Abstract
Objective
Transesophageal echocardiography (TEE) has proven its efficiency in assessing hemodynamics in patients by its ability to evaluate cardiac function and fluid responsiveness. Classically, it requires quantitative measurements, whereas in routine practice TEE is used in our unit especially as a qualitative procedure. We assessed the accuracy of this qualitative central hemodynamic evaluation obtained by TEE at the bedside.
Design and setting
Prospective study conducted in a medical ICU between September 2004 and April 2005. All TEE examinations performed in consecutive patients hospitalized for septic shock and mechanically ventilated for an associated acute lung injury were eligible for evaluation. Intensivists trained in echocardiography were asked to classify (a) respiratory changes in the superior vena cava (SVC), (b) left ventricular (LV) systolic function, (c) right ventricular (RV) end-diastolic size, and (d) shape and kinetics of the interventricular septum (IVS). A post-hoc quantitative evaluation was then performed by a trained investigator unaware of the patients' status.
Results
We evaluated 83 examinations in 30 patients. Qualitative evaluation was easily able to distinguish patients with significant or nonsignificant SVC respiratory changes, normal, moderately or markedly depressed LV systolic function, and nondilated or dilated right ventricle. Acute cor pulmonale was also well recognized.
Conclusion
By its ability accurately to evaluate hemodynamic status qualitative TEE could be useful for intensivists in managing circulatory failure in septic shock, rendering the more time-consuming quantitative evaluation useless.
Similar content being viewed by others
Introduction
Bedside echocardiography has progressively gained acceptance in the medical intensive care environment. We have extensively described the application of this technique in two recent clinical commentaries, illustrated with numerous video sequences plus comments [1, 2]. In mechanically ventilated patients transesophageal echocardiography (TEE) is particularly easy. Using this technique daily with a specific apparatus in our unit, our team has totally replaced right heart catheterization. This strategy constitutes a major advance in hemodynamic evaluation for the most critically patients, permitting easy distinction of the various, frequently associated causes of circulatory failure, i.e., left and right ventricular dysfunction, hypovolemia, vasoplegia, the latter being diagnosed by a process of elimination. It also avoids inaccurate invasive evaluations [3] and the numerous complications related to right heart catheterization [4].
However, echocardiography is an imaging method which first gives a qualitative evaluation of hemodynamic status. While some quantitative measurements, such as Doppler flow velocity, may be useful in a cardiological setting, qualitative measurements are especially required, in our opinion, to adequately manage septic patients exhibiting hemodynamic instability [2].
This prospective study was carried out in consecutive septic patients exhibiting hemodynamic instability to assess the accuracy of the qualitative central hemodynamic evaluation obtained by TEE performed at the bedside by a senior intensivist. This qualitative evaluation was compared with a post-hoc quantitative evaluation performed from the digitized recording by a fully trained investigator unaware of patient status.
Materials and method
A prospective evaluation of bedside TEE was performed between September 2004 and April 2005 in our medical intensive care unit. All TEE examinations performed in patients hospitalized for septic shock and mechanically ventilated for an associated acute lung injury were eligible for evaluation. Occurring in a septic context, shock was defined as the requirement for norepinephrine infusion to maintain arterial pressure higher than 90 mmHg as measured by a radial catheter. During examination the senior physician responsible for the patients (C.C., K.C., O.P.) was asked to complete a questionnaire, thus performing a qualitative evaluation. These senior physicians all had at least 1 year's experience of daily practice of bedside echocardiography in our unit, and they all performed at least 60 TEE examinations. None of them was a cardiologist. Current study does not induce any change in our routine practice. The study was presented to the CCPPRB of Ambroise Pare Hospital and the need for informed consent was waived.
Qualitative evaluation
Echo-Doppler studies were performed with a Sequoia C 256 (Siemens, Pennsylvania, USA) equipped with a multiplane 5-MHz transesophageal echocardiographic transducer, using a routine procedure [2]. All patients were sedated and perfectly adapted to the respirator. Using the signal from the respirator, airway pressure was displayed on the screen of the echo-Doppler device, accurately timing cardiac events during the respiratory cycle. Only three echocardiographic views were required, a long-axis view of the superior vena cava (SVC) using the M-mode [5], a four-chamber, long-axis transesophageal view of the heart, and a short-axis view of the left ventricle (LV) by a transgastric approach.
