Abstract
Some undesired nanoparticles, such as malformed structures or incomplete core/shell Fe3O4-Au nanocomposites, might be formed when Fe3O4-Au core/shell nanocomposites are being synthesized. These impurities should be separated before any applications are performed. In this investigation, magnetic cores (Fe3O4) were synthesized using a conventional fabrication method involving coprecipitation of Fe2+ and Fe3+. Carboxyl-capped magnetite-gold composite nanoparticles, measuring ≤50 nm, were then synthesized using a plant extract (Eucalyptus camaldulensis). The prepared carboxyl-capped magnetite-gold composite nanoparticles were further subjected to acid treatment for 18 h and characterized with different instrumentation methods. The results of the investigation showed that acid treatment can be applied successfully to separate defect-free Fe3O4-Au core/shell nanoparticles from different types of Fe3O4-Au nanocomposites, without any considerable changes in their physiochemical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Magnetic nanoparticles (MNPs) have been known as one of the nanostructures that provide the widest uses in biomedicine[1–5]. For example, MNPs that are composed of magnetite (Fe3O4) or maghemite (γ-Fe2O3) have unique thermal, chemical, and magnetic properties that make these particles particularly well suited for medical applications[5, 6]. However, producing nanoparticles (NPs) with high stability and biocompatibility presents one of the greatest challenges. To address this, MNPs should be covered with an external quite inert shell, in order to protect the magnetic core against chemical changes. In addition, the surfaces of these particles should be functionalized with organic molecules, to allow them to bind chemically to other biomolecules such as DNA, proteins, amino acids, etc.[7–9]. Gold is a chemically quite inert element, and no single acid dissolves it. This metal is resistant to chemical reactions that take place in biological fluids and is a very useful element for providing a coating layer to protect MNPs[10, 11]. Also, gold metal does not decrease the magnetic properties of Fe3O4 MNPs and, consequently, will not limit the applications in which these particles are used[12, 13].
At present, a number of different chemical and biological methods have been reported for fabricating magnetite Fe3O4-Au composite NPs[4, 6, 9]. These nanocomposites contain both defect-free Fe3O4 core@Au shell MNPs and other types of Fe3O4-Au nanocomposites such as malformed structures or incomplete core/shell Fe3O4-Au nanocomposites (Figure1). As mentioned above, the importance of the external inert shell in protecting the magnetic core is well established, and different reports have been published on the synthesis of Fe3O4 core@Au shell MNPs[4, 13]. However, in these studies, no further treatments such as acid washing have been applied for separating the defect-free Fe3O4 core@Au MNPs from other types of Fe3O4-Au nanocomposites. An intact gold layer on the Fe3O4 nucleus can protect the core against acid wash treatment. In contrast, Fe3O4 minerals in malformed structures or other structures with incomplete inert shell will be dissolved with exposure to acid. In the current study, carboxyl-capped Fe3O4-Au composite MNPs were prepared by a combined chemical and biological reducing method[14]. Hydrochloric acid (HCl) was used to dissolve all types of Fe3O4-Au composite MNPs, except for Fe3O4@Au core/shell MNPs. In the next step, the remaining Fe3O4 MNPs with defect-free gold shell, which has been designated in this report as ‘Fe3O4 core@Au shell MNPs,’ were separated from the reaction mixture with a magnet and fully characterized using different instrumentation methods.
Results and discussion
This study reports a simple acid wash treatment for separating defect-free Fe3O4 core@Au shell MNPs from other types of Fe3O4-Au composite NPs (i.e., malformed structures and incomplete core/shell MNPs) (Figure1). First, crude magnetite Fe3O4-Au nanocomposites were synthesized using the method described above, and their shapes and sizes were studied by transmission electron microscopy (TEM). Figure2 shows two representative TEM images recorded from the drop-coated film of the Fe3O4-Au nanocomposites that were fabricated by a recently described semi-biosynthesis method[14]. Two dark and gray particles with sizes of ≤50 nm can be observed in the TEM images that have been illustrated in Figure2a,b.
Transmission electron microscopic images. Of both magnetic-gold composite nanoparticles (dark particles) and bare Fe3O4magnetite nanoparticles (gray particles), recorded by different magnification (a and b). Lower illustrations show energy-dispersive spectroscopy patterns of these gray (c) or black (d) nanoparticles.
