Abstract
Introduction
Transthoracic echocardiography (TTE) is a useful tool for minimally invasive hemodynamic monitoring in the ICU. Dynamic indices (such as the inferior vena cava distensibility index (dIVC)) can be used to predict fluid responsiveness in mechanically ventilated patients. Although quantitative use of the dIVC has been validated, the routinely used qualitative (visual) approach had not been assessed before the present study.
Methods
Qualitative and quantitative assessments of the dIVC were compared in a prospective, observational study. After operators with differing levels in critical care echocardiography had derived a qualitative dIVC, the last (expert) operator performed a standard, numeric measurement of the dIVC (referred to as the quantitative dIVC). Two groups of patients were separated into two groups: group (dIVC < 18%) and group (dIVC ≥ 18%).
Results
In total, 114 patients were assessed for inclusion, and 97 (63 men and 34 women) were included. The mean sensitivity and specificity values for qualitative assessment of the dIVC by an intensivist were 80.7% and 93.7%, respectively. A qualitative evaluation detected all quantitative dIVCs >40%. Most of the errors concerned quantitative dIVCs of between 15% and 30%. In the dIVC <18% group, two qualitative evaluation errors were noted for quantitative dIVCs of between 0 and 10%. The average of positive predictive values and negative predictive values for qualitative assessment of the dIVC by residents, intensivists and cardiologists were 83%, 83%, and 90%; and 92%, 94%, and 90%, respectively. The Fleiss kappa for all operators was estimated to be 0.68, corresponding to substantial agreement.
Conclusion
The qualitative dIVC is a rather easy and reliable assessment for extreme numeric values. It has a gray zone between 15% and 30%. The highest and lowest limitations of the gray area are rather tedious to define.
Despite reliability of the qualitative assessment when it comes to extreme to numerical values, the quantitative dIVC measurement must always be done within a hemodynamic assessment for intensive care patients. The qualitative approach can be easily integrated into a fast hemodynamic evaluation by using portable ultrasound scanner for out-of-hospital patients.
Similar content being viewed by others
Introduction
The management of patients with acute circulatory failure requires knowledge of the preload dependence state. Dynamic indices predict the response to volume expansion with greater accuracy than do static indices. This assessment is very important because inadequate fluid replacement or inappropriate infusion of catecholamines can lead to significant adverse effects[1]. Several of these dynamic indices (including those derived by echocardiography) have been validated in ICU patients[2–8]. Indeed, transthoracic echocardiography (TTE) is a valuable, minimally invasive, rapid hemodynamic monitoring tool for use in the ICU. TTE provides information not only on dynamic indices of preload dependence but also on overall contractility of the heart, filling pressures, and the impact of many diseases (septic shock, acute respiratory distress syndrome, cardiogenic shock, and so on). Echography for measuring hemodynamic parameters are easy to learn, and the corresponding indices have been assessed on several occasions[9–12]. Of these, the superior vena cava collapsibility index (cSVC)[5] and the inferior vena cava distensibility index (dIVC) have been widely validated[6, 7, 13–17].
Several studies have demonstrated the acceptability of qualitative (visual) measurement of the left ventricular ejection fraction when compared with quantitative reference measurements[18, 19]. Similarly, Vieillard-Baron et al.[20] validated the use of qualitative transesophageal echocardiography in the hemodynamic assessment of septic shock, which included the cSVC as an indicator of preload dependency. In clinical practice, many operators solely perform qualitative analyses of data from bedside echocardiography measurements, considered as valid as the quantitative measurement, and less time consuming. To the best of our knowledge, qualitative measurement of the dIVC in mechanically ventilated ICU patients has not been compared with quantitative measurement. Although this index is easily calculated on ultrasound with the use of the mean value from three measures[21–23], the threshold for discriminating between nonresponders and responders is small (12% or 18%, depending on the equation and the IVC analysis site used)[6, 7]. The quantitative assessment is completed by one individual operator who calculated the average of three measures. This can be considered time consuming.