The physicians were asked to assess the following parameters in terms of the grades below: (a) SVC respiratory changes (0, no respiratory variation; 1, minor respiratory variations; 2, major respiratory variations); (b) LV systolic function (0, normal; 1, moderately depressed; 2, severely depressed; (c) right ventricular (RV) diastolic size (0, normal; 1, moderately enlarged; 2, markedly enlarged; and (d) presence of RV pressure overload according to the shape and kinetics of the interventricular septum (IVS; 0, absent, defined by normal shape and kinetics; 1, present, defined by dyskinesia). When grading, our physicians used what they had learned in our unit during their at least 1 year's experience of bedside echocardiography. We have previously reported that in our usual practice we consider a major SVC respiratory change as a partial or complete collapse of the vessel, a minor change as a variation of less than 30%, a severely depressed LV systolic function as an LV ejection fraction or an LV fractional area contraction below 40%, and a markedly dilated RV as an RV larger than the LV [1, 2].
Patient population
The study was carried out in 30 patients (22 men, 8 women; mean age 65 ± 11 years; mean Simplified Acute Physiology Score II 62 ± 23); no patient had experienced previous cardiac or respiratory disease. All required norepinephrine infusion, 15 dobutamine, and 3 epinephrine. During the study period 83 TEE examinations were performed (2.8 ± 2.9 per patient, range 1–6). The number of examinations per investigator was 30 for C.C., 30 for K.C., and 23 for O.P. Only five patients had an unchanged hemodynamic profile on TEE examination throughout their clinical course, which corresponded to 12 echocardiographic procedures. In-hospital mortality rate for the overall series was 57%.
Quantitative evaluation
Echocardiographic images were recorded on video tape, and a computer-assisted quantitative evaluation was performed by a trained investigator unaware of the patient's status and the result of qualitative evaluation. Using the two-dimensional view to direct the M-mode beam across the maximal diameter, SVC diameter was measured during the respiratory cycle. The collapsibility index of SVC, i.e., the inspiratory decrease in SVC diameter, was determined as: maximal diameter on expiration–minimal diameter on inspiration)/maximal diameter on expiration, expressed as a percentage [5]. Left ventricular areas (A) at end-systole (ES) and at end-diastole (ED) were measured from the four-chamber view of the cardiac chambers. Left ventricular long-axis was measured at ES and ED as the distance from the apex to the midpoint of the mitral valve ring, and the LVES and LVED volumes (V) and ejection fraction (EF) were calculated using the single-plane, area-length formula [6]. On the same view RV end-diastolic area was measured, and end-diastolic area ratio (EDA ratio) was calculated as previously described [7]. Left ventricular end-systolic area and LVEDA were measured from a short-axis cross-sectional view of the LV at the midpapillary muscle level, and LV fractional area contraction (FAC) was calculated as (LVEDA-LVESA)/LVEDA. On the same view a first diameter, D1, bisecting and perpendicular to the plane of IVS, and a second diameter, D2, perpendicular to D1, and thus parallel to the IVS, were measured. From these diameters, an end-systolic eccentricity index (D2/D1) was calculated, as proposed by Ryan et al. [8].
Statistical analysis
Statistical calculations were performed using the Statgraphics plus package (Manugistics, Rockville, Md., USA). Data are expressed as mean ± 1 SD. A p value less than 0.05 is considered as statistically significant. Between-group comparisons were performed by analysis of variance followed by Bonferroni's multiple comparison procedure. Using measurement of interrater agreement (κ) [9] we compared qualitative gradation a priori to quantitative classification carried out a posteriori. LV systolic function was classified as class 0 if LVEF was 50% or greater, class 1 if between 40% and 50%, and class 2 if below 40%. SVC respiratory changes were classified as class 0 if SVC collapsibility index was below 30%, class 1 if between 30% and 60%, and class 3 if above 60%. Finally, RV size was classified as class 0 if EDA ratio was below 0.6, class 1 if between 0.6 and 1, and class 2 if above 1.