It should be noted that Fe3O4 magnetite NPs are seen as a gray color because elemental gold (Au) has a higher electron density than Fe3O4 and, consequently, more electrons are transmitted in bright field imaging[15]. The analysis of the gray particles by energy-dispersive spectroscopy (EDS) confirmed the presence of Fe and O element signals (Figure2c). The peaks shown in Figure2c are attributed to Fe and O elements, and this confirmed the existence of iron-oxide NPs (copper and carbon peaks existed due to the grid used for TEM imaging). The EDS pattern of the dark particles indicated the presence of gold, iron, and oxygen, as illustrated in Figure2d. The EDS experiment was repeated to analyze different other dark NPs, and the obtained EDS spectra confirmed that the other dark NPs were also made up of both elemental gold and Fe3O4 magnetite ingredients (Figure3a,b). In addition, further analysis of some gray particles (Figure3a) by EDS showed a gold element signal with lower density, confirming the presence of other types of Fe3O4-Au nanocomposites, especially malformed Fe3O4-Au composite NPs (Figure3c).
Another transmission electron micrograph of magnetic-gold composite nanoparticles, prepared by the semi-biosynthetic method (a). Lower illustration shows energy-dispersive spectroscopy patterns of a core/shell Fe3O4-Au composite nanoparticle (b) and other types of Fe3O4-Au nanocomposites, especially malformed Fe3O4-Au composite nanoparticles (c).
In the next step, a simple dissolution method using hydrochloric acid (1 N) was performed to separate acid-stable magnetite particles, coated with an intact gold layer, from those containing bare Fe3O4 MNPs, malformed structures or incomplete core/shell nanocomposites (Figure1). It should be noted that defect-free Fe3O4 core@Au shell MNPs are stable against acid and can easily be separated after the acid wash process by applying an external magnetic field. In contrast, during acid wash treatment, malformed structures or incomplete Fe3O4@Au core/shell MNPs are decomposed to gold metal and soluble ferric iron (Figure1). In addition, bare Fe3O4 MNPs are also completely dissolved in HCl solution (1 N) (Figure1). Figure4a shows a test tube containing Fe3O4-Au nanocomposites that has been mixed with HCl and incubated for 18 h. Also, Figure4b,c demonstrates two tubes containing acid-stable NPs before and after separation by a magnet.
The images of different test tubes that contain Fe 3 O 4 -Au composite MNPs. In exposure with 1 N hydrochloric acid (a) and after separation with external magnetite field (b). Tube (c) shows the defect-free core/shell fraction of Fe3O4-Au nanocomposites separated by a magnet and washed three times with distilled water.
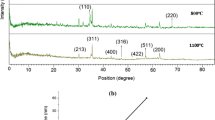
The upper illustration in Figure5 shows different TEM images of the acid-stable NPs after washing with distilled water. As shown in this illustration, dark NPs with sizes of less than 50 nm can be observed in TEM images, indicating different magnetite NPs with higher electron density. The chemical composition of these NPs was studied using the X-ray diffraction (XRD) method and is depicted in the lower illustration in Figure5. The Fe3O4 composite MNPs were observed after the acid leaching process and verified the connection between MNPs and elemental gold (also lower illustration in Figure5). Some negligible impurities may be remained after acid leaching, so advanced characterization methods such as high resolution TEM or elemental mapping is necessary to differentiate between the core/shell structure and other impurities. The magnetite properties of prepared Fe3O4-Au composite MNPs before and after acid wash treatment with HCl (1 N) were also investigated, using the vibrating sample magnetometer (VSM) method. The magnetic hysteresis curves of bare (a) Fe3O4 MNPs, (b) Fe3O4-Au composites MNPs, and (c) Fe3O4 core@Au shell MNPs are demonstrated in Figure6 and show magnetite saturation conditions for all magnetite nanomaterials. No considerable change was observed in superparamagnetism properties of this magnetite material (Fe3O4) either before or after the acid washing process with HCl (1 N) for 18 h (Figure6b,c). Furthermore, we have tested HCl solutions with higher normality of 2, 3, 4, and 5 N for the dissolution of non-core/shell composite NPs, but in all experiments, non-magnetic properties were observed from the remaining nanomaterials (data not shown).
Representative transmission electron microscopic images. Of Fe3O4-Au composite nanoparticles treated with hydrochloric acid (1 N) for 18 h at room temperature and separated by an external magnetic field (upper illustration). Lower illustration shows X-ray diffraction pattern of these nanoparticles verifying the connection of magnetite nanoparticles with elemental gold.
The existence of a carboxyl group on the surface of the prepared magnetite-gold nanocomposites fabricated by a semi-biosynthetic with ethanol extract of Eucalyptus camaldulensis has previously been demonstrated by the Fourier transform infrared spectroscopy (FTIR) method[10] and confirmed in this investigation (Figure7a). IR peaks, centered at 3,430 and 1,720 cm−1, confirmed the presence of a carboxyl group on the surface of the semi-biosynthesized Fe3O4-Au nanocomposites (Figure7a). Furthermore, this carboxyl group can also be detected on the surface of Fe3O4-Au core/shell composites MNPs after acid wash treatment (Figure7b) showing that carbonyl groups have not been chemically changed or degraded during acid treatment of Fe3O4-Au composite MNPs.
