, nuclear translocation of Nrf2 and promotion of SOD2 expression, resulting in high ATP production and protection against mitochondrial fragmentation. Exosomal miR-23b and miR-21 can alleviate oxidative stress, leading to a reduction in neuroinflammation and providing neuroprotective effects by PTEN/PI3K/AKT/Nrf2 pathway. ADSCs-exosomes could attenuate inflammation and oxidative stress induced by high glucose in podocytes through the upregulation of FAM129B and reactivation of the Nrf2-HO-1 pathway, FAM129B competes with Nrf2 and binds to Keap1, leading to reduced Nrf2 ubiquitination and thereby activation of the Nrf2 pathway. MSCs-exosomes are able to protect against acute liver injury through activation of the P62-Keap1-Nrf2 pathway. P62 serves as an important regulator located upstream of the Keap1-Nrf2 pathway. In response to oxidative stress, P62 competitively interacts with the Nrf2-binding site of Keap1 and inhibits the Nrf2 ubiquitination. It has been demonstrated that miR-100-5p-enriched exosomes have capability to decrease oxidative stress through the regulation of the Nox4-ROS-Nrf2 axis. miR-130a-3p suppresses Nrf2 methylation and upregulates Nrf2 expression through DNMT1 inhibition to activate HIF1α/ACTA1 axis, thereby improving angiogenesis. In addition, exosomes are able to upregulate the expression level of SIRT1 which its restoration results in an increase in Nrf2 and HO-1. Exosomal circ-ITCH suppresses ferroptosis and enhances the angiogenesis by the Nrf2 activation. circ_0072464 shuttled by BMSC-derived EVs can reduce ferroptosis through miR-431 inhibition and the subsequent increase in miR-431-mediated Nrf2 expression. Exosomes loaded with miR-194 alleviate damage caused by ischemia by increasing the Nrf2/HO-1 activation, leading to the downregulation of ferroptosis. Exosome-circAkap7 lessens oxidative stress against ischemic damage via increasing nuclear transcription of Nrf2 by absorbing miR-155-5p. ACTA1, Skeletal muscle actin alpha 1; ceRNAs, competing endogenous RNAs; DNMT1, DNA methyltransferase 1; DPN, Diabetic peripheral neuropathy; HIF1α, Hypoxia inducible factor 1 subunit alpha; HO-1, heme oxygenase-1; KEAP1, Kelch-like ECH-associated protein 1; MSCs, mesenchymal stem cells; Nox4, NADPH oxidase 4; Nrf2, factor nuclear factor-erythroid 2-related factor 2; PI3K, The phosphoinositide 3-kinase; PTEN, Phosphatase and tensin homolog deleted on chromosome 10; SIRT1, silent information regulator 1; SOD; Superoxide dismutase, VEGFA, vascular endothelial growth factor-A
Diabetic peripheral neuropathy (DPN) is known as the most common form of neuropathy in world and its prevalence rate rises with the prolonged duration of diabetes over time [63]. DPN is a common complication related to both type 1 and type 2 diabetes. This condition results in disabling neuropathic pain and in severe cases, lower extremity amputation and imposes a substantial economic burden on society [64]. Research has indicated the pivotal role of Nrf2 in various pathways that impact the development of diabetic neuropathy [65]. Furthermore, Nrf2 is involved in regulating cellular redox homeostasis, inflammatory responses and improving mitochondrial function, all of which contribute to its neuroprotective effects in DPN [66]. Recent findings provided evidence demonstrating that delivering miR-130a-3p via EVs derived from ADSC is able to activate Nrf2/hypoxia inducible factor 1 subunit alpha (HIF1α)/ skeletal muscle actin alpha 1 (ACTA1) axis. This activation occurs by inhibiting DNA methyltransferase 1 (DNMT1) to improve DPN through the suppression of apoptosis in schwann cells (Fig. 1) [67]. Furthermore, another study has demonstrated that Nrf2 overexpression in schwann cells contributes to the repair of peripheral nerve injury in DPN [68]
Diabetic foot ulcers (DFU) have serious implications, often resulting in high mortality and morbidity rates [69], along with imposing a substantial socioeconomic burden [70]. Previous research has indicated that DFU development is influenced by multiple factors such as peripheral arterial disease, neuropathy and infections [71]. The wound healing process is marked by numerous events, such as inflammation, angiogenesis and the extracellular matrix (ECM) remodeling. As a result, the pathophysiology of DFU is extraordinarily complicated [72]. Patients' hyperglycemic state set off DFU pathophysiological hallmarks, which include abnormal vascular development, neuropathy, and immunological response [73]. Hyperglycaemia can lead to dysfunction in vascular endothelial cells by elevated plasma thromboxane A2 and reduced levels of vasodilators. This condition results in ischemia and ulcers. Therefore, it is crucial to regulate blood vessel formation and integrity to prevent the DFU development [74]. In hyperglycemic conditions, the dysfunction and senescence of endothelial progenitor cells (EPCs) result in higher levels of reactive oxygen species (ROS) and hinder DFU healing [75]. EPCs with their capacity to differentiate directly into endothelial cells, have a vital role in the process of revascularization during wound healing [76, 77]. Diabetic mice with Nrf2 knockout compared with the diabetic wild-type littermates exhibited delayed wound closure along with increased oxidative DNA injury and apoptosis [78]. It also has been demonstrated that overexpression of Nrf2 is able to protect diabetic EPCs against dysfunction induced by oxidative stress in vitro [79]. Besides, Nrf2 activation pharmaceutically improves the function of EPCs and accelerates diabetic wound healing in rodents [80, 81]. Loss of Nrf2/ARE activity contributes to increased oxidative stress, that could worsen the impaired function of endothelial cells and abnormal angiogenesis which typically observed in diabetes [82]. Studies have revealed the regulatory Nrf2 role in the regulation of vascular endothelial growth factor-A (VEGFA) [83]. Evidence from an earlier study showed that the Nrf2 has capability of regulation of endothelial proliferation and vascular growth through mechanisms dependent on VEGFA [84]. Increased levels of Nrf2 and VEGFA provide protection against injuries related to diabetic foot ulcers. This protection is achieved by inhibiting oxidative stress and inflammaion [85] and promoting microvascular formation [86]. Human ASC-exosomes were found to mitigate premature senescence in EPCs triggered by high glucose levels. Additionally, these exosomes improved wound healing in diabetic rats [87]. In this research, overexpressed Nrf2 of ASC-exosome led to a significant reduction in ulcer size and granulation tissue formation. This effect was accompanied by an elevated level of growth factor expression and improved angiogenesis achieved through the modulation of various proteins [87].
CircHIPK3 is a type of non-coding RNA that operates through different types of mechanisms, such as interacting with miR, serving as a sponge for RNA binding protein, acting as templates for protein translation, or directly regulating gene expression [88]. Research has provided evidence indicating the involvement of circHIPK3 in many different physiological and pathological mechanisms [89]. circHIPK3 is considered as a crucial factor in various vascular conditions, including diabetic wounds and atherosclerosis [88, 90]. It has been reported that human umbilical cord mesenchymal stem cells (hUC-MSCs) derived circHIPK3 overexpressing exosomes are able to protect cells from injuries induced by high glucose and boost angiogenesis in diabetic wounds. This protective impact is likely mediated through the direct inhibition of miR-20b-5p activity which leads to elevated expressions of Nrf2 and VEGFA. As a result, targeting the circHIPK3/miR-20b-5p/Nrf2/VEGFA axis could be a novel approach for the DFU treatment (Fig. 1) [74]. It has also been demonstrated that exosomal circRNA-itchy E3 ubiquitin protein ligase (circ-ITCH) derived from bone marrow- derived stem cell (BMSCs) suppressed ferroptosis and enhanced the human umbilical vein endothelial cells (HUVECs) angiogenesis by the Nrf2 pathway activation through the recruitment of TATA-Box-binding protein associated factor 15 (TAF15) protein. As a result, this mechanism accelerated the DFU wound healing (Fig. 1) [91]. In addition, in the study by Wang et al., exosomes produced by BMSC were found to enhance processes such as EPC tube formation, neovascularization, collagen deposition and re-epithelialization. Notably, these positive effects were inhibited in the absence of Nrf2. It has also been reported that positive effects of these exosomes were ampilified when treated with a combination of exosomes derived by BMSC and tert-butylhydroquinone (tBHQ), a small-molecule activator of Nrf2. These findings indicate that using Nrf2 activators in conjunction with BMSC-exosomes could present a novel approach for treating chronic diabetic wounds [45].
