Abstract
Background
Testing for human epidermal growth factor receptor-2 (HER-2) in breast cancer is performed by either immunohistochemistry (IHC) or in situ hybridization (ISH). The growth factor receptor-bound protein-7 (GRB7) gene is in close proximity to HER-2 on chromosome 17q11-12 and codes a signal transduction molecule shown to be an independent adverse marker in breast cancer.
Methods
HER-2 and GRB7 protein expression from 613 frozen breast tumors was determined by Western analysis. HER-2 protein results were confirmed with IHC. Commercial HER-2 FISH was performed on a subset of tumors with multi-probe FISH used to assess the extent of HER-2 gene amplification. mRNA expression was determined by Multi-plex RT-PCR.
Results
Seven tumors with GRB7 protein over-expression scored HER-2 FISH amplified but had no HER-2 protein over-expression. Four of the 7 tumors showed elevated GRB7 but not HER-2 mRNA over-expression. The breast cancer cell line HCC3153 did not over-express HER-2 protein but showed HER-2 FISH amplification of a limited segment around the HER-2 gene. Ten breast cancer tumors from the TCGA database had gene copy number increases around HER-2 without HER-2 mRNA or protein over-expression.
Conclusions
A subset of human breast cancers that test positive with FISH for HER-2 gene amplification do not over-express HER-2 protein. One mechanism for this discordance is the incomplete amplification of the smallest HER-2 region of chromosome 17q11-12, which includes GRB7. HER-2 gene amplification without protein over-expression is clinically significant because patients with such tumors are unlikely to benefit from HER-2 targeted therapy.
Similar content being viewed by others
Introduction
Amplification of chromosome 17q11-12 occurs in about 20-25% of breast tumors leading to over-expression of the human epidermal growth factor receptor 2 gene (HER-2 or ERBB2) (Slamon et al. [1987]; Slamon et al. [1989]; Ross et al. [2003]). The HER-2 gene encodes a tyrosine kinase receptor and is the best-studied gene present in the amplicon. Because chromosome 17q11-12 amplification was initially detected in frozen breast tumor specimens by Southern blot analysis using a HER-2 probe, it is historically known as HER-2 amplification (Tandon et al. [1989]; Kallioniemi et al. [1992]). Chromosome 17q11-12 amplification has been subsequently found to correlate with HER-2 over-expression on both the mRNA and protein levels in a molecularly fully characterized breast tumor cohort (Press et al. [2002]). Most studies of chromosome 17q11-12 amplification have focused on the HER-2 gene such that HER-2 gene amplification and HER-2 protein over-expression have come to be recognized as important markers of clinically aggressive breast cancer and the target of specifically directed therapies (Press et al. [2008]; Goldenberg [1999]; Xia et al. [2002]).
HER-2 protein, when over-expressed, is the molecular target for specific therapies such as Trastuzumab, a humanized monoclonal antibody that binds to the extracellular domain of the HER-2 protein (Goldenberg [1999]), and Lapatinib a small molecule inhibitor of the intracellular tyrosine kinase domain of both HER-2 and epidermal growth factor receptor (HER-1) (Xia et al. [2002]; Kim & Murren [2003]). Considerable data indicate that HER-2 protein over-expression is required for the responsiveness to either therapy (Press et al. [2008]; Mass et al. [2005]; Di Leo et al. [2008]). Both Trastuzumab and Lapatinib have received approval by the FDA for the treatment of HER-2 positive breast cancer and are associated with improved clinical outcome in metastatic (Slamon et al. [2001]; Geyer et al. [2006]) and, for Trastuzumab, early stage HER-2 positive breast cancer (Romond et al. [2005]; Piccart-Gebhart et al. [2005]).
Despite success in treating HER-2 positive breast cancer patients with these therapies, considerable debate continues to exist regarding which method of testing of HER-2 represents the best assessment of a patient’s HER-2 status (Bartlett et al. [2001]; Wolff et al. [2007]; Sauter et al. [2009]; Press et al. [2005]; Hammock et al. [2003]; Troxell et al. [2006]; Tse et al. [2011]; Pauletti et al. [1996]; Pauletti et al. [2000]; Perez et al. [2012]). The FDA has approved immunohistochemical (IHC) assay methods (Herceptest and Pathway), fluorescence in situ hybridization (FISH) assays (PathVysion; INFORM; and FISH pharmDx) and the newer chromogenic in situ hybridization (CISH) assays (SPOT-Light; INFORM dual CISH; and CISH pharmDx). The American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) recently created a set of joint guidelines for the laboratory evaluation of HER-2 status (Wolff et al. [2007]). They recommend either using IHC assays for initial evaluation of HER-2 status followed by reflex testing by FISH for some IHC categories (i.e. 2+) or utilization of FISH in initial testing (Wolff et al. [2007]).