However, it should be noted that the magnetite properties of Fe3O4-Au composite MNPs was not optimized during this study, and further optimization process should be performed on the magnetization of the Fe3O4-Au composite MNPs which makes them better candidate for biomedical applications. Also, no assay was carried out to determine the biocompatibility of the above Fe3O4-Au core/shell composites MNPs and merit further investigation.
Conclusions
Different chemical or biological methods have been reported for synthesizing Fe3O4 MNPs and Fe3O4 core@Au shell MNPs[16–20]. However, when Fe3O4-Au core/shell nanocomposites are synthesized, some undesired NPs, such as malformed structures or incomplete core/shell Fe3O4-Au nanocomposites, may be formed. These types of MNPs are categorized as impurities and need to be removed from the final product. In the current study, an acid wash treatment has been developed to remove all undesired nanomaterials (Figure1). The physiochemical properties of isolated Fe3O4-Au composite MNPs before and after acid wash treatment were investigated by different characterization methods, such as TEM, EDS, XRD, VSM, and FTIR. The results show that the acid wash process did not change the surface chemistry and magnetite properties of Fe3O4 core@Au shell MNPs and can be used to reduce impurities and obtain the defect-free core/shell fraction of Fe3O4-Au composite MNPS.
Methods
Semi-biosynthesis of magnetite Fe3O4-Au nanocomposites
Carboxyl-capped magnetite Fe3O4-Au nanocomposites were prepared at room temperature, using the previously described methods[14, 21]. For this purpose, FeCl3·6H2O (0.487 g) and FeSO4·7H2O (0.25 g) were precisely weighted and dissolved in 200 ml of deionized water.
The mixture was then treated through a drop-by-drop addition of 10 ml NaOH solution (0.15 M), with mixing (200 rpm), under an argon atmosphere. The final pH of the reaction mixture was adjusted to pH12, with an excess volume of NaOH (0.15 M). The reaction mixture was then further incubated for 30 min at 80°C. The resulting black precipitate was separated by centrifugation (8,000×g). The separated MNPs were washed four times with deionized water. The prepared Fe3O4 MNPs were separated from the solution with a magnet. The reduction of Au3+ onto the surface of Fe3O4 MNPs was carried out using an ethanol extract prepared from E. camaldulensis leaves[14].
The E. camaldulensis leaves were air-dried at room temperature and then pulverized (50 g). The ethanol extract was prepared by macerating the powder (250 g) for 72 h with three changes of the solvent (1,000 ml) at room temperature. The combined solvent extracts were evaporated to yield a brownish or greenish viscous residue. A stock solution (10 mg/ml) of the plant extract was prepared in ethanol for further experiments and reserved in the refrigerator, at 4°C. Chloroauric acid was purchased from Merck, Darmstadt, Germany. MNPs (20 mg) were dispersed in 30 ml of aqueous HAuCl4 (chloroauric acid) solution (1 mM) and mixed with 1 ml of an ethanol extract of E. camaldulensis (10 mg/ml). The resulting mixture was allowed to stand for 45 min at room temperature, and then the prepared magnetite Fe3O4-Au nanocomposites were separated from the colloid by a magnet. Finally, the NPs were washed three times with distilled water and subjected to the next experiments.
Acid wash treatment
HCl solution (1 N) was used to remove bare Fe3O4 MNPS or other malformed structures from magnetite Fe3O4-Au nanocomposites (Figure1). Prepared composite NPs (100 mg) were mixed with HCl solution (600 ml, 1 N) and incubated for 18 h at room temperature. Then, the remaining composite NPs were separated from the solution by an external magnetic field. These separated NPs were washed three times with deionized water and subjected to further characterization.
Characterizations
All samples were characterized by transmission electron microscopy (model EM 208 Philips, Amsterdam, The Netherlands), EDS, XDS (Philips X' Pert Pro), and VSM. The surface chemistry of the composite MNPs before and after acid treatment was also studied by the FTIR(Nicolet Magna 550, Madison, WI, USA).
References
Corchero JL, Villaverde A: Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009, 27: 468–476. 10.1016/j.tibtech.2009.04.003
Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V: Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials 2008, 29: 4012–4021. 10.1016/j.biomaterials.2008.07.004
Chertoka B, Moffatb BA, Davida AE, Yua F, Bergemannc C, Rossb BD, Yang VC: Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29: 487–496. 10.1016/j.biomaterials.2007.08.050
Mahmoudi M, Milani AS, Stroeve P: Synthesis, surface architecture and biological response of superparamagnetic iron oxide nanoparticles for application in drug delivery: a review. Int. J. Biomed. Nanosci. Nanotechnol. 2010, 1: 164–201. 10.1504/IJBNN.2010.034651
Mahmoudi M, Simchi A, Imani M: Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J. Iran. Chem. Soc. 2010, 7: S1-S27. 10.1007/BF03246181
Lu Q, Yao K, Xi D, Liu Z, Luo X, Ning Q: Synthesis and characterization of composite nanoparticles comprised of gold shell and magnetic core/cores. J. Magn. Magn. Mater. 2006, 301: 44–49. 10.1016/j.jmmm.2005.06.007
Tsuji M, Nishio M, Jiang P, Miyamae N, Lim S, Matsumoto K, Ueyama D, Tang X: Role of chloride ions in the formation of Au@Ag core-shell nanocrystal structures by using a microwave-polyol method. Colloid. Surface. A. 2008, 317: 247.