Skeletal system diseases
Osteoporosis and intervertebral disc degeneration (IDD) are prevalent conditions, both among younger and elderly population which significantly affect life quality [92, 93]. Osteoporosis or low bone mineral density affects about 44 million Americans, which accounts for 55% of people 50 years of age and older [94]. Osteoporosis can cause bones to become more fragile, making them susceptible to low-energy fractures. Usually, these fractures happen in regions of the skeleton with high trabecular content and weight-bearing condition such as hip, spine and the wrist [95, 96]. Oxidative stress play a role in pathophysiology of osteoporosis and can lead to significant cellular injury, including cell apoptosis, necrosis and autophagy [97, 98]. Additionally, overproduction of ROS can trigger disruption of mineral tissue homeostasis and the remodeling process of bone by inducing oxidative stress [99, 100]. Studies have shown the key role of Nrf2 in osteoporosis. According to recent studies, Nrf2 plays an significant role in bone tissue homeostasis and its activation can triger antioxidant responses against ROS, thus regulating osteoporosis occurrence [101, 102]. Exosomes derived from MSC have been demonstrated to improve bone loss in animal models of bone defects like osteoporosis and osteonecrosis [103, 104]. ADSCs-exosomes have been shown to reduce ROS accumulation and mitochondria dysfunction in osteoblasts. Pretreatment with ADSCs-exosomes could induce Nrf2 expression in dexamethasone -stimulated osteoblasts showing that these exosome insert their anti-apoptotic effects via Nrf2. Thus, ADSCs-exosomes could reduce apoptosis and oxidative stress by modulating Nrf2/HO-1 expression following dexamethasone exposure and prevent the progression of glucocorticoid-induced osteoporosis in vivo [11].
IDD is known by serious spinal symptoms, such as low back pain and sciatica syndrome [105] which is resulted from degenerative disc conditions [106]. Treatment approaches for IDD include ECM generation and pro-anabolic treatment via regulating ROS microenvironment [107]. ROS functions as an important factor in the IDD progression because of its’ degenerative environment [108] and exosomes are able to inhibit the elevated ROS by the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome [109]. According to reports, Nrf2 expression level is low in the nucleus pulposus of degenerated disk [110] and activation of Nrf2 in the region could alleviate IDD progression through mobilizing the intrinsic antioxidant capacity of cells and cosequenltly reduction of excessive ROS [111,112,113]. BMSC-derived exosomes have the potential to alleviate IDD process through regulating Keap1/Nrf2 pathway which results in a reduction in excessive levels of ROS [114]. Current research findings show that the occurrence of ferroptosis in nucleus pulposus cells is closely linked to IDD pathogenesis, suggesting that targeting ferroptosis with the inhibition of lipid peroxidation via Nrf2 overexpression could be a promising and novel approach for the treatment of IDD [115, 116]. It has been demonstrated that circ_0072464 shuttled by BMSC-derived EVs can reduce ferroptosis in nucleus pulposus cells by miR-431 inhibition and the subsequent increase in miR-431-mediated Nrf2 expression in both vitro and in vivo models. According to these results, circ_0072464 could serve as a promising therapeutic option for the IDD treatment (Fig. 1) [115].
Respiratory diseases
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are known as common life-threatening lung diseases and syndromes of severe respiratory failure with substantial morbidity and mortality rates in critically ill patients [117, 118]. ALI is marked by extensive inflammation within the lungs, along with the accumulation of inflammatory cells especially macrophages and neutrophils. This accumulation causes injury to both lung endothelial cells and epithelial barriers [119]. Individuals with pneumonia or sepsis are highly vulnerable to ALI, indicating a direct association with infection [120]. Cell-based therapies like the administration of human amniotic mesenchymal stem cells (hAMSCs) can have positive outcomes in ALI treatment and provide protection against lung inflammation induced by lipopolysaccharides (LPS) [119, 121, 122]. However, the therapeutic effects of stem cells-based treatment pose challenges due to complex procedures involved in isolating, purifying, and storing these cells in a sterile manner. Additionally, inflammatory environments do not support the transplanted stem cells survival. Therefore, the use of sEVs could serve as an alternative treatment option to mimic the beneficial effects of stem cells [123] and have the ability to provide protection in a wide range of inflammatory diseases [124]. It has been found that activation of Nrf2 confers protection against LPS-induced lung injury [125] and its’ overexpression could mitigate the injury [122].
Therefore, modulation of Nrf2 expression could offer a new method for treating ALI. It is also reported that Nrf2 could slow down lung injury development through the activation of antioxidant genes and modulation of NLRP3 inflammasome in lung inflammation in vitro model induced by lipopolysaccharides (LPS) [125, 126].
Macrophages and pulmonary microvascular endothelial cells (PMVECs) are the most important mediators and victims of ALI [127]. When a pathogen invades, alveolar macrophages become activated and shift to a M1 phenotype (pro-inflammatory). These activated alveolar macrophages release inflammatory cytokines like interleukin 1 (IL-1), tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) [128, 129]. Overproduction of inflammatory cytokines and ROS elevation leads to membrane and DNA damage of PMVECs [130]. Shen et al. showed that ADSCs exosomes have the ability to regulate Keap1/Nrf2 pathway within macrophages in lung tissues. This regulatory process ultimately results in a protective effect against lung injury induced by cecal ligation and puncture [49]. Nrf2 regulates numerous cytoprotective genes like glutathione Peroxidase 4 (GPX4) [116]. The antioxidant enzyme GPX4 functions as a negative regulator of ferroptosis through diminishing lipid peroxidation [131, 132]. Studies have revealed that the delivery of miR-125b-5p through exosomes derived from ADSCs can attenuate PMVECs ferroptosis induced by inflammation during sepsis-induced ALI through the regulation of Keap1/Nrf2/GPX4 expression, therefore improve the ALI (Fig. 1) [133]. BMSC-exosomes have been shown to decrease ALI related in cardiopulmonary bypass by mitigating inflammatory responces and oxidative stress. The mechanism behind this effect likely involves nuclear factor kappa B (NF-κB) p65 pathway as well as the protein kinase B (Akt)/Nrf2/HO-1 signaling pathways [29]. It was found that hUC-MSCs‐derived exosomes play a pivotal role in promoting Nrf2 expression and nuclear translocation. This effect is mediated through the miR-199a-5p transduction and its targeted binding with caveolin1. Consequently, this process increases the antioxidant enzyme expression within the cells of the lung, effectively regulating oxidative stress induced by sulfur mustard. Sulfur mustard is a chemical warfare agent known for the production of blister formation and can result in a series of systemic damages, particularly severe ALI [134].
Growing research has revealed that BMSCs possess biological properties that modulate the immune system and offer therapeutic benefits in alleviating ARDS, mainly through the secretion of their exosomes [135, 136]. BMSCs-exosomes have the ability to produce beneficial outcomes, such as enhancing the epithelial barrier repair in pulmonary alveoli [134]. They could prevent apoptosis induced by hyperoxia in type II alveolar epithelial cells (AECIIs) [137]. AECIIs are lung epithelium stem cells which have essential secretory and regenerative functions and maintain lung homeostasis [138]. Various studies have shown that the AECIIs apoptosis plays a crucial role in the development of sepsis-induced ARDS [139]. It has been described that BMSC-exosomes effectively reduce sepsis-caused apoptosis in AECIIs and consequently ARDS by recovering the mitochondrial dysfunction mediated by Nrf2 [140].
Cardiac diseases
Myocardial infarction (MI) occurs due to a sudden decrease or cessation of blood flow in a coronary artery which leads to severe and prolonged acute ischemia in a specific area of the heart muscle, ultimately resulting in myocardial necrosis [141, 142]. MI, known as the most common cardiovascular disease, is a serious threat to life and health [143]. There is growing evidence that BMSCs transplantation can be considered as a promising treatment for MI due to their anti-inflammatory, anti-fibrosis and angiogenic properties [144]. However, animal models and clinical trials have discovered the limited effectiveness of BMSCs for MI that may be attributed to the poor local microenvironment and high levels of inflammation reactions within ischemic tissue, leading to engrafted cells death following administration of BMSCs [145, 146].
It has been demonstrated that the MSCs paracrine effect promotes angiogenesis, myocardial tissue repair, immunosuppression and stem cell homing through secretion of EVs, transforming growth factor beta T helper 1 (TGF-β), TNF-a, VEGF and other growth factors and cytokines [147]. ADSC-derived Exosomes could promote angiogenesis in the ischemic region and protect cardiac myocytes from excessive oxidative stress in the infarct area [148,149,150]. Additionally, exosomes have the potential to suppress inflammation and improve cardiac function and fibrosis in an animal model of MI [151]. Oxidative stress serves as a crucial mechanism in myocardial damage following MI [143]. An imbalance between the ROS production and their removal by body's antioxidant mechanisms leads to the macromolecules damage and redox signaling interruption that affect structure and function of heart, leading to myocardial hypertrophy, impaired contractile function, and fibrosis seen in chronic heart failure [152]. The Nrf2/ARE axis act as key signaling molecules in preventing oxidative damage of cardiac cell [153] and protects the heart against cardiac dysfunction and maladaptive remodeling [154, 155]. Previous studies demonstrated that Nrf2 signaling is involved in the onset and progression of numerous heart disorders, including MI, myocarditis and atrial fibrillation [156, 157]. Nrf2/HO-1 activation upregulates the transcription of several endogenous antioxidants and protects cardiomyocytes from damage caused by oxygen radicals [158]. Exosomes derived from fibronectin type III domain-containing protein 5 (FNDC5)-preconditioned BMSCs play a protective role against MI through anti-inflammatory effects and macrophage polarization. These effects are mediated by the NF-κB signaling pathway and the Nrf2/HO-1 axis [46]. Chen et al. reported that exosome isolated from human-induced pluripotent stem cells (iPSCs) -derived MSCs could increase the survival of cardiomyocytes, improve cardiac function, reduce the extent of heart tissue damage and suppress oxidative stress. However, these positive effects of exosomes were notably reversed when LY294002, an inhibitor of the Akt/Nrf2/HO-1 pathway, was used. This suggests that exosomes could potentially improve MI triggered by severe acute pancreatitis by activating the Akt/Nrf2/HO-1 pathway [159]. The myocardial tissue of rats with atrial fibrillation exhibited reduced levels of Nrf2 and HO-1. It was revealed that Nrf2-overexpressing BMSC-exosomes could inhibit arrhythmias caused by atrial fibrillation, myocardial fibrosis, inflammation and apoptosis by activating the Nrf2/HO-1 axis [24]. Moreover, exosomes derived from silent information regulator 2 homolog 1 (Sirt1)-overexpressing ADSCs could contribute to tube formation, cell migration, and the recruitment of endothelial progenitor cells (EPCs) to the site of repair. This process facilitates the repair of the injured cardiac area through the Nrf2/CXCL12/CXCR7 pathway [160]. Sirt1 is a deacetylase dependent protein on nicotinamide adenine dinucleotide (NAD +) which regulates acetylation of specific transcription factors such as Nrf2 to prevent damage caused by oxidative stress and inflammation [161, 162].