In addition to HER-2, there are a number of other chromosome 17q11-12 genes, including closely neighboring GRB7, which may be amplified and over-expressed concurrently with HER-2 (Luoh [2002]; Kao & Pollack [2006]; Kauraniemi & Kallioniemi [2006]; Bai & Luoh [2008]; Stein et al. [1994]; Glynn et al. [2010]). The GRB7 gene codes for a multi-domain signal transduction molecule, and is known to play important roles in tumor growth and migration (Shen & Guan [2004]). The GRB7 protein can interact with HER-2 and multiple other signaling proteins, including receptor and non-receptor tyrosine kinases (Shen & Guan [2004]). Located less than 15 kb away from the HER-2 gene, the GRB7 gene is contained well within the smallest amplified region of the HER-2 amplicon on chromosome 17q11-12. Amplification of GRB7 and other neighboring genes is typically associated with increased transcriptional activation and may have significant prognostic and predictive value in breast cancer treatment independent of the HER-2 gene (Ramsey et al. [2011]; Nadler et al. [2010]).
Though they are usually over-expressed together, our Western blotting analysis of 563 breast tumors from a well-annotated breast tumor repository found a significant discordance in GRB7 and HER-2 protein over-expression, allowing us to investigate the independent prognostic significance of GRB7 protein over-expression in breast cancer (Ramsey et al. [2011]). We found that patients whose tumors had isolated GRB7 protein over-expression had the worst prognosis. In addition, our studies revealed that protein over-expression of GRB7 is a stronger independent adverse prognostic factor than HER-2 over-expression (Ramsey et al. [2011]), a conclusion which is seemingly at odds with the widely held perspective of the role of HER-2 protein over-expression as the sole driver of adverse prognosis in chromosome 17q11-12 amplified breast tumors. The role of GRB7 protein in breast cancer was recently further validated when GRB7 was found to be the only significant adverse prognostic factor from among 394 gene candidates examined in a uniformly treated patient population with triple negative breast cancer, an especially aggressive form of the disease (Sparano et al. [2011]; Giricz et al. [2011]).
With the current study we seek to elucidate possible mechanisms of isolated GRB7 over-expression and to explore resultant implications for current HER-2 testing and treatment regimens.
Methods
Tissues, cell culture, and databases
Tumor specimens analyzed in this study were derived from the Oregon Health and Science University (OHSU) Knight Cancer Institute Breast Cancer Repository (Ramsey et al. [2011]; Christianson et al. [1998]; Saez et al. [2006]). These tumors were originally submitted by community hospitals in Oregon, Washington and Alaska to the College of American Pathologists (CAP) - certified OHSU Hormone Receptor Laboratory between 1985-1998, for the purpose of routine evaluation of estrogen and progesterone receptor proteins. Tumors were snap-frozen within thirty minutes of biopsy or mastectomy. Frozen tissues remaining following the completion of the analysis were banked and have been maintained continuously frozen at –80 C. A waiver of informed consent and HIPAA authorization for research use of remaining tissue was approved by Institutional Review Boards (IRB) at the Oregon Health and Science University and community hospitals in accordance with federal and local privacy laws (OHSU IRB # IRB00000211).
Snap-frozen primary breast tumor tissues were also purchased from the National Disease Research Interchange (NDRI). Tumor pathology was verified and anonymized at the sites of origin and obtained according to protocols approved by local Institutional Review Boards.
Human breast cancer cell lines including HCC 3153 were obtained from ATCC and the laboratory of Dr. Joe Gray (OHSU Knight Cancer Institute) and maintained in RPMI with 10% FCS (Neve et al. [2006]). Protein extraction and Western blotting analysis for HER-2 and GRB7 protein expression were performed as previously described (Ramsey et al. [2011]).
The Cancer Genome Atlas (TCGA) project includes a database of comprehensive genomic and epigenetic information currently for 847 primary breast tumors. Data are deposited in standard formats in the TCGA Data Coordinating Center and are available on line through a data portal (Cancer Genome Atlas [2012]).
