Abstract
In this work, transparent heterojunction between zinc oxide (ZnO) and poly(N-vinyl carbazole) (PVK) was fabricated by solution processing techniques such as spin-coating and dip-coating techniques; then, its performance was studied using current (I)-voltage (V) measurement at room temperature. Before fabricating the heterojunction, initially, the growth characteristics of both thin films were independently optimized on a well-cleaned glass substrate, then its structural properties, optical properties, and surface topography were characterized using an X-ray diffractometer, UV-VIS-NIR spectrophotometer, and atomic force microscope, respectively. The structural analysis confirms the existence of a PVK thin film in amorphous nature and ZnO thin film in hexagonal crystal structure. The transparent nature of the heterojunction was found to be more than 85% in the visible and NIR regions with the absorption onset in the ultraviolet region. The observed experimental results explored the possibilities of fabricating ZnO/PVK transparent heterojunction by solution-based routes on a transparent fluorine-doped tin oxide substrate for transparent electronics applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Materials which are displaying the remarkable combination of high electrical conductivity and optical transparency already form the basis of many important technological applications including flat panel displays, solar energy capture, and other optoelectronic devices [1]. Transparent electronic devices such as bipolar transistors and diodes can be fabricated with the help of p-n transparent junction. After the discovery of the first transparent semiconductor delafossite CuAlO2 in 1997 by Kawazoe et al. [2], the development of a series of p-type transparent materials and transparent p-n junction devices such as UV-light-emitting diodes was triggered. Very recently, Snure et al. [3] discovered another p-type transparent oxide (CuBO2) that also belongs to the delafossite family but exhibits better room-temperature electrical conductivity (1.65 S/cm). Zinc oxide (ZnO) is a transparent n-type semiconductor material having a wide bandgap (e.g., approximately 3.37 eV at room temperature) with large exciton binding energy of approximately 60 meV [4]. Such properties make them well suited for the realization of many optoelectronic applications. Poly(N-vinyl carbazole) (PVK) is one of the famous holes conducting polymeric material with amorphous nature and widely utilized in the fabrication of blue light-emitting diodes [5]. It has, by linear chains of repeated molecular groups (H2C–HC) n with pendant carbazole side groups [(C6H4)2NH], arranged randomly around the same chain. Since the electrical and optical properties of films strongly depend on their microstructure, stoichiometric nature, and impurities present, each deposition technique with its associated parameters yields films of different properties. Depending on the deposition method, the substrate can have a significant influence on the properties of the films. Particularly, the thermal expansion coefficient of the substrate affects the orientation of crystallites in the films and its electrical properties. Solution processing is envisioned as a key technology in the low-cost production of electronic devices on both conducting and non-conducting substrates. In this report, the formation of transparent heterojunction between ZnO and PVK thin films by solution processing routes and their performance were discussed.
Methods
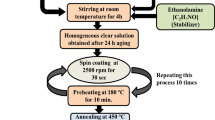
All the precursor and solvent materials were used in as-received condition without any further purification. Transparent PVK solution was prepared in dichloromethane (DCM) by dissolving 0.5 g of PVK in 20 ml of DCM at room temperature. The well-cleaned substrates (glass or fluorine-doped tin oxide (FTO)) were dip-coated using freshly prepared polymer sol and then baked at 80°C for 15 min to get the PVK films. In the case of ZnO thin films, the sol was prepared [6] using the following procedure: isopropyl alcohol (136.4 ml) and diethanolamine (5.2 ml) were stirred for 15 min at room temperature; then, zinc acetate dehydrate (13.38 g) was added and stirred once again for 60 min then refluxed at 70°C for 30 min, cooled, and aged for 6 h. The use of metal acetate instead of the commonly used alkoxides will reduce the problem of moisture sensitivity like significant factors in assessing the effectiveness of a process. A commercially available flat motor with a fan which is able to rotate at 2,000 rpm on 12 V DC supply was used to spin coat the ZnO sol on the substrates. After deposition, the films were baked in a box furnace at 450°C for 10 min and allowed to reach room temperature before introducing the next layer. The coating process was repeated thrice to improve the crystalline nature of the ZnO film. Structural analysis of both thin films was carried out using a Philips X-ray diffractometer (Philips, Amsterdam, Netherlands). The atomic force microscopy studies were carried out in contact mode using Veeco Dimension 3100 SPM unit (Veeco Instruments Inc., Plainview, NY, USA), and a UV–VIS-NIR spectrophotometer was used to study the optical behavior of thin films between the wavelength region 300 to 1,100 nm. The commercial scientific software Origin 6.1 was used to plot the spectra and, in some cases, even for the analysis of spectra.
Results and discussions
X-ray diffraction patterns of the dip-coated PVK, Joint Committee on Powder Diffraction Standards (JCPDS) pattern (89–1397) of the ZnO sample, and spin-coated ZnO thin films on glass substrates are shown in Figure 1. The amorphous nature of the PVK thin film was revealed from the analysis. All the peaks of the spin-coated ZnO film XRD pattern are perfectly indexed to the hexagonal phase of ZnO and are in good agreement with the JCPDS file 89–1397. The peaks which are shown in Figure 1c with 2θ values of 31.73°, 34.37°, 36.21°, 47.48°, 56.53°, 62.77°, and 67.86° correspond to the crystal planes of (100), (002), (101), (102), (110), (103), and (112), respectively. Both of the samples were further examined for their microstructural and optical properties using atomic force microscopy (AFM) and UV–VIS-NIR spectrophotometer. The AFM images of both films are shown in Figure 2. Within the scanned region, both films have an appreciable network structure, without porosity, pin holes, and cracks. As compared with the ZnO thin film, PVK does not have any features in the AFM image which also confirm the amorphous nature of the film. In general, films obtained by sol–gel coating with different microstructural and physical properties are achievable by varying the solution preparation parameters such as aging the solution prior to the coating.
