Summary
The innate immune system uses a variety of germline-encoded pattern-recognition receptors that recognize conserved microbial structures or pathogen-associated molecular patterns, such as those that occur in the bacterial cell-wall components peptidoglycan and lipopolysaccharide. Recent studies have highlighted the importance of Toll-like receptors (TLRs) as a family of pattern-recognition receptors in mammals that can discriminate between chemically diverse classes of microbial products. First identified on the basis of sequence similarity with the Drosophila protein Toll, TLRs are members of an ancient superfamily of proteins, which includes related proteins in invertebrates and plants. TLRs activate innate immune defense reactions, such as the release of inflammatory cytokines, but increasing evidence supports an additional critical role for TLRs in orchestrating the development of adaptive immune responses. The sequence similarity between the intracellular domains of the TLRs and the mammalian interleukin-1 and interleukin-18 cytokine receptors reflects the use of a common intracellular signal-transduction cascade triggered by these receptor classes. But more recent findings have demonstrated that there are in fact TLR-specific signaling pathways and cellular responses. Thus, TLRs function as sentinels of the mammalian immune system that can discriminate between diverse pathogen-associated molecular patterns and then elicit pathogen-specific cellular immune responses.
Similar content being viewed by others
Gene organization and evolutionary history
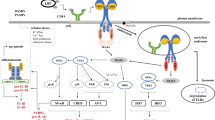
The Drosophila genome contains nine genes that encode Toll and related receptors (dToll1 - dToll9), whereas ten Toll-like receptor (TLR) genes have been identified in mice and humans [1,2,3,4,5,6]. The human and murine TLR2 genes and the murine TLR4 gene have two 5' non-coding exons followed by a third coding exon. In contrast, the human TLR4 gene has an additional 5' non-coding exon [7]. Gene-mapping studies have revealed that TLR genes are dispersed throughout the mammalian genome. Specifically, human TLR genes reside on chromosomes 4 (TLR1, TLR 2, TLR 3, TLR6 and TLR10), 9 (TLR4), 1 (TLR5), X (TLR7 and TLR8) and 3 (TLR9; see Figure 1). DNA sequence comparisons of genes encoding Toll-related proteins in Drosophila, reptiles, birds and in mammals have revealed that the genes are well conserved and have evolved independently from a common ancestor gene [8].
Structural features of human members of the TLR protein family and the archetypal Drosophila Toll protein. Toll and its relatives are characterized by an amino-terminal extracellular leucine-rich repeat (LRR) domain, which is probably involved in ligand binding, and an intracellular Toll/interleukin-1 receptor (TIR) domain required for signal transduction. Known ligands of different TLRs and chromosomal locations of the human TLR genes are indicated. Red arrows indicate a possible dimerization between TLR1, TLR2 and TLR6. TLR9 is normally expressed intracellularly. Abbreviations: MALP-2, macrophage-activating lipopeptide-2; LAM, lipoarabinomannan; details of other ligands mentioned in the figure are discussed in the text.
Characteristic structural features
As shown in Figure 1, TLRs are a family of type I transmembrane receptors characterized by an extracellular amino terminus. They have an amino-terminal leucine-rich repeat (LRR) domain and a carboxy-terminal intracellular tail containing a conserved region called the Toll/interleukin-1 receptor (TIR) homology domain. The extracellular domain contains a varying number of LRR domains, which are presumably involved in ligand binding but may also be necessary for TLR dimerization. The extracellular domain of TLR4 is highly polymorphic compared with the transmembrane and proximal cytoplasmic domains of the protein [9]. In addition, the extracellular domain of TLR4 contains an 82 amino-acid region that is highly variable and contributes to species-specific differences in recognition of lipopolysaccharide (LPS), the prototypic TLR4 ligand [10]. The intracellular TIR domain region spans over 200 amino acids and itself contains three highly conserved regions [11]. The TIR domain mediates protein-protein interactions between the TLRs and signal-transduction components (see the Signaling mechanism section); it is the defining motif of the TLR/interleukin-1 receptor superfamily, which includes the receptors for the cytokines interleukin (IL)-1 and IL-18 and is likely to be one of the earliest signaling domains to have evolved [8]. A TIR-like motif is also present in several plant receptors that are known to confer disease resistance [11], including the N protein, which conveys resistance to the tobacco mosaic virus, or the L6 flax rust resistance protein. Although the IL-1 receptor and TLRs have very different extracellular domains, their TIR domains allow both receptor types to activate similar signal-transduction pathways.