HER-2 fluorescence in situ hybridization (FISH) analysis
HER-2 FISH was performed using the FDA-approved Abbott PathVysion kit on 4 μm frozen sections. Our protocol was adjusted from the FDA recommended protocol to fit within the confines of our study. This kit contains a probe for the HER-2 locus (17q12, SpectrumOrange) and the chromosome 17 centromere (CEP17) as the chromosome 17 control probe (D17Z1,17p11.1-q11.1, SpectrumGreen). OCT compound was removed by three 8-12 hour washes in 4°C 70% EtOH. Following removal of OCT compound, slides were fixed in 3:1 methanol:acetic acid for 5 minutes and baked at 95°C for 5 minutes. The preparations were then pepsin treated, denatured, hybridized, washed, and counterstained according to the manufacturer’s instructions. Fluorescence was visualized on a CytoVysion image capture system (Applied Imaging, San Jose, CA) with Nikon E800 (Nikon, Melville, NY) microscope. HER-2 and CEP17 signals were enumerated in 25 to 60 cells and a total HER-2/CEP17 ratio was calculated. HER-2 signal counts greater than 10 were considered too numerous to count and scored as >10, as defined by clinical laboratory standards. A ratio of >2.2 was considered to be amplified, <1.8, not amplified, and 1.8 to 2.2, equivocal, based on ASCO/CAP guidelines.
Multiple probe FISH
We developed a multi-probe FISH analysis to examine the extent of HER-2 gene amplification using two flanking probes that define the centromeric and telemetric ends of the smallest amplified region of the HER-2 region; BAC probe 3014 and 3079 respectively. Clones were purchased from Invitrogen as GeneHogs (HS996) cultures in glycerol stocks and grown in LB broth with chloramphenicol. BACs were prepared using a Qiagen Midi kit according to protocol. Sequencing was done to confirm clone using primers T7 and Sp6. Purified BAC DNA from 3014 and 3079 were labeled with an Abbott Nick Translation kit. Purified 5′ probe #3014 was hybridized and labeled with Abbott SpectrumGreen (green fluorescence). Purified 3′probe #3079 was labeled with Abbott SpectrumOrange (orange fluorescence), and the commercially available chromosome 17 centromere probe with Abbott SpectrumAqua (aqua fluorescence). Coalescence of red and green fluorescence signals yield yellow signals indicating that sequences represented by both probes are amplified in one contiguous unit. FISH scores represent the ratio of BAC 3014 and BAC 3079 probes to the chromosome 17 centromeric region (D17Z1).
BAC Probe #3014 begins 35 kb upstream of the HER-2 gene promoter and extends further upstream encompassing the centromeric end of the smallest region of amplification (Staaf et al. [2010]). The sequence of one end of the BAC insert has been previously reported (aq154496). We sequenced both ends of its insert and verified that BAC #3014 does reside upstream of HER-2 and that the insert spans 72 kb.
BAC probe #3079 begins in the middle of GRB7 gene and extends further downstream and away from the HER-2 gene towards the telomere. Both ends of the BAC insert have been sequenced and verified as reported (AQ121537 and AQ 121760). BAC #3079 maps immediately downstream and 15 kb away from the 3′-end of the HER-2 transcription unit. #3079 represents the telemetric end of the smallest region of amplification. The size of insert of #3079 is 136 kb.
HER-2 immunohistochemistry (IHC)
HER-2 IHC was performed by the College of American Pathologist (CAP) certified OHSU Clinical Immunohistochemistry Laboratory using an FDA approved kit with modifications for frozen tissues. Four mm frozen sections were acetone fixed, and stained on an FDA-approved Ventana XT instrument using anti-HER-2/neu 4B5 Rabbit Monoclonal Antibody (PathwayTM, Ventana, Tucson AZ) and a protocol optimized for frozen tissue. As slides were not formalin fixed, antigen retrieval was not necessary. Prediluted primary antibody was applied for 16 min at 37 degrees. Washing, secondary antibody incubation, and detection were performed using standard instrument protocols with Ultraview detection (Ventana).