Liver diseases
Acute liver injury, a serious metabolic dysfunction, results from significant damage to hepatic cells and is frequently observed in various severe liver diseases [163]. The application of MSCs-exosome has shown positive results in different exprimental models of liver diseases, such as liver fibrosis, hepatocellular carcinoma and drug-induced acute liver injury [164,165,166]. Researchers observed that glutathione peroxidase 1 (GPX1) derived from hUC-MSCs exosomes effectively lower the levels of ROS and malondialdehyde (MDA) in liver and inhibit apoptosis induced by oxidative stress in liver failure [167]. In addition, ADSCs-derived exosomes could also alleviate ROS and MDA contents in the liver and enhance the activity of superoxide dismutase (SOD) in hepatic ischemia–reperfusion (I/R) injury [168]. Therefore, exosomes derived from MSC can be considered as an effective therapeutic intervention for liver diseases. p62-Keap1-Nrf2 pathway serves as a crucial controller in minimizing iron toxicity in hepatocellular carcinoma cells through the activation of gene transcription related to iron and ROS metabolism [169]. P62 is a key upstream regulator of the Keap1-Nrf2 axis which competitively interacts with Nrf2-binding site of Keap1 and inhibits Nrf2 ubiquitination, thereby stimulating the transcription of downstream genes [170]. P62-Keap1- Nrf2 signaling pathway plays a key role in the maintenance of redox homeostasis [171]. It has been documented that pretreated MSCs-exosome elicits better transplantation and therapeutic efficacy. A recent study found that exosomes derived from baicalin-pretreated MSCs are able to protect against acute liver injury induced by the D-galactosamine and lipopolysaccharide (D-GaIN/LPS) through activation of the P62-Keap1- Nrf2 pathway (Fig. 1) [172].
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease that [173] is linked to metabolic disorders, like insulin resistance, obesity and type 2 diabetes [174]. NAFLD has the potential to advance into non-alcoholic steatohepatitis (NASH) and, over time, develop into cirrhosis [175]. MSCs-exosomes in fatty liver have been reported to be hepatoprotective by lipid metabolism regulation and modulation of oxidative stress and inflammation [26, 176]. Keap-1 deletion results in the enhancement of Nrf2 nuclear translocation which is followed by Ho-1 gene expression which finally results in reduction of the ROS generation [177]. Additionally, suppression of Keap-1 was shown to reduce inflammasome activation induced by metabolic stress, oxidative stress and impaired lipid metabolism in the hepatocytes [178]. It has been demonstrated that exosomal miR-24-3p derived from MSCs by targeting Keap-1 in hepatocytes treated with palmitate, could inhibit the fatty acid synthesis and NF-kB signaling pathway and improve activation of Nrf2 axis, thereby exerting a therapeutic potential against NAFLD (Fig. 1) [179].
NASH, a more severe subtype of NAFLD, is identified by steatosis, ballooning degeneration of hepatocytes, inflammation and the hepatocytes fibrosis [180]. This is a progressive disorder that can eventually cause cancer and cirrhosis of the liver [181]. Oxidative stress has been characterized as an important factor in the progression of NASH [182, 183]. Research exhibited that EVs derived from human liver stem cells could decrease hepatic TNF-α and IL-1β levels in animals with NASH [184]. Kang et al. demonstrated key role of Nrf2 signaling pathway in the treatment of NASH by stem cell derived exosomes. They reported that hUC-MSCs exosomes provide positive therapeutic effects including anti-lipid deposition, anti-oxidative stress and anti-inflammatory through Nrf2/NQO-1 pathway in NASH experimental model [185].
Neurological injury
Neurological diseases refer to a wide range disorders affecting the central and peripheral nervous systems which make them the leading cause of disease burden world wide [186, 187]. However, the available and approved treatment options for these conditions are limited in comparison to other injured areas in the body [188, 189]. Exosomes have been shown to have a significant impact on treating neurodegenerative diseases, nerve injuries, and other neurological disorders [190]. Despite earlier studies suggesting that MSCs and EVs derived from MSCs can suppress oxidative injury [31, 191], their effectiveness in mitigating oxidative neuronal dysfunction and structural damage is not fully understood. Exosomes insert strong antioxidant effect like GPX enzyme activation and ferroptosis inhibition [161] which could ameliorate ROS induced neuronal injury [192]. In the research conducted by Li X et al. it was showed which exosomal miR-194 derived from MSC exerts neuroprotection after oxygen–glucose deprivation/reoxygenation (OGD/R) -induced neuronal injury which is an in vitro ischemic stroke model [193]. Nrf2-mediated therapies exert protective effects against various neurological problems in response to oxidative stress [15, 64]. The results from in vitro and in vivo experiments reveal that exosomes derived from circAkap7-modified ADSCs, referred to as exosomal circAkap7, could exert neuroprotection against ischemic damage by enhancing ATG12 (Autophagy Related 12)-mediated autophagy and mitigate oxidative stress by activation of Nrf2 nuclear transcription (Fig. 1) [194]. In an in vitro model of hypoxia/reperfusion injury, the co-culture of neurons with EVs derived from neural stem/progenitor cells (NPCs) prevented the apoptosis of neurons via the induction of the Nrf2 nuclear translocation that in turn regulates the expression of oxidative stress-induced kinases [195]. MSC-derived exosomes containing miR-194 are able to reduce injury after OGD/R via suppressing CNC homology 1 (Bach1) expression and Nrf2/HO-1 pathway activation through the miR-194 delivery to endothelial cells of brain vessels that leads to the downregulation of ferroptosis (Fig. 1) [193]. EVs derived from human neural stem cells (hNSC) possess the ability to inhibit apoptosis induced by oxidative stress and promote axons growth and angiogensis after ischemia induced neuronal injury. They could also enhance Nrf2 translocation to the cell nucleus in order to up-regulate antioxidant enzymes which results in intracellular ROS reduction [195]. Methotrexate (MTX) ia a chemotherapeutic agent that can cause neurotoxic effects on the central nervous system (CNS) [196] and it has been reported thet exosomes from ADSCs could alleviate MTX induced neurotoxicity through the activation of the Nrf2-ARE signaling pathway [196]. In addition, it has been identified that the protective effect conferred by MSCs-exosome against cognitive deficits in aged mice linked to their ability to prevent ferroptosis in the hippocampus by the modulation of the SIRT1/Nrf2/HO1 signaling pathway [197]. MSCs-exosome can reduce early brain damage and improve cognitive impairment following subarachnoid hemorrhage. It has been demonstrated that BMSC-exosomal miR-23b could alleviate oxidative stress and pyroptosis that modulate neuroinflammation and insert neuroprotection after intracerebral hemorrhage. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is considered as a target gene responsible for mediating the anti-inflammatory and antioxidant properties of miR-23b through modulating the Nrf2 signaling pathway and activating the NLRP3 inflammasom (Fig. 1) [198]. Exosomes derived from MSC have the wide therapeutic potential for improvement of neurological disorders which are triggered through astrocytosis by the activation of Nrf2-NF-kB signaling pathway [20]. The results suggest that miR-100-5p-enriched trophoblast stage-derived MSCs (T-MSCs) exosomes have a protective effect against the loss of dopaminergic neurons. These exosomes contribute to the maintenance of nigrostriatal system function, improvement of motor impairments, and reduction of oxidative stress by the regulation of the NADPH oxidase 4 (Nox4)-ROS-Nrf2 axis (Fig. 1) [199].