Localization and function
TLR distribution
Consistent with their role in pathogen recognition, TLR family members are expressed by cells involved in the first line of host defense, including neutrophils, macrophages, dendritic cells, dermal endothelial cells and mucosal epithelial cells. TLR2 and TLR4, which are the major receptors for bacterial lipoproteins and LPS, respectively, are also expressed on B and T cells, which mediate the more complex adaptive immunity via a large repertoire of antigen-specific immunoglobulin-class receptors [12]. With the exception of TLR9, which is an intracellular receptor, most TLRs are expressed on the cell surface. Interestingly, the subcellular localization of TLR4 has been shown to differ in macrophages and intestinal epithelial cells. In macrophages, TLR4 is expressed on the cell surface and is internalized following engagement of the ligand, whereas in epithelial cells it resides in the Golgi apparatus [13].
TLR agonists and their recognition
The first indication that TLRs may function as pattern-recognition receptors in mammals came from the discovery that the phenotype of lps mice, which do not show the systemic inflammatory response usually triggered by LPS, resulted from loss-of-function mutation in the TLR4 gene, thus establishing TLR4 as a receptor for the LPS of Gram-negative bacteria [14]. Subsequent studies using TLR4 knockout mice corroborated this finding [15]. Ex vivo studies, later confirmed by gene-knockout experiments, showed that there are additional agonists for TLRs. A variety of chemically diverse pathogen-associated molecular patterns are now known to be TLR agonists, as indicated in Figure 1. Studies using TLR2 and TLR4 knockout mice have revealed that these receptors mediate cellular responses to cell-wall components of Gram-positive and Gram-negative bacteria, respectively [16]. These components include LPS, other bacterial glycolipids, peptidoglycan, and bacterial lipoproteins. TLR4 has also been implicated in the recognition of coat proteins (for example, protein F) of the respiratory syncytial virus (RSV), which is a major cause of bronchiolitis in infants [17]. TLR3, TLR5 and TLR9 have been implicated in the recognition of viral double-stranded RNA, bacterial flagellin and bacterial CpG DNA, respectively [18,19,20]. Recently, TLR7 was shown to be involved in cellular activation by small anti-viral compounds, including imidazoquinoline compounds, although the natural ligand for this receptor remains unknown [21]. In addition to their role in pathogen recognition, recent evidence suggests that some TLRs respond to endogenous factors produced by stressed or damaged cells. These factors include heat-shock proteins (Hsps) [22,23,24], fragmentation products of the extracellular matrix components fibronectin and hyaluronan [25,26], and mammalian chromatin [27]. The putative capacity of TLRs to heterodimerize may explain, at least in part, how TLRs are capable of recognizing so many chemically diverse agonists.
Little is known about how microbial products activate TLRs. In Drosophila, Toll does not directly bind microbial products, but instead binds a proteolytic fragment of the secreted growth factor Spaetzle. The current view is that microbial antigens interact with a yet unidentified recognition receptor upstream of Toll to activate zymogens in the fly's hemolymph, ultimately leading to cleavage of Spaetzle to its active Toll ligand form [12]. In contrast, TLR4-dependent recognition of LPS by mammalian cells requires two accessory proteins: the high-affinity LPS acceptor protein CD14, a glycosylphosphatidylinositol (GPI)-anchored protein, and the small secreted protein MD2, which associates with the extracellular domain of TLR4 [28,29,30]. Physical contact between LPS and TLR4 was recently demonstrated [31,32], but to date, there is no evidence for direct ligand binding and recognition for all other TLRs.