Multi-plex RT-PCR assay
RNA was isolated from frozen tumor slices using an Ambion RNAqueous kit. The ProtoScript MuLV Taq RTPCR kit was used for the detection of GRB7 and HER-2 mRNAs from a single first strand cDNA synthesis followed by PCR amplification with HER-2 or GRB7 specific primers and β- actin as internal control (Invitrogen), respectively. The primer concentrations for amplification of GRB7 was 2 μM, HER-2; 1 μM and β-actin; 3 μM. Thermal cycling was initiated at 94°C for 5 minutes, followed by 34 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 2 minutes. Final extension was 70°C for 5 minutes. Detection was by agarose gel visualization. The sequence and product length of the oligonucleotide primer pairs used in this study are as follows: β-actin, forward primer 5′- AAG AGA GGC ATC CTC ACC CT-3′, reverse primer 5′- TAC ATG GCT GGG GTG TTG AA - 3′, product length 218 bp. GRB7 (growth factor receptor-bound protein 7) forward primer 5′ - AGG AAA CTT CGA GAG GAG GA- 3′, reverse primer 5′ - TTG GAC TCG TTC ACA TCT GC -3′, product size 847 bp. HER-2 (human epidermal growth factor receptor 2) forward primer 5′ - GGA AAC CTG GAA CTC ACC TA- 3′, reverse primer 5′ - TTG GTG TCT ATC AGT GTG AGA - 3′, product size 388 bp.
Results
Previous results of Western analysis (Figure 1a and Additional file 1: Table S1) of primary breast cancer extracts from the OHSU Knight Cancer Institute Breast Cancer Tumor Repository for the presence of GRB7 and HER-2 proteins reported that GRB7 over-expression was found in the absence of HER-2 over-expression in 30 of 564 or 5% of tumors (Ramsey et al. [2011]). When tumors with this discordant GRB7/HER-2 protein expression pattern were analyzed for HER-2 gene amplification with an FDA approved, commercially available FISH assay (PathVysion, Abbott Labs), 6 of 27 samples scored amplified for HER-2 (Table 1). Additionally, one frozen tumor specimen among 50 obtained from National Disease Research Interchange (NDRI) was also found to have isolated GRB7 protein over-expression and scored HER-2 FISH amplified.
GRB7 and HER-2 expression in frozen breast tumor tissues. a GRB7 and HER-2 Western Blot Analysis. Densitometric analysis is presented in Additional file 1: Table S1. b HER-2 IHC testing of HER-2 FISH positive tumors which do not over-express HER-2 protein by Western blot analysis. Sample 012 is positive control with 3+ HER-2 over-expression; sample 002 is negative control with 0 HER-2 expression. Magnification bars represent 50 micron. c GRB7 and HER2 mRNA expression by multiplex RT-PCR. Top panel: GRB7 and β-actin, Bottom panel: HER-2 and β-actin. Amplified: positive control with HER-2 and GRB 7 over-expression. Non-amplified: negative control - without either HER-2 or GRB7 over-expression. H2O: negative control without RT product. X: 4 tumors over-expressing GRB7 but not HER-2. O: 1 tumor over-expressing both GRB7 and HER-2.
IHC analysis of the seven discordant tumors with a HER-2 immunohistochemistry (IHC) assay revealed scores of either 0 or 1+ for HER-2 protein expression. Some of these tumors exhibited a granular cytoplasmic pattern of HER-2 staining but the intensity and pattern were distinctly different from the 3+ strongly positive membrane staining pattern (Figure 1b) (Taylor et al. [1998]).
In order to examine the transcript levels of HER-2 and GRB7, we developed a multi-plex RT-PCR assay with β- actin as an internal control (Figure 1c). Among the 7 tumors, 4 showed elevated GRB7 mRNA expression but no elevated HER-2 mRNA expression. Two tumors demonstrated neither GRB7 nor HER-2 mRNA over-expression, while results for the seventh tumor indicated both HER-2 and GRB7 mRNA over-expression (Table 2). Sequencing of the coding region of HER-2 for the seventh tumor was conducted and revealed no apparent deleterious sequence changes in the coding region.
Multi-probe FISH analysis, designed to examine the extent of HER-2 gene amplification in samples with apparent HER-2 gene amplification but no concurrent HER-2 protein over-expression, used two flanking probes that define the centromeric and telemetric ends of the smallest amplified segment of the HER-2 region; BAC probe 3014 and 3079 respectively (Figure 2a) (Staaf et al. [2010]). One sample out of 7 demonstrated decreased levels of gene amplification with these flanking probes; a ratio drop from 2.5 to below 2.0, indicating a lack of complete amplification of the smallest amplified region from HER-2 in this sample (Sample ID 131) (Figure 2b).