The relevance and reproducibility of a qualitative approach for calculating the dIVC has not previously been established. We therefore sought to assess the accuracy of a qualitative approach of dIVC and compare it with that of the quantitative method. We also sought to evaluate the impact of the operator's level of experience on the accuracy of the qualitative approach.
Materials and methods
Patients
This was a prospective, observational study performed in a surgical ICU at a university hospital. The noninterventional study's objectives and data-collection procedures were approved by the Institutional Review Board (IRB) for human subjects at our hospital (N°20/2010 Commission d’Evaluation Ethique des Recherches Non Interventionnelles, Amiens University Hospital, Amiens, France). Informed consent was waived because the IRB considered the protocol to be part of usual care in clinical practice.
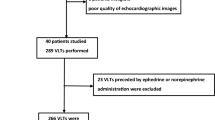
The main inclusion criteria were as follows: (a) patients undergoing TTE for hemodynamic assessment; (b) age older than 18 years; and (c) sedated and mechanically ventilated patients who were synchronized with the respirator (the Servo-i, Maquet, Germany). The main exclusion criteria were as follows: (a) patients with changes in hemodynamic status during the various measurement (changes in catecholamine doses, fluid expansion, and so on), and (b) poor visualization of the IVC by even the most experienced operator.
Study protocol
The operators who performed a qualitative assessment of dIVC had various levels of experience and training in TTE: (a) four anesthesiology residents (n = 4), considered as operators with a basic level of experience (level 1) of critical care echocardiography (less than 30 TTEs), (b) two intensivists (n = 2) with an advanced level of experience (level 2) in critical care echocardiography, and (c) two cardiologists (n = 2) with high levels of expertise (level 3) in critical care echocardiography. The levels of echocardiography skills and knowledge were those described in the guidelines issued by the American College of Chest Physicians and the French Society of Intensive Care[9] and the international expert statement on training standards for critical care ultrasonography[10].
To avoid getting biased results by the different actors, the qualitative measure was completed by a resident, then an intensivist, and last by the cardiologist. The quantitative assessment was completed at the end of the data collection and solely by one individual who calculated the average of three measures.
Qualitative evaluation
The IVC was visualized with a subcostal sonographic approach. Distensibility was assessed in M-mode (coupled to the 2D mode) just upstream of the junction of the hepatic veins, once the M-mode cursor was perpendicular to the IVC. The ultrasound system used was an EnVisor (Philips, Suresnes, France) with an S4-2 Hz probe. Operators performing echocardiographic examination of the IVC were blinded to the patient's fluid-responsiveness status. Each operator had to answer exactly three questions: Is the variation of dIVC greater than 18%, less than 18%, or not viewable?
Quantitative evaluation
The dIVC was measured last by the most expert operator (that is, one of the two cardiologists). The image was obtained as described earlier, and the dIVC was calculated according to the following equation: (maximum diameter on inspiration _ minimum diameter on expiration)/minimum diameter on expiration. The speed of the trace was 12.5 mm/sec.
The quantitative reference cut-off value used for assessing preload dependence was 18%, in line with the study by Barbier et al.[7]. Patients with dIVC <18% were considered to be likely nonresponders in the event of fluid challenge. No fluid challenges were performed in our study. The quantitative dIVC analyzed here was the mean of three evaluations carried out successively by the same operator.
The respiratory pulse pressure variation (PPvariation) was expressed as a percentage and calculated according to the following equation: PPvariation = ((systolic blood pressure (SBP)max - diastolic blood pressure (DBP)max) - (SBPmin - DBPmin))/((SBPmax - DBPmax) + (SBPmin - DBPmin))/2).
Statistical analysis
Quantitative variables are expressed as mean ± standard deviation and compared with a Student t test. A P value <0.05 was considered statistically significant.
Qualitative variables are expressed as percentage and compared with χ2 or the Fisher test.