TLR function
Collectively, TLRs function to alert the immune system to the presence of microorganisms. Engagement of TLRs with their ligands leads to the production of various pro-inflammatory cytokines, chemokines, and effector molecules, depending on the cell type that is activated [33,34,35]. TLR knockout mice have been used to study the roles of TLRs in the immune response against different pathogens in vivo. For example, TLR2-deficient mice are highly susceptible to lethal infections with the sepsis-causing pathogen Staphylococcus aureus [36]. In contrast, infections with Haemophilus influenzae or with RSV persist longer in the lungs of TLR4-deficient mice than in those of control animals [17,37].
Signaling mechanism
Activation of signaling through TIR domains results in recruitment of the cytoplasmic adaptor proteins MyD88 and TOLLIP (Toll interacting protein) [38] (Figure 2). The IL-1 receptor-associated kinases IRAK-1 and IRAK-2 interact with the death domain of MyD88, a motif found in many apoptosis-inducing signaling molecules, and are recruited to the TLR complex. TOLLIP can also recruit IRAKs to the complex, albeit with different kinetics. Upon recruitment, IRAK-1 and IRAK-2 associate with TNF receptor-associated factor 6 (TRAF6), another adaptor protein. Very recently, two groups [39,40] have identified another serine-threonine kinase, termed IRAK-4, as an early component of the TLR-signaling cascade, possibly acting upstream of IRAK-1. TRAF6 induces activation of TGF-β-activated kinase (TAK1) and the mitogen-activated protein (MAP) kinase kinase MKK6, which, in turn, activate the transcription factor NF-κB, the c-Jun N-terminal kinase (JNK) and the p38 MAP kinase. The importance of MyD88 and TRAF6 in TLR signaling has been confirmed by targeted gene disruption. Homologs of many molecules involved in TLR signaling in mammals have been identified in Drosophila.
TLR signal transduction pathways. All TLR proteins utilize the adapter protein MyD88 to activate a signaling pathway leading to the activation of MAP kinases and the transcription factor NF-κB in a TRAF-6-dependent manner. These signaling events culminate in expression of the pro-inflammatory cytokines IL-1β and TNF-α. TLR4 uses an additional adapter molecule, called TIRAP or Mal, to induce the expression of IL-6 and IFN-β. Via an autocrine/paracrine mechanism, IFN-β engages the type I IFN receptor (IFNAR), which leads to the activation of the Jak and Tyk kinases. These kinases phosphorylate the transcription factor STAT1 at tyrosine 701 and serine 727, thus allowing STAT1 to translocate to the nucleus. Nuclear STAT1, together with NF-κB, activates the STAT1-dependent genes inducible nitric oxide synthase (iNOS) and IFN-γ-inducible protein (IP-10). The + symbols indicate that the two contributing signal transduction pathways must be triggered concomitantly in order to get gene activation.
An additional TLR4 signaling pathway was revealed through the observation that certain LPS-induced responses did not require MyD88 [41]. Subsequently, two groups [39,40] identified a molecule called TIR domain-containing adapter protein (TIRAP) or MyD88-adapter-like (Mal), which interacts with TLR4 and mediates MyD88-independent TLR4 signaling. Although downstream components of this pathway remain to be identified, engagement of TLR4 with its ligand was recently shown to induce the secretion of the anti-viral interferon-β (IFN-β) via a TIRAP/Mal-dependent, but MyD88-independent, mechanism [42]. Autocrine or paracrine production of IFN-β was also shown to be required for the expression of selected genes that could not be induced via engagement of TLR2, including inducible nitric oxide synthase (iNOS) or interferon inducible protein 10 (IP10; Figure 2).