Age-related diseases
Aging is considered as an inevitable biological process that results in progressive decline of tissue and organ function [200] and is determined by the senescent cells accumulation in numerous tissues which result in the disruption of homeostasis and decline in regenerative capacity. This phenomenon is associated with the expression of senescence-associated β-galactosidase, the cyclin-dependent kinase inhibitors P16 and P21, elevated oxidative stress levels, and other hallmarks [200, 201]. Angiogenesis plays a critical role in process of wound healing and tissue regeneration through the restoration of blood supply and the delivery of nutrients to damaged area [202]. Endothelial cells as a key element in angiogenesis, experience function impairment as they undergo senescence [203, 204] and a higher presence of these cells are present in aged tissues [203]. It has been shown that there is an age-dependent difference in wound healing process between old and young individuals because of inadequate local angiogenesis and impaired tissue repair in aged poeple [203, 205]. The therapeutic capacity of stem cells-derived exosomes for diseases related to aging is barely reported. Recently, stem cells-derived exosomes have gained great attention in a number of studies in aging-related diseases [206] because of their pro-angiogenic effects at the injury sites [207, 208]. In a recent investigation [209] it was revealed that exosomes from ADSCs have the ability to mitigate the senescence characteristics in osteoarthritic osteoblasts. Chen et al. investigated the impact of human embryonic stem cells (hESCs) ‐exosomes on HUVECs undergoing senescence triggered by D‐galactose. They discovered that chronic treatment with hESCs ‐exosomes could diminish aging indicators and restore impaired functions, including migration, proliferation and tube formation by the transfer of miR‐200a [47].
Oxidative stress is considered as a contributor to the aging process and Nrf2 signaling could be considered as a potential defense mechanism against oxidative stress by senescence control [210]. Older cells have a lower basal Nrf2 protein expression level than cells from young adults which highlightes the importance of Nrf2 activity in determining species longevity [211, 212]. Suppression of Nrf2 expression in "young" cells leads to noticeable impairment in cellular function, while enhancing Nrf2 activity was proven to effectively counteract cellular senescence and render them similar to young cell [178, 213]. Exosomes derived from MSCs were observed to significantly alleviate aging-related senescence of CD4 + T cells. This reduction in senescence was attributed to the exosomes ability to decrease oxidative damage, lower senescence-associated secretory phenotype (SASP) expression, diminish aging-related proteins such as p53, and other markers of aging. miR-21 downregulates PTEN and boosts the activation of phosphoinositide 3-kinases (PI3K) and AKT that lead to Nrf2 gene expression (Fig. 1) [214]. Pressure ulcers, especially in people who are elderly, are known to exhibit poor healing due to age-associated changes in skin tissue [202, 203]. It has been demonstrated that aging cells have higher levels of KEAP1, and it is thought that Keap1 overexpression influences activity of Nrf2 in in the elderly [178, 210]. On the other hand, the increased Nrf2 activity observed in long-lived species is attributed in part to a reduction in Keap1 expression [215]. Treatment with the hESCs-derived exosomes was shown to speed up healing of pressure ulcer and stimulate angiogenesis at sites of wound through rejuvenating endothelial cell senescence by Nrf2 activation in aged mice. This anti-aging effects occurs via the transfer of miR-200a to senescent endothelial cells causing Keap1 down-regulation and subsequent Nrf2 expression, which is a vital pathway in anti-aging processes (Fig. 1) [47]. Moreover, human periodontal ligament stem cells (PDLSCs)-exosomes may have anti-aging impacts through the transfer of miR-141-3p to downregulate KEAP1 expression and consequently activate the Nrf2 antioxidant pathway (Fig. 1) [30].
Acute kidney injury (AKI)
AKI is a commoncondition marked by a sudden decrease in renal function. Various pathological factors, including I/R, sepsis, trauma and exposure to nephrotoxic substances can trigger AKI [216, 217]. Kidney I/R is one of the most common causes of AKI. After ischemic damage, renal tubular cells, specifically those in the proximal tubules, undergo different forms of cell death [218]. The mortality rate of AKI is disturbingly elevated, ranging from 24 to 62% [219]. Therefore, various therapeutic approaches, including the transplantation of MSC, have been developed. However, due to some limitations in MSC transplantation, some researchers have recommended an alternative approach termed 'cell-free therapy.' This strategy involves the use of EVs derived from stem cells for injury therapy [220]. The protective role of EVs derived from MSCs in kidney injury has been documented [27, 221]; However, the specific mechanism responsible for this protective action is not yet clearly elucidated.
Following I/R injury, oxidative stress leads to ROS accumulation high concentration in renal tubules which have a greater number of mitochondria compared to other structures within the kidney. Excessive amounts of ROS can result in the fragmentation and disturbance of mitochondria in renal cells that triggers the death of renal cells through both necrosis and apoptosis, accompanied by the secretion of pro-apoptotic proteins [222]. However, mitochondria possess defense systems to inhibit the additional ROS generation, namely antioxidant systems, that critically rely on the Keap1 -Nrf2 signaling pathway. It has been documented that MSC-EVs are able to alleviate I/R-induced AKI and contribute to the preservation of redox homeostasis by promoting the activation of Nrf2/ARE signaling [223, 224]. The researchers in this investigation proposed a hypothesis suggesting that the activation of Nrf2 may be attributed to some miRNAs transported by EVs derived from MSC. Another I/R research supports this mechanism in which human placenta-MSC sEVs were found to deliver miR-200a-3p to tubular epithelial cells. The presence of this miR led to the downregulation of Keap1, nuclear translocation of Nrf2 and promotion of SOD2 expression. This antioxidant defense mechanisms resulted in elevated ATP production and shielded tubular epithelial cells against mitochondrial fragmentation (Fig. 1) [225]. It has also been found that sEVs derived from hUC-MSCs modified with angiotensin-converting enzyme (ACE) can hinder apoptosis, diminish oxidative stress and regulate inflammatory responses, leading to the reduction of renal I/R injury. The probable mechanism for this effect may be related to the activation of the Nrf2/HO-1 pathway [226].
Skin injuries
Skin is the largest organ of the body, serving as a protective agent against environmental toxins and microorganisms as well as preventing dehydration. Additionally, it performs crucial functions like immune surveillance, self-healing and sensory detection. Skin damage often results from acute traumas, infections, chronic wounds, surgical procedures, diabetic ulcers and genetic disorders [227]. As an important paracrine factor secreted from stem cells, exosomes show regenerative functions in a wide range of diseases. Exosomes derived from iPSC-derived MSC are able to improve the wound healing process through enhancing collagen production, angiogenesis and fibroblasts proliferation/migration in human dermatom [228]. Ultraviolet radiation triggers oxidative stress which results in ROS production [229, 230] and causes DNA fragmentation or lipid peroxidation that ultimately results in several skin damages like premature aging, sunburn ore even carcinogenesis [230,231,232]. As a key component, Nrf2 is involved in the regulation of antioxidant enzymes following skin injury [233] and application of an Nrf2 agonist has been shown to improve mottled hyperpigmentation in the photodamaged skin [234]. These results suggest that regulation of Nrf2 activity can be considered as an effective therapeutic approach for treating skin injury. Activating the Nrf2 pathway internally or systemically in response to skin injury triggers a chain reaction, leading to potent antioxidant production such as SOD, GPX, and catalase (CAT) [233, 235]. Exosomes derived from MSCs could insert skin repair after ultraviolet B radiation-induced ROS production via Nrf2 signaling pathway activation which results in expression of cytoprotective antioxidants and DNA damage inhibition [191, 236].
Conclusion
We discussed the therapeutic potentials of stem cell‐derived exosomes with a focus on Nrf2 regulatory role and its potential application as a novel cell-free therapy approach for several human diseases (see Table 1). Application of stem cell-derived exosome opens a new window for repairing, regenerating, and treating a range of disorders by stimulating different pathways. Exosomes therapy is able to modulate and re-program cell function through delivery of biomolecules and therapeutic compounds to different target tissues, indicating the substantial potential of these tiny vesicles. Nrf2 signaling pathway plays a key role in the regulation of exosomes therapeutic potential and exerts therapeutic effects against diseases through the activation of antioxidant signaling pathways to protect the cells from the detrimental effects of oxidative stress. Based on the context discussed above, promotion of the antioxidant properties by the Nrf2 activation with stem cell derived exosomes administration can represent a novel focus for future research efforts. Therefore, Nrf2 activation can be considered as promising therapeutic approach, however there is a need to develop suitable approaches and methods to tailor exosomes with high drug loading capacity, increased target specificity and non–cytotoxic effects.
Availability of data and materials
Not applicable.