Clinical characteristics, ventilator settings, and hemodynamic parameters of patients with quantitative dIVC <18% were compared with patients with quantitative dIVC ≥18%. The sensitivity, specificity, positive predictive value, and negative predictive value of qualitative assessment of the dIVC were calculated. A kappa coefficient for interrater agreement was computed for each operator with respect to the reference value measured by an expert operator. We adopted the Landis and Koch conventions for describing the degree of agreement as a function of the value of kappa[24]. The statistical analysis was performed with the R.2.12.0 software (R Foundation).
Results
Patients
One hundred fourteen patients were screened. Because of poor visualization of the IVC by one or more of the operators, 17 (14.9%) of the 114 patients were not included in the study. Hence, our analysis covered 97 patients (63 men, 34 women; mean age, 67 ± 13 years; mean Simplified Acute Physiology Score II, 40 ± 13). The reason for ICU admission was postcardiac surgery care in 75 (77.3%) cases, septic shock in 18 (18.6%) cases, and multiple trauma in four (4.1%) cases. The patients were separated into two subgroups on this basis: (a) quantitative dIVC <18%; (b) quantitative dIVC ≥18%.
Demographic and hemodynamic parameters
No differences were found between quantitative dIVC <18% and dIVC ≥18% groups in terms of demographic variables and severity scores (Table 1).
The main ventilator settings and hemodynamic parameters are summarized in Tables 2 and3. As expected, responders had significant lower mean blood pressure (P = 0.04) and DBP (P = 0.03) values. The maximum and minimum IVC diameters were lower in responders (P < 0.01), and PPV was higher (P < 0.001).
Comparison of qualitative and quantitative analysis of IVC distensibility
The sensibility, specificity, positive, and negative predictive values were similar in different groups of operators and are presented in Table 4. The experienced and less experienced operators did not display significantly different kappa values.
The average sensitivity and specificity values for qualitative assessment of the dIVC by anesthesiologists (residents and intensivists) were 80.7% and 93.7%. The kappa coefficient for the entire group of operators was calculated to be 0.68, corresponding to substantial agreement. The kappa coefficients for each operator are presented in Table 4.
Qualitative dIVC assessment had a very low error rate for the 73 (75%) patients with quantitative values <15% and >30%. Unfortunately, the error rate was as high as 35% for quantitative values in the range 15% to 30%, which concerned 24 patients (25%) (Figures 1 and2).
Discussion
Our results showed that when compared with quantitative measurement and a threshold of 18%, qualitative assessment of the dIVC displayed good sensitivity and specificity and a substantial degree of interoperator agreement. This index was able to detect the majority of patients with a dIVC >30%.
Barbier et al.[7] reported that the increase in cardiac output during volume expansion is proportional to the measured value of dIVC. Hence, the qualitative assessment of dIVC in our study should have been able to detect (a) the majority of patients with a dIVC >30% and an increase in cardiac output of >15%, and (b) all patients with a dIVC > 40% and an increase in cardiac output of >20% during a fluid challenge test.
Similarly, this qualitative technique enables the detection of probable nonresponders to volume expansion with low error rates. Most of the errors in the qualitative dIVC evaluation occurred close to the threshold of 18%. The data in Figure 2 show that 90% of errors concerned patients with a dIVC of between 10% and 30%, and 65% concerned patients with a dIVC of between 15% and 25%.
As highlighted in the study by Vieillard-Baron et al.[20], norepinephrine may have limited efficacy in septic shock patients with hypovolemia or ventricular dysfunction. The study of Vieillard-Baron et al. found that experienced transesophageal echocardiography operators could assess these parameters qualitatively and that the provision of quantitative measures is potentially time-consuming. Our study gave similar results for the qualitative assessment of dIVC with TTE by operators with varying levels of experience. Thus, TTE can also yield a qualitative assessment of the dIVC, left ventricular ejection fraction, and right ventricular dysfunction.