A multiple-probe FISH analysis of the HER-2 and GRB7 segment from human chromosome 17q11-12. a A 140 kb genomic segment centered on HER-2 from human chromosome 17q11-12. a A 140 kb genomic segment centered on HER-2 from human chromosome 17q11-12. A. The stippled box represents the smallest amplified region from HER-2 previously identified. B. Five genes from this region shown are STARD3, PGAP3, HER-2, GRB7 and IKZF3. C. The mapping positions of two BACs (#3014 and #3079) that define the centromeric and telomeric ends of the smallest amplified region are shown. D. Filled box represents the extent of gene amplification from the HER-2 region for the cell line HCC-3153. b FISH analysis of human breast tumor cells from tumor #031. HER-2 FISH was performed using the Abbott PathVysion kit on 4 μm frozen sections. Probe for the HER-2 locus (17q12, SpectrumOrange) and the chromosome 17 centromere (CEP17) as the chromosome 17 control probe (D17Z1,17p11.1-q11.1, SpectrumGreen). BAC 3079, BAC 3014. Purified 5′probe #3014 was labeled with Abbott SpectrumGreen (green fluorescence). Purified 3′probe #3079 was labeled with Abbott SpectrumOrange (orange fluorescence), and the commercially available chromosome 17 centromere probe with Abbott SpectrumAqua (aqua fluorescence). Coalescence of orange and green fluorescence signals yield yellow signals indicating that sequences represented by both probes are amplified in one contiguous unit.
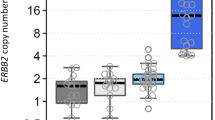
To confirm our observation that at least in some breast cancers apparent HER-2 gene amplification without HER-2 over-expression is due to incomplete, or unproductive, amplification of the smallest amplified HER-2 region, we examined an independent set of primary breast tumor data from The Cancer Genome Atlas Project (TCGA) and a panel of established breast cancer cell lines from SU2C (Stand up to Cancer) (Heiser et al. [2012]). The HCC 3153 cell line, which does not over-express HER-2 or GRB7 RNA, scored amplified by currently available HER-2 FISH testing with a ratio of 2.65 (Figure 3a). Array hybridization revealed that cell line HCC3153 has both HER-2 and GRB7 genes amplified but that the extent of the amplification does not extend to cover the smallest amplified region in its entirety (accession no. EGAS00000000059 in the European Genome-Phenome Archive (EGA); and accession no. E-MTAB-181 in the ArrayExpress) (44). Western analysis confirmed the lack of HER-2 protein over-expression (Figure 3b). HCC3153, therefore, is a cell line model of HER-2 gene amplification without HER-2 over-expression. A survey of gene copy number for 847 primary breast tumors available in the TCGA database found 10 tumors which had gene copy number increases in only part of the 140 kb genomic segment centering around HER-2. These samples have a log2 ratio of 0.5 or higher, a value commonly used as the cut-off for amplification in array analysis, for part of this region from the GISTIC2 database. Five samples out of ten had ratios greater than 1.0, a cut off for high level of 17q11-12 amplification. None of the samples demonstrated HER-2 mRNA over-expression based on RNAseq analysis in TCGA. Data regarding HER-2 protein expression determined by IHC, available for all but one tumor, showed that all specimens scored less than 3+ for HER-2 staining (Table 3).
A human breast cancer cell line with HER-2 gene amplification but no concurrent HER-2 over-expression. a HER-2 gene amplification in breast cancer cell line HCC3153 Red: HER-2 by PathVysion probe; Green: Chromosome 17centromere. b Western analysis of HER-2 protein expression of breast cancer cell lines.
Discussion
Working with a well-annotated frozen breast tumor repository, our laboratory has been studying tumors with amplification of the chromosome 17q11-12 region. As part of these studies, we previously performed Western analysis of the expression of HER-2 and GRB7 protein, the products of two closely linked genes, which are often over-expressed in tandem when 17q11-12 amplification is present. Analysis of these data finds discordance in HER-2 and GRB7 protein over-expression and shows GRB7 protein over-expression to be a stronger independent predictor of adverse prognosis than the over-expression of the HER-2 protein. Additionally, we found that the breast cancer patient population with isolated GRB7 protein over-expression appears to have the worst prognosis (Ramsey et al. [2011]). Studies by others likewise report that GRB7 protein over-expression is an adverse prognostic factor in triple negative breast cancer (Nadler et al. [2010]; Sparano et al. [2011]).