Abbreviations
- ACTA1:
-
Skeletal muscle actin alpha 1
- ADSCs:
-
Adipose-derived stem cells
- AECII:
-
Type II alveolar epithelial cell
- AKI:
-
Acute kidney injury
- Akt:
-
Protein kinase B
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- ATG12:
-
Autophagy Related 12
- Bach1:
-
CNC homology 1
- BMSC:
-
Bone marrow-derived stem cell
- CAT:
-
Catalase
- Circ-ITCH:
-
CircRNA-itchy E3 ubiquitin protein ligase
- CNS:
-
Central nervous system
- CXCL12:
-
C–X–C motif chemokine ligand 12
- CXCR7:
-
C–X–C chemokine receptor type 7
- DFU:
-
Diabetic foot ulcers
- D-GaIN/LPS:
-
D-galactosamine and lipopolysaccharide
- DNMT1:
-
DNA methyltransferase 1
- DPN:
-
Diabetic peripheral neuropathy
- ECM:
-
Extracellular matrix
- EPCs:
-
Endothelial progenitor cells
- EVs:
-
Extracellular vesicles
- FNDC5:
-
Fibronectin type III domain-containing protein 5
- GCL:
-
Glutamate-cysteine ligase
- GPX:
-
Glutathione peroxidase
- hAMSC:
-
Human amniotic mesenchymal stem cells
- HDFs:
-
Human dermal fibroblasts
- hESCs:
-
Human embryonic stem cells
- HIF1α:
-
Hypoxia inducible factor 1 subunit alpha
- hNSC:
-
Human neural stem cells
- HO-1:
-
Heme oxygenase-1
- hUC-MSCs:
-
Human umbilical cord mesenchymal stem cells
- HUVECs:
-
Human umbilical vein endothelial cells
- IDD:
-
Intervertebral disc degeneration
- IL-1:
-
Interleukin 1
- IL-6:
-
Interleukin 6
- iPSCs:
-
Human-induced pluripotent stem cells
- I/R:
-
Ischemia–reperfusion
- KEAP1:
-
Kelch-like ECH-associated protein 1
- LPS:
-
Lipopolysaccharides
- MDA:
-
Malondialdehyde
- MI:
-
Myocardial infarction
- miR:
-
MicroRNAs
- MSC*s:
-
Mesenchymal stem cells
- MTX:
-
Methotrexate
- NAD+ NAFLD:
-
Nicotinamide adenine dinucleotide Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- Nox4:
-
NADPH oxidase 4
- NF-κB:
-
Nuclear factor kappa B
- NLRP3:
-
NLR family pyrin domain containing 3
- NPCs:
-
Neural stem/progenitor cells
- NQO1:
-
NAD(P)H quinone oxidoreductase 1
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- OGD/R:
-
Oxygen–glucose deprivation/reoxygenation
- PDLSCs:
-
Human periodontal ligament stem cells
- PI3K:
-
The phosphoinositide 3-kinase
- PMVECs:
-
Pulmonary microvascular
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- ROS:
-
Reactive oxygen species
- SASP:
-
Senescence-associated secretory phenotype
- sEVs:
-
Small extracellular vesicles
- Sirt1:
-
Silent information regulator 2 homolog 1
- SOD:
-
Superoxide dismutase
- TAF15:
-
TATA-Box-binding protein associated factor 15
- tBHQ:
-
Tert-butylhydroquinone
- TGF-β:
-
Transforming growth factor beta
- T-MSCs:
-
Trophoblast stage-derived MSCs
- TNFα:
-
Tumor necrosis factor alpha
- UV:
-
Utlraviolet
- VEGFA:
-
Vascular endothelial growth factor-A
References
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells cancer and cancer stem cells. Nature. 2001;414(6859):105–11.
Jeong J-O, Han JW, Kim J-M, Cho H-J, Park C, Lee N, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108(11):1340–7.
Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36(3):469–84.
Rani S, Ritter T. The exosome—a naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2016;28(27):5542–52.
Trisko J, Fleck J, Kau S, Oesterreicher J, Holnthoner W. Lymphatic and blood endothelial extracellular vesicles: a story yet to be written. Life. 2022;12(5):654.
Liu C, Li Y, Han G. Advances of mesenchymal stem cells released extracellular vesicles in periodontal bone remodeling. DNA Cell Biol. 2022;41(11):935–50.
Lu Y, Wang L, Zhang M, Chen Z. Mesenchymal stem cell-derived small extracellular vesicles: a novel approach for kidney disease treatment. Int J Nanomedicine. 2022;17:3603–18.
Chen L, Qu J, Mei Q, Chen X, Fang Y, Chen L, et al. Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Res Ther. 2021;12:1–15.
Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9(1):1735249.
Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):6466.
Yao X-w, Liu Z-y, Ma N-f, Jiang W-k, Zhou Z, Chen B, et al. Exosomes from adipose-derived stem cells alleviate dexamethasone-induced bone loss by regulating the Nrf2/HO-1 axis. Oxid Med Cell Longev. 2023;2023:1.
Izadpanah M, Yalameha B, Sani MZ, Cheragh PK, Mahdipour M, Rezabakhsh A, et al. Exosomes as theranostic agents in reproduction system. Adv Biol. 2023;8:2300258.
Nooshabadi VT, Khanmohammadi M, Shafei S, Banafshe HR, Malekshahi ZV, Ebrahimi-Barough S, et al. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochem Biophys Rep. 2020;23: 100792.
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145.
Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128: 110237.
Tenchov R, Sasso JM, Wang X, Liaw W-S, Chen C-A, Zhou QA. Exosomes: nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802–46.
Alemi F, Sadeghsoltani F, Fattah K, Hassanpour P, Malakoti F, Kardeh S, et al. Applications of engineered exosomes in drugging noncoding RNAs for cancer therapy. Chem Biol Drug Des. 2023;102(5):1257–75.
Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30(4):656–73.
Xu H-K, Chen L-J, Zhou S-N, Li Y-F, Xiang C. Multifunctional role of microRNAs in mesenchymal stem cell-derived exosomes in treatment of diseases. World J Stem Cells. 2020;12(11):1276.
Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956.
Long Q, Upadhya D, Hattiangady B, Kim D-K, An SY, Shuai B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci. 2017;114(17):E3536–45.
Otero-Ortega L, Laso-García F, Gómez-de Frutos M, Fuentes B, Diekhorst L, Díez-Tejedor E, et al. Role of exosomes as a treatment and potential biomarker for stroke. Transl Stroke Res. 2019;10(3):241–9.
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–28.
Xu L, Fan Y, Wu L, Zhang C, Chu M, Wang Y, et al. Exosomes from bone marrow mesenchymal stem cells with overexpressed Nrf2 inhibit cardiac fibrosis in rats with atrial fibrillation. Cardiovasc Ther. 2022;2022:1.
Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6):10847–54.
Cheng L, Yu P, Li F, Jiang X, Jiao X, Shen Y, et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34(6):1697–708.
Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255: 117719.
Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, Segaliny AI, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–88.
Zhang T-Y, Zhang H, Deng J-Y, Gong H-R, Yan Y, Zhang Z, et al. BMMSC-derived exosomes attenuate cardiopulmonary bypass-related acute lung injury by reducing inflammatory response and oxidative stress. Curr Stem Cell Res Ther. 2023;18(5):720–8.
Liu M, Chen R, Xu Y, Zheng J, Wang M, Wang P. Exosomal miR-141–3p from PDLSCs alleviates high glucose-induced senescence of PDLSCs by activating the KEAP1-NRF2 signaling pathway. Stem Cells Int. 2023;2023:1.
Ding C, Qian C, Hou S, Lu J, Zou Q, Li H, et al. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to prevent reactive oxygen species generation in POI. Mol Ther Nucleic Acids. 2020;21:37–50.
Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25(22):5474.
Johnson DA, Johnson JA. Nrf2—a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–67.
Khassafi N, Tameh AA, Mirzaei H, Rafat A, Barati S, Khassafi N, et al. Crosstalk between Nrf2 signaling pathway and inflammation in ischemic stroke: mechanisms of action and therapeutic implications. Exp Neurol. 2023;373:114655.
Panieri E, Saso L. Potential applications of NRF2 inhibitors in cancer therapy. Oxid Med Cell Longevity. 2019;2019:1.
Carlson J, Price L, Deng H. Nrf2 and the Nrf2-interacting network in respiratory inflammation and diseases. In: Deng H, editor. Nrf2 and its modulation in inflammation. Cham: Springer; 2020. p. 51–76.
Cheng L, Zhang H, Wu F, Liu Z, Cheng Y, Wang C. Role of Nrf2 and its activators in cardiocerebral vascular disease. Oxid Med Cell Longevity. 2020;2020:4683943.
Tu W, Wang H, Li S, Liu Q, Sha H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10(3):637.
Fan Z, Wirth A, Chen D, Wruck C, Rauh M, Buchfelder M, et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371-e.
Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40(13):e00099-e120.
Mohan S, Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed Pharmacother. 2018;108:1866–78.
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R. Nrf2–ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104.
Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, et al. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radical Biol Med. 2018;121:78–85.
Vahidinia Z, Tameh AA, Karimian M, Zare-Dehghanani Z, Moradi F, Joghataei MT. Calcitriol ameliorates brain injury in the rat model of cerebral ischemia-reperfusion through Nrf2/HO-1 signalling axis: An in silico and in vivo study. J Stroke Cerebrovasc Dis. 2022;31(6): 106331.
Wang L, Cai Y, Zhang Q, Zhang Y. Pharmaceutical activation of Nrf2 accelerates diabetic wound healing by exosomes from bone marrow mesenchymal stem cells. Int J Stem Cells. 2022;15(2):164–72.
Ning H, Chen H, Deng J, Xiao C, Xu M, Shan L, et al. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res Ther. 2021;12(1):1–15.