The quality of the dIVC evaluation appeared to be good and did not depend greatly on the operator's level of echocardiographic experience. This finding is in agreement with the literature. Clinical work shows that it is possible to teach nonintensivists and noncardiologists how to perform basic transthoracic and transesophageal echocardiography[11, 12, 25] over a 12-month period by setting simple, standardized diagnostic goals. The principle of learning simple, standardized echocardiography skills goes beyond the field of intensive care. It has already been shown that a 4-hour course on the ultrasound analysis of inferior vena cava (with 20 clinical cases) can significantly improve the clinical diagnosis of vascular overload by residents in internal medicine[26]. Similarly, ultrasound analysis of the inferior vena cava makes it possible for nephrologists with little experience of ultrasound the better to assess blood-volume status between sessions for dialysis patients with chronic renal failure[27].
In the present study, the fact that the residents and intensivists did not differ significantly in terms of the recorded sensitivity and specificity values further suggests that learning is rapid. If used to evaluate hemodynamic status, serial quantitative measurement of the dIVC might help to improve the accuracy of qualitative analysis alone. Although it appears that the qualitative assessment of dIVC is easy to learn, further experience may increase the technique's reliability and reduce the error rate.
The cut-off used for assessing preload dependence with quantitative dIVC was 18%[7]: this is an arbitrary value that correctly evaluates the majority of patients. However, the application of a "gray zone" approach to PPvariation for prediction of fluid responsiveness in mechanically ventilated patients identifies a range of PPvariation values (from 9% to 13%)[28]. For dIVC evaluation, this gray zone is around 18%: volemic evaluation is difficult, and the benefit of fluid resuscitation is subject to debate. Just as with quantitative dIVC assessment, qualitative dIVC assessment is unreliable for patients with a volemic status in this gray zone; these patients should therefore receive an overall assessment (the clinical context, the passive leg-raising maneuver, respiratory pulse-pressure variation, and so on). With the exception of the gray zone (within which no single marker is sufficiently reliable), our study shows that the qualitative approach to dIVC assessment can be easily integrated into an overall hemodynamic assessment, when it comes to extreme to numeric values.
The quantitative dIVC requires less time with an evolutive ultrasonographic machine available in the intensive care units. The gain of accuracy of the quantitative dIVC measures has improved with the technique and can reduce its gray zone around 18%.
Limitations
Our study has several limitations.
No fluid assessment was completed to find changes in qualitative dIVC or quantitative dIVC, nor in cardiac output. The qualitative dIVC was measured according to the hemodynamic status of patients when assessing different measures, nor was the study random, nor was it our main objective.
Our failure rate for visualization of the IVC was 14.9%. This rate is close to the literature values[29, 30]. However, our rates took account of all patients in whom at least one of the operators (with different levels of expertise) could not visualize the IVC (as recommended by the current guidelines). In addition, most of the admissions to our ICU are related to postcardiac surgery care; the presence of chest tubes can interfere with the operator's view and make it difficult or impossible to visualize the IVC.
Given that 14.9% of patients were not evaluable and 20.6% (20 patients) are in the gray area of dIVC, between 15% and 30%, about 40% of patients are not evaluable by reliable visual methods. Nevertheless, a limit exists for all assessments preload dependency with the inferior vena cava, because these limits also apply to quantitative dIVC if we consider the "principle of the gray area".
Although our qualitative assessment was performed by just eight operators with different levels of experience of TTE, the study involved a large enough number of patients to confirm the technique's utility under different load and volume-expansion conditions. However, the large number of measurements and the satisfactory correlations between qualitative and quantitative dIVC assessments by the various operators confirmed that it is possible to use the qualitative-technique assessment on a daily basis. Our least experienced operators (level 1) had performed an average of 30 TTE examinations. An operator must understand some basic technical details of TTE before he or she can perform a dIVC assessment, because it is not always easy to obtain a good view and identify the best measurement site. Validation of qualitative dIVC assessment by a larger number of operators would nevertheless be of value.