Rosenholm JM, Zhang J, Sun W, Gu H: Large-pore mesoporous silica-coated magnetite core-shell nanocomposites and their relevance for biomedical applications. Micropor. Mesopor. Mat. 2011,145(1–3):14–20.
Maltas E, Ozmen M, Cingilli Vural H, Yildiz S, Ersoz M: Immobilization of albumin on magnetite nanoparticles. Mater. Lett. 2011,65(23–24):3499–3501.
Sun Q, Reddy BV, Marquez M, Jena P, Gonzalez C, Wang Q: Theoretical study on gold-coated iron oxide nanostructure: Magnetism and bioselectivity for amino acids. J. Phys. Chem. C 2007, 111: 4159–4163. 10.1021/jp067095x
Cho SJ, Idrobo JC, Olamit J, Liu K, Browning ND, Kauzlarich SM: Growth mechanisms and oxidation resistance of gold-coated iron nanoparticles. Chem. Mater. 2005, 17: 3181–3186. 10.1021/cm0500713
Liu XG, Geng DY, Liang JM, Zhang ZD: Magnetic stability of Al2O3-coated FCC-Co nanocapsules. J. Alloy Compound. 2008, 465: 8–14. 10.1016/j.jallcom.2007.10.128
Tartaj P, Morales MP, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ: The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2003, 36: 182–197. 10.1088/0022-3727/36/13/202
Haratifar E, Shahverdi HR, Shakibaie M, MollazadehMoghaddam K, Amini M, Montazeri H, Shahverdi AR: Semi-biosynthesis of magnetite-gold composite nanoparticles using an ethanol extract of Eucalyptus camaldulensis and study of the surface chemistry. J. Nanomater. 2009, 2009: 5.
Yan A, Liu X, Qiu G, Wu H, Yi R, Zhang N, Xu J: Solvothermal, synthesis and characterization of size-controlled Fe3O4 nanoparticles. J. Alloy Compound. 2008, 458: 487–491. 10.1016/j.jallcom.2007.04.019
Li Y, Xu G, Zhu YL, Ma XL, Cheng HM: SnO2/In2O3 one-dimensional nano-core-shell structures: synthesis, characterization and photoluminescence properties. Solid State Commun. 2007, 142: 441–444. 10.1016/j.ssc.2007.03.031
Lu H, Salabas EL, Schuth F: Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46: 1222–1244. 10.1002/anie.200602866
Qiua JD, Penga HP, Lianga RP, Xiab XH: Facile preparation of magnetic core–shell Fe3O4@Au nanoparticle/myoglobin biofilm for direct Biosensors and Bioelectronics. Biosense. Bioelectron. 2010, 25: 1447–1453. 10.1016/j.bios.2009.10.043
Pradhan A, Jones RC, Caruntu D, O’Connor CJ, Tarr MA: Gold–magnetite nanocomposite materials formed via sonochemical methods. Ultrason. Sonochem. 2008, 15: 891–897. 10.1016/j.ultsonch.2008.01.004
Lima J, Tiltona RD, Eggemanc A, Majetichc SA: Design and synthesis of plasmonic magnetic nanoparticles. J. Magn. Magn. Mater. 2007, 311: 78–83. 10.1016/j.jmmm.2006.10.1169
Martínez-Mera I, Espinosa-Pesqueira ME, Pérez-Hernández R, Arenas-Alatorre J: Synthesis of magnetite (Fe 3 O 4 ) nanoparticles without surfactants at room temperature. Mater. Lett. 2007, 61: 4447–4451. 10.1016/j.matlet.2007.02.018
Acknowledgements
This work has been supported by the Pharmaceutical Sciences Research Center, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. Also, the authors kindly thank the team of Scribendi Company for the language editing and proofreading assistance provided for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ARS is the leader of the group and the main writer of this study's manuscript. HM and AA both participated in synthesis and characterization of nanoparticles. Also, AA and HS commented on EDS and XRD experiments. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Montazeri, H., Amani, A., Shahverdi, H.R. et al. Separation of the defect-free Fe3O4-Au core/shell fraction from magnetite-gold composite nanoparticles by an acid wash treatment. J Nanostruct Chem 3, 25 (2013). https://doi.org/10.1186/2193-8865-3-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-25