Chen B, Zhang J, Zhu Q, Sun Y, Yang Y, Niu X, et al. Human embryonic stem cells derived exosomes promote tissue regeneration in aged mice by rejuvenating senescent endothelial cells. SSRN J. 2019. https://doi.org/10.2139/ssrn.3335004.
Lerner N, Chen I, Schreiber-Avissar S, Beit-Yannai E. Extracellular vesicles mediate anti-oxidative response—in vitro study in the ocular drainage system. Int J Mol Sci. 2020;21(17):6105.
Shen K, Jia Y, Wang X, Zhang J, Liu K, Wang J, et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021;165:54–66.
Bekele BB, Manzar MD, Alqahtani M, Pandi-Perumal SR. Diabetes mellitus, metabolic syndrome, and physical activity among Ethiopians: a systematic review. Diabetes Metab Syndr. 2021;15(1):257–65.
Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. BioMed Res Int. 2021;2021:1.
Venuta F, Rendina EA. Combined pulmonary artery and bronchial sleeve resection. Oper Tech Thorac Cardiovasc Surg. 2008;13(4):260–73.
Natesan V, Kim S-J. Diabetic nephropathy–a review of risk factors, progression, mechanism, and dietary management. Biomol Ther. 2021;29(4):365.
Xia X, Wang Y, Zheng JC. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl Neurodegener. 2022;11(1):1–31.
Peng L, Chen Y, Shi S, Wen H. Stem cell-derived and circulating exosomal microRNAs as new potential tools for diabetic nephropathy management. Stem Cell Res Ther. 2022;13(1):25.
Jin J, Wang Y, Zhao L, Zou W, Tan M, He Q. Exosomal miRNA-215-5p derived from adipose-derived stem cells attenuates epithelial–mesenchymal transition of podocytes by inhibiting ZEB2. Biomed Res Int. 2020;2020:1–14.
Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60(11):3055–66.
Liu Y, Uruno A, Saito R, Matsukawa N, Hishinuma E, Saigusa D, et al. Nrf2 deficiency deteriorates diabetic kidney disease in Akita model mice. Redox Biol. 2022;58: 102525.
Xu X, Sun J, Chang X, Wang J, Luo M, Wintergerst KA, et al. Genetic variants of nuclear factor erythroid-derived 2-like 2 associated with the complications in Han descents with type 2 diabetes mellitus of Northeast China. J Cell Mol Med. 2016;20(11):2078–88.
Ren P, Qian F, Fu L, He W, He Q, Jin J, et al. Adipose-derived stem cell exosomes regulate Nrf2/Keap1 in diabetic nephropathy by targeting FAM129B. Diabetol Metab Syndr. 2023;15(1):1–10.
Cheng K-C, Lin R-J, Cheng J-Y, Wang S-H, Yu J-C, Wu J-C, et al. FAM129B, an antioxidative protein, reduces chemosensitivity by competing with Nrf2 for Keap1 binding. EBioMedicine. 2019;45:25–38.
Zeng G, Lian C, Li W, An H, Han Y, Fang D, et al. Upregulation of FAM129B protects cardiomyocytes from hypoxia/reoxygenation-induced injury by inhibiting apoptosis, oxidative stress, and inflammatory response via enhancing Nrf2/ARE activation. Environ Toxicol. 2022;37(5):1018–31.
Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40(6):828–49.
Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–48.
Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology. 2017;25:393–402.
Xu C, Hou B, He P, Ma P, Yang X, Yang X, et al. Neuroprotective effect of salvianolic acid A against diabetic peripheral neuropathy through modulation of Nrf2. Oxid Med Cell Longevity. 2020;2020:1.
Chen J, Li G, Liu X, Chen K, Wang Y, Qin J, et al. Delivery of miR-130a-3p through adipose-derived stem cell-secreted evs protects against diabetic peripheral neuropathy via DNMT1/NRF2/HIF1α/ACTA1 axis. Mol Neurobiol. 2023;60(7):3678–94.
Tang W, Chen X, Liu H, Lv Q, Zou J, Shi Y, et al. Expression of Nrf2 promotes schwann cell-mediated sciatic nerve recovery in diabetic peripheral neuropathy. Cell Physiol Biochem. 2018;46(5):1879–94.
Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes. 2016;7(7):153.
Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc. 2010;100(5):335–41.
Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(S1):S3–6.
Tentolouris N, Edmonds ME, Jude EB, Vas PR, Manu CA, Tentolouris A, et al. Understanding diabetic foot disease: current status and emerging treatment approaches. Front Endocrinol. 2021;12:753181.
Aumiller WD, Dollahite HA. Pathogenesis and management of diabetic foot ulcers. Jaapa. 2015;28(5):28–34.
Liang ZH, Lin SS, Pan NF, Zhong GY, Qiu ZY, Kuang SJ, et al. UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet Med. 2023;40(2): e14968.
Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag care (Langhorne, Pa). 2014;23(7):31–8.
Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology. 2005;20(1):36–42.
Kaushik K, Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21(11):1137–50.
Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, et al. An essential role of NRF2 in diabetic wound healing. Diabetes. 2016;65(3):780–93.
Wang RY, Liu LH, Liu H, Wu KF, An J, Wang Q, et al. Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int J Mol Med. 2018;42(3):1327–40.
Fan J, Liu H, Wang J, Zeng J, Tan Y, Wang Y, et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J Cell Mol Med. 2021;25(2):652–65.
Sun X, Wang X, Zhao Z, Chen J, Li C, Zhao G. Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochem. 2020;122(8): 151649.
Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:1.
Kuosmanen SM, Kansanen E, Kaikkonen MU, Sihvola V, Pulkkinen K, Jyrkkänen H-K, et al. NRF2 regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res. 2018;46(3):1124–38.
da Costa RM, Rodrigues D, Pereira CA, Silva JF, Alves JV, Lobato NS, et al. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front Pharmacol. 2019;10:382.
Li M, Yu H, Pan H, Zhou X, Ruan Q, Kong D, et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front Pharmacol. 2019;10:1099.
Sun N, Ning B, Hansson KM, Bruce AC, Seaman SA, Zhang C, et al. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci Rep. 2018;8(1):17509.
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14.
Zhang L, Wang Y, Yu F, Li X, Gao H, Li P. CircHIPK3 plays vital roles in cardiovascular disease. Front Cardiovasc Med. 2021;8: 733248.
Wen J, Liao J, Liang J, Chen X-p, Zhang B, Chu L. Circular RNA HIPK3: a key circular RNA in a variety of human cancers. Front Oncol. 2020;10:773.
Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y, et al. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J Mol Endocrinol. 2020;65(3):69–84.
Chen J, Li X, Liu H, Zhong D, Yin K, Li Y, et al. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabetic Med. 2023;40: e15031.
Wang Y-XJ, Griffith J, Ma H, Kwok A, Leung J, Yeung D, et al. Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos Int. 2011;22:91–6.
Phetfong J, Sanvoranart T, Nartprayut K, Nimsanor N, Seenprachawong K, Prachayasittikul V, et al. Osteoporosis: the current status of mesenchymal stem cell-based therapy. Cell Mol Biol Lett. 2016;21(1):1–20.
Foundation NO. America’s bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation; 2002. p. 1–55.
Lewiecki EM, Borges JLC. Bone density testing in clinical practice. Arq Bras Endocrinol Metabol. 2006;50:586–95.
Riegger J, Schoppa A, Ruths L, Haffner-Luntzer M, Ignatius A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. 2023;28(1):76.
Li H, Li D, Ma Z, Qian Z, Kang X, Jin X, et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy. 2018;14(10):1726–41.
Liu H-D, Ren M-X, Li Y, Zhang R-T, Ma N-F, Li T-L, et al. Melatonin alleviates hydrogen peroxide induced oxidative damage in MC3T3-E1 cells and promotes osteogenesis by activating SIRT1. Free Radical Res. 2022;56(1):63–76.
Lin H, Gao X, Chen G, Sun J, Chu J, Jing K, et al. Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun. 2015;460(2):422–7.
Bartell SM, Kim H-N, Ambrogini E, Han L, Iyer S, Serra Ucer S, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5(1):3773.
Ibáñez L, Ferrándiz ML, Brines R, Guede D, Cuadrado A, Alcaraz MJ. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid Med Cell Longevity. 2014;2014:1.
Park CK, Lee Y, Kim KH, Lee ZH, Joo M, Kim H-H. Nrf2 is a novel regulator of bone acquisition. Bone. 2014;63:36–46.
Tan S, Wong J, Sim S, Tjio C, Wong K, Chew J, et al. Mesenchymal stem cell exosomes in bone regenerative strategies—a systematic review of preclinical studies. Mater Today Bio. 2020;7: 100067.
Zhang Z, Zhao S, Sun Z, Zhai C, Xia J, Wen C, et al. Enhancement of the therapeutic efficacy of mesenchymal stem cell-derived exosomes in osteoarthritis. Cell Mol Biol Lett. 2023;28(1):75.
Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57: 100978.
An HS, Anderson PA, Haughton VM, Iatridis JC, Kang JD, Lotz JC, et al. Introduction: disc degeneration: summary. Spine. 2004;29(23):2677–8.