UV–VIS-NIR spectrophotometer transmittance spectra of both thin films are shown in Figure 3. The transparent nature was found to be more than 85% in the visible and near infrared region for both films with the absorption onset in the ultraviolet region. The shape of the absorption edge in both thin films are solely due to the electronic transition from the top of the valence band to the bottom of the conduction band. For a direct bandgap semiconductor, such as ZnO, the absorbance in the vicinity of the onset is given [7] by
where α is the absorption coefficient, C is a constant, hν is the photon energy, and Εgbulk is the bandgap. Hence, the direct bandgap energy calculated using the above formula with its absorption onset wavelength of 390 nm is 3.2 eV, which agrees well with the value reported in the literature [8].
Realization of heterojunction formation between n-ZnO thin film with other materials such as p-SrCu2O2[9], p-SiC [10], p-Si [11], and p-GaN [12] initiated us to explore similar possibilities with polymer films. In order to fabricate a transparent heterojunction, ZnO thin film was deposited first on FTO substrate then characterized; the observed XRD pattern is shown as an inset in Figure 4. Substrate contribution due to the crystalline nature of the FTO substrate was evident from the XRD measurement. The optical quality was investigated using UV–VIS-NIR spectrophotometer, and it was shown in Figure 4. The oscillatory character of the films grown on FTO substrate may be due to interference effects between the sample and the FTO layer in the visible region. Then, on the top of ZnO film, a thin layer of PVK was deposited using the same protocol mentioned previously. I-V measurement was performed to study the junction formation; the observed spectra are shown in Figure 5. The obtained forward and reverse bias breakdown characteristics confirm the junction formation between these layers. It helped us to realize the successful fabrication of a transparent diode by a simple solution-based film growth process.
Conclusions
Transparent ZnO/PVK heterojunction by solution processing techniques was successfully fabricated on solid substrates. Both of the films are uniform; more than 85% of optical transparency was achieved in the visible region, and atomic force microscopic investigation reveals our films are pin-hole and crack-free. Transparent heterojunction formation with low breakdown voltage (approximately 2.5 V) between ZnO and PVK films was confirmed by I-V measurement. All these experimental results confirm the possibilities of achieving heterojunction by solution-based routes.
References
Edwards PP, Porch A, Jones MO, Morgan DV, Perks RM: Basic materials physics of transparent conducting oxides. Dalton Trans. 2004, 39: 2995.
Kawazoe H, Yasukawa M, Hyodo H, Kurita M, Yanagi H, Hosono H: P-type electrical conduction in transparent thin films of CuAlO 2 . Nature 1997, 389: 939. 10.1038/40087
Snure M, Tiwari A: CuBO 2 : a p-type transparent oxide. Appl Phys Lett 2007, 91: 092123. 10.1063/1.2778755
Maksimov O: Recent advances and novel approaches of p-type doping of zinc oxide. Rev Adv Mater Sci 2010, 24: 26.
Pisharady SK, Menon CS, Sudarshanakumar C: Optical and electrical properties of carbazole thin film. J Mater Sci 2005, 40: 2047. 10.1007/s10853-005-1230-6
Tahar RBH, Tahar NBH: Crystallographic orientation in pure and aluminum-doped zinc oxide thin films prepared by sol–gel technique. J Am Ceram Soc 2005, 88: 1725. 10.1111/j.1551-2916.2005.00268.x
Wang J, Yin S, Zhang Q, Saito F, Sato T: Photocatalytic degradation of organic dyes with Er3+:YAlO 3 /ZnO composite under solar light. J Mater Chem 2003, 13: 2348. 10.1039/b303420h
Searson PC, Pesika NS, Stebe KJ: Determination of the particle size distribution of quantum nanocrystals from absorbance spectra. Adv Mater 2003, 15: 1289. 10.1002/adma.200304904
Ohta H, Orita M, Hirano M, Hosono H: Fabrication and characterization of ultraviolet-emitting diodes composed of transparent p-n heterojunction, p-SrCu 2 O 2 and n-ZnO. J Appl Phys 2001, 89: 5720. 10.1063/1.1367315
Yuen C, Yu SF, Lau SP, Rusli , Chen TP: Fabrication of n-ZnO:Al/p-SiC(4H) heterojunction light-emitting diodes by filtered cathodic vacuum arc technique. Appl Phys Lett 2005, 86: 241111. 10.1063/1.1947889
Chen P, Ma X, Yang D: Ultraviolet electroluminescence from ZnO/p-Si heterojunctions. J Appl Phys 2007, 101: 053103. 10.1063/1.2464185
Alivov YI, Van Nostrand JE, Look DC, Chukichev MV, Ataev BM: Fabrication and characterization of n-ZnO/p-AlGaN heterojunction light-emitting diodes on 6H-SiC substrates. Appl Phys Lett 2003, 83: 2943. 10.1063/1.1615308
Acknowledgments
The authors are grateful to Prof. Dr. B. S. Murty, Department of Metallurgical and Materials Engineering, Indian Institute of Technology Madras, Chennai, 600 036, India, for the atomic force microscopy measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both authors contributed equally to this manuscript and read and approved its final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Thanigainathan, P., Paramasivan, C. Realization of ZnO/PVK transparent heterojunction by solution processing routes. Int Nano Lett 3, 9 (2013). https://doi.org/10.1186/2228-5326-3-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-9