Results
Evaluation of SVC collapsibility
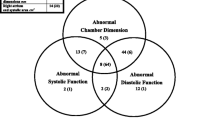
Qualitative evaluation of SVC respiratory changes are presented with the corresponding mean values of SVC collapsibility index in Table 1. A discernible change during tidal ventilation was absent in 57 examinations while minor respiratory changes were observed in 22. Major changes were only observed in 4 examinations. Distribution of individual values by grade is presented in Fig. 1. In particular, all patients graded as group 1 (minor changes) had an SVC collapsibility index no higher than 36%, whereas complete collapse of the vessel was seen in all patients graded as group 2 (major changes). Interrater agreement was classified as moderate (weighted κ = 0.54 ± 0.079).
Evaluation of LV systolic function
Qualitative evaluation of LV systolic function is presented with the corresponding mean values of LVEF in Table 1. Distribution of individual values of LVEF and LVFAC by grade is presented in Fig. 2. Only 2 of the 38 patients graded as grade 0 (normal LV systolic function) had an LVEF between 45% and 50%. All 21 patients graded as grade 2 (severely depressed LV systolic function) had an LVFAC below 40%, whereas one patient had an LVEF between 40% and 45%. Finally, only one of the 24 patients graded as grade 1 had an LVEF and an LVFAC above 50%. Interrater agreement was classified as good (κ ± 0.72, 95% CI 0.59–0.85).
Individual values of left ventricular ejection fraction (LVEF) in the long axis (above) and left ventricular area contraction (LVFAC) in the short axis (below) in the three qualitative groups: grade 0 normal systolic function; grade 1 moderately depressed systolic function; grade 2 severely depressed systolic function
Evaluation of RV diastolic size
Qualitative evaluation of RV diastolic size is presented with the corresponding mean values of the EDA ratio in Table 1. The diastolic size of the RV was judged normal in 70 examinations and moderately dilated in 13. No patient exhibited a major RV dilatation at any time. Figure 3 reports the distribution of EDA ratios by grade. All but one patient graded as grade 1 (moderately dilated RV) had an EDA ratio of 0.6 or more. Interrater agreement was classified as good (κ = 0.74, 95% CI 0.54–0.94).
Evaluation of RV pressure overload
Qualitative evaluation of septal kinetics is presented in Table 1. Normal kinetics of the IVS were observed in 81 examinations, while dyskinesia, suggesting RV pressure overload, was noted in only two examinations in two different patients. Quantitative evaluation by calculation of the eccentricity index, also presented in Table 1, corroborated the qualitative evaluation.
Discussion
Daily management of sepsis-related circulatory failure in our unit has been guided for more than 10 years by bedside echocardiographic examination. Our strategy has been extensively described in a recent clinical commentary [2]. Low arterial pressure in a septic context is usually thought to result from abnormally low vascular tone [10]. Treatment of this specific circulatory disorder by norepinephrine infusion is now widely accepted [11]. However, there are three associated conditions that can limit the efficacy of norepinephrine infusion: hypovolemia, depression of LV systolic function, and abnormal RV afterloading [2]. The present report shows that these three conditions can be easily diagnosed by bedside TEE.
In sepsis the detection of fluid responsiveness before fluid administration is of great practical interest, particularly to avoid useless and likely harmful fluid challenge. Invasive and noninvasive parameters have been proposed for this purpose [12, 13]. Recently we demonstrated that patients with an SVC collapsibility index below 30% are never “fluid responsive,” whereas all patients with an index above 60% are [5]. As illustrated here, qualitative evaluation allowed clear separation of patients according to their collapsibility index, avoiding useless fluid administration in a majority of cases. Because in our previous study [5] two patients were responders with respective SVC collapsibility index values of 31% and 33%, we cannot exclude, as shown in Fig. 1, that fluid infusion actually induced an increase in cardiac index in five patients graded as having “minor respiratory variations.” In this situation quantitative measurement of SVC collapsibility index could help the decision. Because this was below 36%, patients did not receive fluids. However, this only represents 6% of the 83 procedures, and in three of these cases the mean LVEF was 24%, suggesting the need for a treatment other than fluids, as inotropic drugs, to correct circulatory failure.