Frapin L, Clouet J, Delplace V, Fusellier M, Guicheux J, Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv Drug Deliv Rev. 2019;149:49–71.
Feng C, Yang M, Lan M, Liu C, Zhang Y, Huang B, et al. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid Med Cell Longevity. 2017;2017:1.
Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L, et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1–15.
Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10(7):510.
Lin J, Chen J, Zhang Z, Xu T, Shao Z, Wang X, et al. Luteoloside inhibits IL-1β-induced apoptosis and catabolism in nucleus pulposus cells and ameliorates intervertebral disk degeneration. Front Pharmacol. 2019;10:868.
Luo X, Huan L, Lin F, Kong F, Sun X, Li F, et al. Ulinastatin ameliorates IL-1β-induced cell dysfunction in human nucleus pulposus cells via Nrf2/NF-κB pathway. Oxid Med Cell Longevity. 2021;2021:1.
Wang K, Hu S, Wang B, Wang J, Wang X, Xu C. Genistein protects intervertebral discs from degeneration via Nrf2-mediated antioxidant defense system: an in vitro and in vivo study. J Cell Physiol. 2019;234(9):16348–56.
Xu G, Lu X, Liu S, Zhang Y, Xu S, Ma X, et al. MSC-derived exosomes ameliorate intervertebral disc degeneration by regulating the Keap1/Nrf2 axis. Stem Cell Rev Rep. 2023;19:1–16.
Yu X, Xu H, Liu Q, Wang Y, Wang S, Lu R, et al. circ_0072464 shuttled by bone mesenchymal stem cell-secreted extracellular vesicles inhibits nucleus pulposus cell ferroptosis to relieve intervertebral disc degeneration. Oxid Med Cell Longevity. 2022;2022:1.
Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23: 101107.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49.
Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc. 2015;12(1):S3–8.
Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–52.
Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31(6):1607–11.
Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011;27(3):719–33.
Zhang S, Jiang W, Ma L, Liu Y, Zhang X, Wang S. Nrf2 transfection enhances the efficacy of human amniotic mesenchymal stem cells to repair lung injury induced by lipopolysaccharide. J Cell Biochem. 2018;119(2):1627–36.
Ren K. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology. 2019;107(3):271–84.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem cells. 2017;35(4):851–8.
Wei J, Chen G, Shi X, Zhou H, Liu M, Chen Y, et al. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem Biophys Res Commun. 2018;500(3):790–6.
Xu L, Zhu Y, Li C, Wang Q, Ma L, Wang J, et al. Small extracellular vesicles derived from Nrf2-overexpressing human amniotic mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting NLRP3. Biol Direct. 2022;17(1):35.
Liu D, Huang S-Y, Sun J-H, Zhang H-C, Cai Q-L, Gao C, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9(1):1–19.
van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450–64.
Luo P, Zhang Q, Zhong T-Y, Chen J-Y, Zhang J-Z, Tian Y, et al. Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect. Mil Med Res. 2022;9(1):1–16.
Wang L, Mehta S, Ahmed Y, Wallace S, Pape MC, Gill SE. Differential mechanisms of septic human pulmonary microvascular endothelial cell barrier dysfunction depending on the presence of neutrophils. Front Immunol. 2018;9:1743.
Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radical Biol Med. 2020;152:175–85.
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–92.
Shen K, Wang X, Wang Y, Jia Y, Zhang Y, Wang K, et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023;62: 102655.
Gong C, Gu Z, Zhang X, Xu Q, Mao G, Pei Z, et al. HMSCs exosome-derived miR-199a-5p attenuates sulfur mustard-associated oxidative stress via the CAV1/NRF2 signalling pathway. J Cell Mol Med. 2023;27(15):2165–82.
Gao X, Patel DJ. NMR studies of echinomycin bisintercalation complexes with d (A1-C2-G3-T4) and d (T1-C2-G3-A4) duplexes in aqueous solution: sequence-dependent formation of Hoogsteen A1. Cntdot. T4 and Watson-Crick T1. Cntdot. A4 base pairs flanking the bisintercalation site. Biochemistry. 1988;27(5):1744–51.
Deng H, Wu L, Liu M, Zhu L, Chen Y, Zhou H, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020;54(6):828–43.
Yang W, Huang C, Wang W, Zhang B, Chen Y, Xie X. Bone mesenchymal stem cell-derived exosomes prevent hyperoxia-induced apoptosis of primary type II alveolar epithelial cells in vitro. PeerJ. 2022;10: e13692.
Chen Q, Liu Y. Heterogeneous groups of alveolar type II cells in lung homeostasis and repair. Am J Physiol Cell Physiol. 2020;319(6):C991–6.
Li H, Niu X, Shi H, Feng M, Du Y, Sun R, et al. circHECTD1 attenuates apoptosis of alveolar epithelial cells in acute lung injury. Lab Invest. 2022;102(9):945–56.
Li Z, Zheng B, Liu C, Zhao X, Zhao Y, Wang X, et al. BMSC-derived exosomes alleviate sepsis-associated acute respiratory distress syndrome by activating the Nrf2 pathway to reverse mitochondrial dysfunction. Stem Cells Int. 2023;2023:1.
Kapur NK, Thayer KL, Zweck E. Cardiogenic shock in the setting of acute myocardial infarction. Methodist Debakey Cardiovasc J. 2020;16(1):16.
Edupuganti MM, Ganga V. Acute myocardial infarction in pregnancy: current diagnosis and management approaches. Indian Heart J. 2019;71(5):367–74.
Xiao H, Wu D, Yang T, Fu W, Yang L, Hu C, et al. Extracellular vesicles derived from HBMSCs improved myocardial infarction through inhibiting zinc finger antisense 1 and activating Akt/Nrf2/HO-1 pathway. Bioengineered. 2022;13(1):905–16.
Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302–10.
Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020;577(7790):405–9.
Hu M, Guo G, Huang Q, Cheng C, Xu R, Li A, et al. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: the role of injured cardiomyocytes-derived exosomes. Cell Death Dis. 2018;9(3):357.
Lalu MM, Mazzarello S, Zlepnig J, Dong YY, Montroy J, McIntyre L, et al. Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): a systematic review and meta-analysis. Stem Cells Transl Med. 2018;7(12):857–66.
Liu X, Li Q, Niu X, Hu B, Chen S, Song W, et al. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int J Biol Sci. 2017;13(2):232.
Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836.
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell death discovery. 2019;5(1):79.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119.
Costa S, Reina-Couto M, Albino-Teixeira A, Sousa T. Statins and oxidative stress in chronic heart failure. Rev Port Cardiol (English Edition). 2016;35(1):41–57.
Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, et al. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8:71–85.
Hu C-M, Chen Y-H, Chiang M-T, Chau L-Y. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110(3):309–16.
Matsushima S, Kinugawa S, Ide T, Matsusaka H, Inoue N, Ohta Y, et al. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am J Physiol Heart Circ Physiol. 2006;291(5):H2237–45.
Wu X, Huang L, Liu J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy. Exp Ther Med. 2021;22(1):1–10.
Yarmohammadi F, Hayes AW, Karimi G. The cardioprotective effects of hydrogen sulfide by targeting endoplasmic reticulum stress and the Nrf2 signaling pathway: a review. BioFactors. 2021;47(5):701–12.
Xie S, Deng W, Chen J, Wu Q-Q, Li H, Wang J, et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. 2020;16(1):12.
Chen M, Chen J, Huang W, Li C, Luo H, Xue Z, et al. Exosomes from human induced pluripotent stem cells derived mesenchymal stem cells improved myocardial injury caused by severe acute pancreatitis through activating Akt/Nrf2/HO-1 axis. Cell Cycle. 2022;21(15):1578–89.
Huang H, Xu Z, Qi Y, Zhang W, Zhang C, Jiang M, et al. Exosomes from SIRT1-overexpressing ADSCs restore cardiac function by improving angiogenic function of EPCs. Mol Ther Nucleic Acids. 2020;21:737–50.
Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19(1):1–29.
Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 2019;24:1–10.
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869–81.
Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346-e.
Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):1–13.
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:1–9.
Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25(2):465–79.
Zhang Y, Li Y, Wang Q, Zheng D, Feng X, Zhao W, et al. Attenuation of hepatic ischemia-reperfusion injury by adipose stem cell-derived exosome treatment via ERK1/2 and GSK-3β signaling pathways. Int J Mol Med. 2022;49(2):1–12.
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–84.
Suzuki T, Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free Radical Biol Med. 2015;88:93–100.
Mousa AM, El-Sammad NM, Hassan SK, Madboli AENA, Hashim AN, Moustafa ES, et al. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement Altern Med. 2019;19:1–13.
Zhao S, Huang M, Yan L, Zhang H, Shi C, Liu J, et al. Exosomes derived from baicalin-pretreated mesenchymal stem cells alleviate hepatocyte ferroptosis after acute liver injury via the Keap1-NRF2 pathway. Oxid Med Cell Longevity. 2022;2022:8287227.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22.
Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Yang M, Cui Y, Song J, Cui C, Wang L, Liang K, et al. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem Biophys Res Commun. 2021;546:74–82.
Yan C, Sun W, Wang X, Long J, Liu X, Feng Z, et al. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol Nutr Food Res. 2016;60(5):1139–49.
Zhao X-J, Yu H-W, Yang Y-Z, Wu W-Y, Chen T-Y, Jia K-K, et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–37.
Du X, Li H, Han X, Ma W. Mesenchymal stem cells-derived exosomal miR-24-3p ameliorates non-alcohol fatty liver disease by targeting Keap-1. Biochem Biophys Res Commun. 2022;637:331–40.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–83.
Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol. 2019;70(3):531–44.
Vergani L. Fatty acids and effects on in vitro and in vivo models of liver steatosis. Curr Med Chem. 2019;26(19):3439–56.
Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants. 2021;10(2):174.
Bruno S, Pasquino C, Sanchez MBH, Tapparo M, Figliolini F, Grange C, et al. HLSC-derived extracellular vesicles attenuate liver fibrosis and inflammation in a murine model of non-alcoholic steatohepatitis. Mol Ther. 2020;28(2):479–89.
Kang Y, Song Y, Luo Y, Song J, Li C, Yang S, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radical Biol Med. 2022;192:25–36.
Vahidinia Z, Joghataei MT, Beyer C, Karimian M, Tameh AA. G-protein-coupled receptors and ischemic stroke: a focus on molecular function and therapeutic potential. Mol Neurobiol. 2021. https://doi.org/10.1007/s12035-021-02435-5.
Vahidinia Z, Karimian M, Joghataei MT. Neurosteroids and their receptors in ischemic stroke: from molecular mechanisms to therapeutic opportunities. Pharm Res. 2020;160:105163.
Alessandrini M, Preynat-Seauve O, De Bruin K, Pepper MS. Stem cell therapy for neurological disorders. S Afr Med J. 2019;109(8b):S71–8.
Khassafi N, Zahraei Z, Vahidinia Z, Karimian M, Azami TA. Calcitriol pretreatment attenuates glutamate neurotoxicity by regulating NMDAR and CYP46A1 gene expression in rats subjected to transient middle cerebral artery occlusion. J Neuropathol Exp Neurol. 2022;81(4):252–9.
Hill AF. Extracellular vesicles and neurodegenerative diseases. J Neurosci. 2019;39(47):9269–73.
Wang T, Jian Z, Baskys A, Yang J, Li J, Guo H, et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257: 120264.
Shao C, Chen Y, Yang T, Zhao H, Li D. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Rev Rep. 2022;18(3):1127–42.
Li X, Zhang X, Liu Y, Pan R, Liang X, Huang L, et al. Exosomes derived from mesenchyml stem cells ameliorate oxygen-glucose deprivation/reoxygenation-induced neuronal injury via transferring MicroRNA-194 and targeting Bach1. Tissue Cell. 2021;73: 101651.
Xu L, Ji H, Jiang Y, Cai L, Lai X, Wu F, et al. Exosomes derived from CircAkap7-modified adipose-derived mesenchymal stem cells protect against cerebral ischemic injury. Front Cell Dev Biol. 2020;8:1066.
Liu Q, Tan Y, Qu T, Zhang J, Duan X, Xu H, et al. Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro. Life Sci. 2020;254: 117772.
Huang T, Tong H, Zhou H, Wang J, Hu L, Wang Y, et al. ADSC-exosomes alleviate MTX-induced rat neuronal damage by activating Nrf2-ARE pathway. J Mol Neurosci. 2022;72(6):1334–44.
Liu J, Huang J, Zhang Z, Zhang R, Sun Q, Zhang Z, et al. Mesenchymal stem cell-derived exosomes ameliorate delayed neurocognitive recovery in aged mice by inhibiting Hippocampus ferroptosis via activating SIRT1/nrf2/HO-1 signaling pathway. Oxid Med Cell Longevity. 2022;2022:1.
Hu L-T, Wang B-Y, Fan Y-H, He Z-Y, Zheng W-X. Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage. Neural Regen Res. 2023;18(3):560.
He S, Wang Q, Chen L, He YJ, Wang X, Qu S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson’s disease model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med. 2023;21(1):747.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194.
Van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–46.
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265rs6-sr6.
Zhao P, Sui BD, Liu N, Lv YJ, Zheng CX, Lu YB, et al. Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 2017;16(5):1083–93.
Mistriotis P, Andreadis ST. Vascular aging: molecular mechanisms and potential treatments for vascular rejuvenation. Ageing Res Rev. 2017;37:94–116.
Strong AL, Bowles AC, MacCrimmon CP, Frazier TP, Lee SJ, Wu X, et al. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Transl Med. 2015;4(6):632–42.
Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):109.
Han C, Zhou J, Liu B, Liang C, Pan X, Zhang Y, et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater Sci Eng C. 2019;99:322–32.
Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, et al. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-κB/TNF-α signaling pathway. Oxid Med Cell Longevity. 2019;2019:1.
Tofiño-Vian M, Guillén MI, Perez del Caz MD, Castejón MA, Alcaraz MJ. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longevity. 2017;2017:1.
Zhang H, Davies KJ, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–36.
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301(2):H363–72.
Ebrahimi KB, Cano M, Rhee J, Datta S, Wang L, Handa JT. Oxidative stress induces an interactive decline in Wnt and Nrf2 signaling in degenerating retinal pigment epithelium. Antioxid Redox Signal. 2018;29(4):389–407.
Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165(6):1361–74.
Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K, Zhang J, et al. hPMSCs-derived exosomal miRNA-21 protects against aging-related oxidative damage of CD4+ T cells by targeting the PTEN/PI3K-Nrf2 axis. Front Immunol. 2021;12: 780897.
Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci USA. 2015;112(12):3722–7.
Ni J, Hou X, Wang X, Shi Y, Xu L, Zheng X, et al. 3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression. Cell Death Dis. 2019;10(5):355.
Zhao S, Wu W, Liao J, Zhang X, Shen M, Li X, et al. Molecular mechanisms underlying the renal protective effects of coenzyme Q10 in acute kidney injury. Cell Mol Biol Lett. 2022;27(1):57.
Huang J, Cao H, Cui B, Ma X, Gao L, Yu C, et al. Mesenchymal stem cells-derived exosomes ameliorate ischemia/reperfusion induced acute kidney injury in a porcine model. Front Cell Dev Biol. 2022;10: 899869.
Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–61.
Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359.
Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, et al. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol. 2015;26(10):2349.
Sanz AB, Santamari B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol. 2008;19(9):1634–42.
Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, et al. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res. 2016;41(2):119–28.
Lim SW, Kim KW, Kim BM, Shin YJ, Luo K, Quan Y, et al. Alleviation of renal ischemia/reperfusion injury by exosomes from induced pluripotent stem cell-derived mesenchymal stem cells. Korean J Intern Med. 2022;37(2):411.
Cao H, Cheng Y, Gao H, Zhuang J, Zhang W, Bian Q, et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia–reperfusion injury. ACS Nano. 2020;14(4):4014–26.
Zhang J, Su R, Wang Y, Wang H, Li S, Yang X, et al. Protective effect of small extracellular vesicles (EVs) derived from ACE2-modified human umbilical cord mesenchymal stem cells against renal ischemia–reperfusion injury. Nephrology. 2023. https://doi.org/10.1111/nep.14237.
Maver T, Maver U, Kleinschek KS, Raščan IM, Smrke DM. Advanced therapies of skin injuries. Wien Klin Wochenschr. 2015;127:187–98.
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):1–14.
Zhang Z, Zi Z, Lee EE, Zhao J, Contreras DC, South AP, et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat Med. 2018;24(5):617–27.
El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10(8):751–2.
Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18(8):1286–90.
Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298–308.
Schäfer M, Farwanah H, Willrodt AH, Huebner AJ, Sandhoff K, Roop D, et al. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol Med. 2012;4(5):364–79.
Kerns ML, Miller RJ, Mazhar M, Byrd AS, Archer NK, Pinkser BL, et al. Pathogenic and therapeutic role for NRF2 signaling in ultraviolet light–induced skin pigmentation. JCI insight. 2020;5(20): e139342.
Telorack M, Meyer M, Ingold I, Conrad M, Bloch W, Werner S. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genet. 2016;12(1): e1005800.
Gao W, Wang X, Si Y, Pang J, Liu H, Li S, et al. Exosome derived from ADSCs attenuates ultraviolet b-mediated photoaging in human dermal fibroblasts. Photochem Photobiol. 2021;97(4):795–804.
Che J, Wang H, Dong J, Wu Y, Zhang H, Fu L, et al. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci Ther. 2023. https://doi.org/10.1111/cns.14454.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZV and AAT contributed to the conception, design and drafting of the manuscript. SHB, MI and ESH contributed to data collection, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vahidinia, Z., Azami Tameh, A., Barati, S. et al. Nrf2 activation: a key mechanism in stem cell exosomes-mediated therapies. Cell Mol Biol Lett 29, 30 (2024). https://doi.org/10.1186/s11658-024-00551-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11658-024-00551-3