Conclusion
The qualitative assessment of the dIVC has a gray zone between 15% and 30%. The sensitivity and specificity are good for values below 15% and above 30%, regardless of the operator's level of experience in ultrasound. Despite reliability of the qualitative assessment when it comes to extreme to numeric values, the quantitative dIVC measurement must always be done within a hemodynamic assessment for intensive care patients. The qualitative approach can be easily integrated into a fast hemodynamic evaluation by using a portable ultrasound scanner for out-of-hospital patients (evaluation of the dIVC, left or right ventricular dysfunction, and screening for tamponade or pleural effusion).
Key messages
-
Qualitative assessment of the dIVC has a gray zone between 15% and 30%, which should encourage the use of quantitative measurement of the dIVC in intensive care patients.
-
The correlation between qualitative and quantitative dIVC was considered good regardless of the operator's level of experience in ultrasound.
-
Qualitative dIVC assessment can easily take place in out of hospital FAST hemodynamic assessment, with a portable ultrasound scanner.
Abbreviations
- BMI:
-
Body mass index
- cSVC:
-
superior vena cava collapsibility index
- DAP:
-
diastolic arterial pressure
- DBP:
-
diastolic blood pressure
- dIVC:
-
inferior vena cava distensibility index
- HR:
-
heart rate
- ICU:
-
intensive care unit
- IRB:
-
Institutional Review Board
- IVC:
-
inferior vena cava
- MAP:
-
mean arterial pressure
- NPV:
-
negative predictive value
- PEEP:
-
positive end-expiratory pressure
- PP:
-
pulse pressure
- PPlat:
-
plateau pressure
- PPV:
-
positive predictive value
- PPvariation:
-
variation respiratory pulse pressure
- RR:
-
respiratory rate
- SAPS II:
-
Simplified Acute Physiology Score
- SBP:
-
systolic blood pressure
- Se:
-
sensitivity
- Sp:
-
specificity
- TTE:
-
transthoracic echocardiography
- Vt:
-
tidal volume.
References
Murakawa K, Kobayashi A: Effect of vasopressors on renal tissue gas tensions during hemorrhagic shocks in dogs. Crit Care Med 1988, 16: 789-792. 10.1097/00003246-198808000-00012
Charron C, Fessenmeyer C, Cosson C, Mazoit JX, Hebert JL, Benhamou D, Edouard AR: The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg 2006, 102: 1511-1517. 10.1213/01.ane.0000209015.21418.f4
Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL: Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001, 119: 867-873. 10.1378/chest.119.3.867
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M: Diagnosis of central hypovolemia using by using passive leg raising. Intensive Care Med 2007, 33: 1133-1138. 10.1007/s00134-007-0642-y
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F: Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004, 30: 1734-1739.
Feissel M, Michard F, Faller JP, Teboul JL: The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 2004, 30: 1834-1837.
Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, Vieilard-Baron A: Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004, 30: 1740-1746.
Charron C, Caille V, Jardin F, Vieillard-Baron A: Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care 2006, 12: 249-254. 10.1097/01.ccx.0000224870.24324.cc
Mayo PH: American College of Chest Physicians/La Société de Réanimation de Langue Française: statement on competence in critical care ultrasonography. Chest 2009, 135: 1050-1060. 10.1378/chest.08-2305
Expert Round Table on Ultrasound in ICU: International expert statement on training standards for critical care ultrasonography. Intensive Care Med 2011, 37: 1077-1083.
Vignon P, Chastagner C, Francois B, Martaillé JF, Normand S, Bonnivard M, Gastinne H: Diagnostic ability of hand-held echocardiography in ventilated critically ill patients. Crit Care 2003, 7: R84-R91. 10.1186/cc2360
Vignon P, Dugard A, Abraham J, Belcour D, Gondran G, Pepino F, Marin B, François B, Gastinne H: Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med 2007, 33: 1795-1799. 10.1007/s00134-007-0742-8
Guiotto G, Masarone M, Paladino F, Ruggiero E, Scott S, Verde S, Schiraldi F: Inferior vena cava collapsibility to guide fluid removal in slow continuous ultrafiltration: a pilot study. Intensive Care Med 2010, 36: 692-696. 10.1007/s00134-009-1745-4
Michard F, Teboul JL: Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002, 121: 2000-2008. 10.1378/chest.121.6.2000
Jue J, Chung W, Schiller NB: Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation. J Am Soc Echocardiogr 1992, 5: 613-619.