Long suspected, acute LV failure in sepsis was demonstrated by radionuclide angiography in 1984 [14] and corroborated by echocardiography [15, 16]. We previously reported that the usual norepinephrine infusion may also reveal LV failure by partly correcting systemic vascular resistance and then increasing LV afterload [2]. In the case of persistent circulatory failure or following a new hemodynamic degradation LV systolic dysfunction may thus be systematically detected. TEE as a qualitative procedure accurately and easily distinguished patients according to their LV systolic function. All but one patients graded as grade 0 had an LVEF and an LVFAC above 50%, which does not require inotropic drug infusion [2]. Furthermore, all patients graded as grade 2 had severely depressed LV systolic function and an LVEF and an LVFAC below 30%, which frequently needs inotropic drug infusion [2, 17]. Finally, in patients graded as grade 1 the distribution of LVEF and LVFAC values was wider, but all patients except for one had depressed LV systolic function. In this group of patients the decision to infuse an inotropic drug also depends on clinical and laboratory parameters of tissue hypoperfusion, and repeated evaluation of LV systolic function is needed.
RV failure can be observed in sepsis as a result of increased pulmonary vascular resistance [1] or as the consequence of acute myocardial depression, also involving the right ventricle [2]. Of course, these two conditions are usually associated, particularly under mechanical ventilation [2]. RV failure in sepsis would complicate the treatment, rendering fluid administration inefficient [18]. Because under normal conditions the right ventricle works against a circulation with low pressure, i.e., the pulmonary circulation, any acute RV failure induces RV dilatation. Qualitative evaluation of RV size was efficient in our study because the mean EDA ratio in grade 1 patient (patients with a qualitative diagnosis of moderate RV enlargement) was about 0.73, when a normal value is below 0.6 [19]. In certain conditions an excessive right ventricular afterload induces a characteristic echocardiographic pattern, called acute cor pulmonale [20], which is important to diagnose because ventilator settings may need to be adjusted in an attempt to unload the right ventricle [21]. As illustrated here in two patients, this pattern which combines right ventricular enlargement and septal dyskinesia is easy to recognize by TEE. The qualitative assessment of the presence of septal dyskinesia was confirmed by a marked increase in the eccentricity index of the left ventricle, reflecting movement of the IVS toward the left chamber.
Conventional hemodynamic evaluation in sepsis-related circulatory failure is obtained by measurement of quantitative parameters with a pulmonary artery catheter. These quantitative parameters are used by the clinician to decide on a therapeutic strategy, which may be to give fluids, to use a vasoactive agent acting on vascular tone, or an inotropic agent. With this strategy, however, the clinician uses quantitative parameters to perform a qualitative evaluation, the final therapeutic decision being determined by suspicion of relative hypovolemia, an abnormally low vascular tone, or a given degree of myocardial depression. Quantitative measurements can also be provided by echocardiography [1, 2] as the different parameters reported in this study but also Doppler determination of LV stroke index, cardiac output, and respiratory variations in aortic velocities, recently proposed as a parameter of LV preload dependency [22]. However, by virtue of being quantitative these measurements are subject to error and must be made by experienced echocardiographers. Moreover, quantitative measurements require stop-frame analysis. During this analysis some information, such as accurate endocardial outlines, which are easy to observe in real time can in part be lost by freezing of the frame.
Finally, these results should be interpreted in light of the experience of our physicians who were not cardiologists and have no specific certification in echocardiography. They do, however, use bedside echocardiography and especially TEE daily to manage the most severe cases. They all performed alone at least 60 TEE examinations in mechanically ventilated patients, and misclassifications when observed were distributed randomly among them. The level of training an intensivist needs before performing an accurate qualitative TEE evaluation of fluid responsiveness, LV function and RV function remains to be evaluated, although some authors suggest that with minimal training intensivists are able to assess LV systolic function accurately using TEE [23].