Frontiers
It is clear that engagement of TLRs activates a variety of inflammatory and innate immune responses in mammals. Ongoing efforts in many laboratories have led to the identification of TLR-specific signaling components and cellular responses, and more will be discovered in the future. It is also likely that TLRs work in combination with additional pattern-recognition receptors and co-receptors to add further diversity to their functions in vivo. How the host integrates the information that is signaled through TLRs and any co-receptors will ultimately control the progression of the immune response to pathogens. Understanding this process will undoubtedly lead to the development of novel therapeutics and immune adjuvants.
References
Chuang T, Ulevitch RJ: Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001, 1518: 157-161. 10.1016/S0167-4781(00)00289-X. The cloning of the human TLR10 gene.
Du X, Poltorak A, Wei Y, Beutler B: Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000, 11: 362-371. The identification of mouse TLR7, TLR8 and TLR9.
Chuang TH, Ulevitch RJ: Cloning and characterization of a subfamily of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000, 11: 372-378. The cloning and identifcation of human TLR7, TLR8 and TLR9.
Medzhitov R, Preston-Hurlburt P, Janeway CA: A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997, 388: 394-397. 10.1038/41131. This is the first report showing a role for TLRs in mammalian immunity.
Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Takeda K, Akira S: TLR6: a novel member of an expanding toll-like receptor family. Gene. 1999, 231: 59-65. 10.1016/S0378-1119(99)00098-0. This paper reports the cloning of human and mouse TLR6.
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF: A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998, 95: 588-593. 10.1073/pnas.95.2.588. The authors describe the molecular cloning of five human TLRs (TLR1-TLR5).
Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B: PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000, 275: 9773-9781. 10.1074/jbc.275.13.9773. The authors show that the transcription factors PU.1 and ICSBP participate in the basal regulation of human TLR4 in myeloid cells.
Kimbrell DA, Beutler B: The evolution and genetics of innate immunity. Nat Rev Genet. 2001, 2: 256-267. 10.1038/35066006. The authors discuss the high degree of similarities at the molecular level between the Drosophila and mammalian immune system.
Smirnova I, Poltorak A, Chan EK, McBride C, Beutler B: Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4). Genome Biol. 2000, 1: research002.1-002.10. 10.1186/gb-2000-1-1-research002. This study shows that TLR4 is a polymorphic protein both in different animal species and strains.
Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI: Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002, 3: 354-359. 10.1038/ni777. This paper identifies a region of the human TLR4 that is hypervariable across species and that discriminates between different acylation states of Pseudomonas aeruginosa LPS.
O'Neill LA, Dinarello CA: The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000, 21: 206-209. 10.1016/S0167-5699(00)01611-X. The authors describe in detail the IL1-receptor/TLR superfamily.
Imler JL, Hoffmann JA: Toll receptors in innate immunity. Trends Cell Biol. 2001, 11: 304-311. 10.1016/S0962-8924(01)02004-9. The authors review the current understanding of the role of Toll-related receptors in both Drosophila and mammalian immunity.
Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A: Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002, 195: 559-570. 10.1084/jem.20011788. The authors show by immunostaining that TLR4 is located within the Golgi apparatus in an intestinal epithelial cell line.
Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998, 282: 2085-2088. 10.1126/science.282.5396.2085. In this study, the authors prove that the lps and Tlr4 loci are identical.
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S: Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999, 162: 3749-3752. This study corroborates the finding in [14] by using TLR4-knockout mice.
Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S: Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999, 11: 443-451. Using knockout mice, the authors demonstrate that TLR2 and TLR4 are involved in the recognition of cell-wall components of Gram-positive and Gram-negative bacteria, respectively.
Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW: Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000, 1: 398-401. 10.1038/80833. This was the first paper to show that viral products can activate TLRs.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA: Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001, 413: 732-738. 10.1038/35099560. Using gene knockout experiments, the authors identified double-stranded RNA as an agonist for TLR3.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S: A Toll-like receptor recognizes bacterial DNA. Nature. 2000, 408: 740-745. 10.1038/35047123. This paper shows that bacterial DNA containing CpG motifs activates immune cells through TLR9.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A: The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001, 410: 1099-1103. 10.1038/35074106. Using knockout mice, the authors show that TLR5 is involved in the recognition of bacterial flagellin, the principal component of bacterial flagella.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S: Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002, 3: 196-200. 10.1038/ni758. This study shows that imidazoquinoline compounds, which are potent anti-tumor and anti-viral agents, activate immune cells through TLR7.
Ohashi K, Burkart V, Flohe S, Kolb H: Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000, 164: 558-561. This is the first demonstration that TLRs can be activated by endogenous ligands.
Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D: Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001, 89: 244-250. This paper and [24-27] show that endogenous ligands, including mammalian DNA, stimulate TLRs.
Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H: HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002, 277: 15107-15112. 10.1074/jbc.M111204200. See [23].
Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF: The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001, 276: 10229-10233. 10.1074/jbc.M100099200. See [23].
Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC: Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002, 195: 99-111. 10.1084/jem.20001858. See [23].
Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A: Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002, 416: 603-607. 10.1038/416603a. See [23].
Ulevitch RJ, Tobias PS: Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995, 13: 437-457. 10.1146/annurev.iy.13.040195.002253. In this review, the authors provide comprehensive information about the LPS receptor complex.
Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M: MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999, 189: 1777-1782. 10.1084/jem.189.11.1777. This study and [30] established that MD-2 is a required component of the LPS signaling complex.
Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K, Golenbock DT: Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001, 194: 79-88. 10.1084/jem.194.1.79. See [29].
Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B: Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000, 97: 2163-2167. 10.1073/pnas.040565397. This article and [32] indicate that TLR4 directly recognizes LPS.
Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, et al: Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000, 105: 497-504. See [31].
Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN: Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001, 69: 1477-1482. 10.1128/IAI.69.3.1477-1482.2001. The authors of this study and [34] show that different forms of LPS bind to different TLRs, leading to differential response.
Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ: Differential roles of Toll-like receptors in the elicitation of pro-inflammatory responses by macrophages. Ann Rheum Dis. 2001, 60: iii6-12. See [33].
Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP: Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human β-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001, 31: 3131-3137. 10.1002/1521-4141(200111)31:11<3131::AID-IMMU3131>3.0.CO;2-G. The exposure of human epithelial cells to bacterial lipoprotein is shown to induce β-defensin 2 expression.
Takeuchi O, Hoshino K, Akira S: Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000, 165: 5392-5396. This study shows that TLR2- and MyD88-deficient mice are highly susceptible to lethal Staphylococcus aureus infection.
Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM: Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002, 168: 810-815. This study demonstrates a role for TLR4 in sensing and affecting initial innate response to Haemophilus influenzae.
Akira S: Toll-like receptors and innate immunity. Adv Immunol. 2001, 78: 1-56. This is a comprehensive review about TLRs and their signaling pathways.
Horng T, Barton GM, Medzhitov R: TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001, 2: 835-841. 10.1038/ni0901-835. In this paper and [41] a new TIR-domain-containing adaptor molecule, TIRAP/Mal, is characterized, which controls activation of MyD88-independent signaling downstream of TLR4.
Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al: Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001, 413: 78-83. 10.1038/35092578. See [39].
Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S: Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol. 2001, 166: 5688-5694. LPS-induced expression of costimulatory molecules by dendritic cells is maintained in MyD88 knockout mice, indicating that there is a MyD88-independent pathway.
Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN: TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat Immunol. 2002, 3: 392-398. 10.1038/ni774. TLR4-induced β-interferon is shown to be responsible for the differential response that is induced by TLR2 and TLR4 agonists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armant, M.A., Fenton, M.J. Toll-like receptors: a family of pattern-recognition receptors in mammals. Genome Biol 3, reviews3011.1 (2002). https://doi.org/10.1186/gb-2002-3-8-reviews3011
Published:
DOI: https://doi.org/10.1186/gb-2002-3-8-reviews3011