In order to elucidate possible mechanisms involving GRB7 protein over-expression without HER-2 over-expression, we performed a standard clinical FISH analysis for HER-2 gene amplification. Surprisingly, 6 of 27 tumors scored positive for HER-2 gene amplification with this assay. This result was unexpected since these samples did not over-express HER-2 protein by Western analysis. Though these patients were diagnosed as HER-2 positive by FISH analysis, the gold standard for HER-2 clinical laboratory testing, such patients seem unlikely to benefit from HER-2 targeted therapy given the absence of HER-2 protein.
Because the accurate determination of HER-2 status is essential to treatment, we subjected our set of tumors to a standard clinical assay for HER-2 protein by IHC and found those results to be consistent with the lack of HER-2 protein over-expression found with Western analysis. However, using a Multi-plex RT-PCR assay, we found mixed results for HER-2 and GRB7 mRNA expression. Four out of 7 specimens over-expressed GRB7 but not HER-2 mRNA. This result seems consistent with over-expression of the GRB7 protein rather than HER-2 in some tumors. This suggests that GRB7 can be the focus for gene amplification and selective retention of the 17q11-12 segment and lends further evidence to the role of GRB7 protein over-expression as an independent adverse prognostic factor in breast cancer.
Two of 7 samples failed to over-express GRB7 or HER-2 mRNA despite isolated GRB7 protein over-expression. One of the 7 samples (sample ID 213) over-expressed both HER-2 and GRB7 mRNA but sequencing of the coding region of HER-2 mRNA for this tumor found no mutation in its open reading frame, suggesting post-transcriptional regulation of HER-2 expression. The mRNA and protein expression pattern in this tumor is nonetheless consistent with the concept of GRB7 gene being the focus of 17q11-12 amplification and selective retention.
Cases of HER-2 FISH positive but IHC negative tumors have been reported in the past, making up about 2% of all breast cancer patients or 10% of HER-2 FISH positive breast tumors (Sauter et al. [2009]; Lipton et al. [2010]). Much of this discrepancy has been attributed to pre-analytic and analytic issues associated with IHC of paraffin preserved sections. Our finding of frozen breast tumor samples that score positive for HER-2 gene amplification by FISH but do not over-express HER-2 protein by Western analysis nor HER-2 mRNA by RT-PCR, indicates some of these tumors may indeed be true HER-2 FISH false positives- i.e. HER-2 gene amplification without concomitant protein over-expression. Of note, we did not perform HER-2 FISH analysis of all 563 patient samples in our cohort, but based on the distribution and incidence of HER-2 FISH amplified cases in subsets of this tumor cohort we estimate 10% of tumors that were FISH amplified would not over-express HER-2 protein on Western analysis.
In an attempt to understand the mechanism of isolated GRB7 protein over-expression, we speculated that perhaps only a portion of the HER-2 gene is amplified. The HER-2 FISH probe utilized in the commonly employed Abbott PathVysion Kit covers a 190 kb genomic segment of chromosome 17q11-12 that contains both HER-2 and GRB7 genes and may score positive when only part of the HER-2 gene or GRB7 gene alone is amplified. Therefore we developed a multi-probe FISH assay with a set of flanking probes that demarcate the centromeric and telomeric ends of the smallest amplified region, which was previously identified as 90 kb in length (Staaf et al. [2010]). Utilizing this strategy, we were able to perform multi-probe FISH analysis on 6 of the 7 samples. Four of the samples (131, 517, 213 and 031) showed different and lower copy numbers when flanking probes were used rather than the commercial probe, which targets the central portion of the amplified region. In the case of specimen 131, the amplification appears to fall short of covering the smallest amplified region in its entirety, possibly explaining why this sample, though scoring positive for HER-2 gene amplification by current commercial assay, fails to over-express HER-2 protein or HER-2 mRNA. Current HER-2 FISH testing strategy cannot accurately discern the HER-2 amplification status of this sample.
We turned to the SU2C and TCGA databases to search for a similar pattern of HER-2 protein expression and gene amplification in breast cancer cell lines and other patient cohorts. We found that the breast cancer cell line HCC 3153 displays an amplification in only part of the smallest amplified region from the 17q11-12 region based on array analysis, though scoring positive with the standard HER-2 FISH assay. This cell line fails to over-express either HER-2 or GRB7 mRNA or protein. Thus we have identified a cell line model of HER-2 gene amplification without protein or mRNA over-expression that independently confirms our observation using banked frozen tumors.