One of the limitation of our study is that because of the design of the study we were unable to evaluate interobserver variability in both qualitative and quantitative assessment. Such a variability may influence performance of statistical tests according to the relative overlap of some variables.
In conclusion, the present study demonstrates that after suitable training qualitative TEE at the bedside can be a simple and accurate way to assess fluid responsiveness and LV and RV systolic functions. This suggests that intensivists could use it to manage sepsis-related circulatory failure by employing only qualitative evaluation, without the need for more time-consuming quantitative measurements.
References
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2002) Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med 166:1310–1319
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Iberti T, Fischer E, Leibowitz A, Panacek E, Silverstein J, Albertson T (1990) A multicenter study of physician's knowledge of the pulmonary artery catheter. Pulmonary artery catheter study group. JAMA 264:2928–2932
Practice guidelines for pulmonary artery catheterization. A report by the American Society of Anesthesiologists Task Force on pulmonary artery catheterization (1993) Anesthesiology 78:380–394
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F (2004) Superior vena cava collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 30:1734–1739
Triulzi M, Gerard T, Wilkins G, Gilliam L, Gentile L, Weyman A (1985) Normal adult cross-sectional echocardiographic values: left ventricular volumes. Echocardiography 2:153–1697
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F (2001) Acute cor pulmonale in ARDS submitted to protective ventilation: incidence, clinical implications and prognosis. Crit Care Med 29:1551–1555
Ryan T, Petrovic O, Dillon J, Feigenbaum H, Conley M, Armstrong W (1985) An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol 5:918–924
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Siegel J, Greenspan M, Del Guercio L (1967) Abnormal vascular tone, defective oxygen transport and myocardial failure in human septic shock. Ann Surg 165:504–517
Martin C, Viviand X, Leone M, Thirion X (2000) Effect of norepinephrine on the outcome of septic shock. Crit Care Med 28:2758–2765
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky M, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Feissel M, Michard F, Faller JP, Teboul JL (2004) The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 30:1834–1837
Parker M, Shelhamer J, Bacharach S, Green M, Natanson C, Frederick T, Damske B, Parillo J (1984) Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100:483–490
Ozier Y, Guéret P, Jardin F, Farcot JC, Bourdarias JP, Margairaz A (1984) Two-dimensional echocardiographic demonstration of acute myocardial depression in septic shock. Crit Care Med 12:596–599
Jardin F, Fourme T, Page B, Loubières Y, Vieillard-Baron A, Beauchet A, Bourdarias JP (1999) Persistent preload defect in sever sepsis despite fluid loading. A longitudinal echocardiographic study in patients with septic shock. Chest 116:1354–1359
Jardin F, Sportiche M, Bazin M, Bourokba A, Margairaz A (1981) Dobutamine: a hemodynamic evaluation in septic shock. Crit Care Med 9:329–332
Schneider A, Teule G, Groeneveld A, Nauta J, Heidendal G, Thijs L (1988) Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J 116:103–112
Weyman A (1982) Cross sectional echocardiography. Lea & Fibiger, Philadelphia
Jardin F, Dubourg O, Bourdarias JP (1997) Echocardiographic pattern of acute cor pulmonale. Chest 111:209–217
Jardin F, Vieillard-Baron A (2003) Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med 29:1426–1434
Slama M, Masson H, Teboul JL, Arnout ML, Susic D, Frohlich E, Andrejak M (2002) Respiratory variations of aortic VTI: a new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol 283:H1729–H1733
Benjamin E, Griffin K, Leibowitz A, Manasia A, Oropello JM, Geffroy V, DelGiudice R, Hufanda J, Rosen S, Goldman M (1998) Goal-directed transesophageal echocardiography performed by intensivists to assess left ventricular function: comparison with pulmonary artery catheterization. J Cardiothorac Vasc Anesth 12:10–15
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vieillard-Baron, A., Charron, C., Chergui, K. et al. Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient?. Intensive Care Med 32, 1547–1552 (2006). https://doi.org/10.1007/s00134-006-0274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0274-7