Jeffrey RB Jr, Federle MP: The collapsed inferior vena cava: CT evidence of hypovolemia. Am J Roentgenol 1988, 150: 431-432. 10.2214/ajr.150.2.431
Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P: Echocardiography in the intensive care unit: from evolution to revolution. Intensive Care Med 2008, 34: 243-249. 10.1007/s00134-007-0923-5
Randazzo MR, Snoey ER, Levitt MA, Binder K: Accuracy of emergency physician assessment of left ventricular ejection fraction and central venous pressure using echocardiography. Acad Emerg Med 2003, 10: 973-977. 10.1111/j.1553-2712.2003.tb00654.x
Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA: Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med 2002, 9: 186-193. 10.1111/j.1553-2712.2002.tb00242.x
Vieillard-Baron A, Charron C, Chergui K, Peyrousset O, Jardin F: Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient. Intensive Care Med 2006, 32: 1547-1552. 10.1007/s00134-006-0274-7
Moreno FL, Hagan AD, Holmen JR, Pryor TA, Strickland RD, Castle CH: Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol 1984, 53: 579-585. 10.1016/0002-9149(84)90034-1
Mintz G, Kotler M, Parry W, Iskandrian A, Kane S: Real-time inferior vena cava ultrasonography: normal and abnormal findings and its use in assessing right heart function. Circulation 1981, 64: 1018-1025. 10.1161/01.CIR.64.5.1018
Jardin F, Vieillard-Baron A: Ultrasonographic examination of the venae cavae. Intensive Care Med 2006, 32: 203-206. 10.1007/s00134-005-0013-5
Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 1977, 33: 159-174. 10.2307/2529310
Charron C, Prat G, Caille V, Belliard G, Lefèvre M, Aegerter P, Boles JM, Jardin F, Vieillard-Baron A: Validation of a skills assessment scoring system for transesophageal echocardiographic monitoring of hemodynamics. Intensive Care Med 2007, 33: 1712-1822. 10.1007/s00134-007-0801-1
Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Brooks E, Levy A, Kirkpatrick JN, Spencer KT: A comparison by medicine residents of physical examination versus hand-carried ultrasound for estimation of right atrial pressure. Am J Cardiol 2007, 99: 1614-1616. 10.1016/j.amjcard.2007.01.037
Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT: Hand carried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol 2006, 1: 749-753. 10.2215/CJN.00310106
Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot J, Vallet B, Tavernier B: Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology 2011, 115: 231-241. 10.1097/ALN.0b013e318225b80a
Stawicki S, Braslow B, Panebianco N, Kirkpatrick J, Gracias V, Hayden G, Dean A: Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg 2009, 1: 55-61.
Gunst M, Sperry J, Ghaemmaghami V, O’Keeffe T, Friese R, Frankel H: Bedside echocardiographic assessment for trauma/critical care: the BEAT exam. J Am Coll Surg 2008, 3: e1-e3.
Acknowledgements
We thank Sandra Petiot, Clémence Buchalet, and Mathieu Guilbart for their active participation in the acquisition of data. We received no financial support for conducting this study. This study was approved by the Institutional Review Board of Amiens University Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AD and EZ conceived, designed, and coordinated the study and drafted the manuscript. PGG, FL, FT, and MS participated in the coordination of the study and helped in drafting the manuscript. HD and MD performed the statistical analysis and helped in drafting the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Duwat, A., Zogheib, E., Guinot, P.G. et al. The gray zone of the qualitative assessment of respiratory changes in inferior vena cava diameter in ICU patients. Crit Care 18, R14 (2014). https://doi.org/10.1186/cc13693
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc13693