A survey of the TCGA database (N = 847) found 10 tumors with only partial amplification of the HER-2 region based on array hybridization, though these tumors would be expected to score amplified with currently available HER-2 FISH assays. None of these tumors exhibited HER-2 mRNA or protein over-expression. This result indicates in this cohort derived from the TCGA database that about 5% of HER-2 amplification events occur without concurrent HER-2 over-expression and that this is due to partial amplification of the HER-2 region. This observation helps confirm our view that incomplete or partial amplification of the smallest amplified region of HER-2 may be problematic for current FISH and CISH assay methods that use only a single HER-2 probe.
In conclusion, our work provides evidence that the GRB7 gene may be the focus of some cases of the chromosome 17q11-12 amplification and selective retention, further strengthening the prognostic importance of GRB7 protein over-expression in breast cancer. In addition, we have established that a subset (approximately 5-10%) of primary breast cancers, considered as HER-2 amplified by the current FISH assay, are actually HER-2 false positive due to incomplete amplification of the smallest HER-2 region. Given this circumstance, multi-probe FISH may represent a potential strategy to avoid this pitfall and improve HER-2 FISH testing accuracy. Accurate determination of HER-2 status is essential since patients whose tumors have HER-2 gene amplification but no protein over-expression are unlikely to benefit from current HER-2 targeted therapies.
References
Bai T, Luoh SW: GRB-7 facilitates HER-2/Neu-mediated signal transduction and tumor formation. Carcinogenesis 2008, 29: 473-479.
Bartlett JM, Going JJ, Mallon EA, et al.: Evaluating HER2 amplification and overexpression in breast cancer. J Pathol 2001, 195: 422-428. 10.1002/path.971
Cancer Genome Atlas N: Comprehensive molecular portraits of human breast tumours. Nature 2012, 490: 61-70. 10.1038/nature11412
Christianson TA, Doherty JK, Lin YJ, et al.: NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 1998, 58: 5123-5129.
Di Leo A, Gomez HL, Aziz Z, et al.: Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 2008, 26: 5544-5552. 10.1200/JCO.2008.16.2578
Geyer CE, Forster J, Lindquist D, et al.: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006, 355: 2733-2743. 10.1056/NEJMoa064320
Giricz O, Calvo V, Pero SC, et al.: GRB7 is required for triple-negative breast cancer cell invasion and survival. Breast Cancer Res Treat 2011, 133: 607-615.
Glynn RW, Miller N, Kerin MJ: 17q12-21 - the pursuit of targeted therapy in breast cancer. Cancer Treat Rev 2010, 36: 224-229. 10.1016/j.ctrv.2009.12.007
Goldenberg MM: Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther 1999, 21: 309-318. 10.1016/S0149-2918(00)88288-0
Hammock L, Lewis M, Phillips C, Cohen C: Strong HER-2/neu protein overexpression by immunohistochemistry often does not predict oncogene amplification by fluorescence in situ hybridization. Hum Pathol 2003, 34: 1043-1047. 10.1053/S0046-8177(03)00409-X
Heiser LM, Sadanandam A, Kuo WL, et al.: Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci USA 2012, 109: 2724-2729. 10.1073/pnas.1018854108
Kallioniemi OP, Kallioniemi A, Kurisu W, et al.: ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA 1992, 89: 5321-5325. 10.1073/pnas.89.12.5321
Kao J, Pollack JR: RNA interference-based functional dissection of the 17q12 amplicon in breast cancer reveals contribution of coamplified genes. Gene Chromosome Canc 2006, 45: 761-769. 10.1002/gcc.20339
Kauraniemi P, Kallioniemi A: Activation of multiple cancer-associated genes at the ERBB2 amplicon in breast cancer. Endocr Relat Cancer 2006, 13: 39-49. 10.1677/erc.1.01147
Kim TE, Murren JR: Lapatinib ditosylate GlaxoSmithKline. IDrugs 2003, 6: 886-893.
Lipton A, Kostler WJ, Leitzel K, et al.: Quantitative HER2 protein levels predict outcome in fluorescence in situ hybridization-positive patients with metastatic breast cancer treated with trastuzumab. Cancer 2010, 116: 5168-5178. 10.1002/cncr.25430
Luoh SW: Amplification and expression of genes from the 17q11–q12 amplicon in breast cancer cells. Cancer Genet Cytogenet 2002, 136: 43-47. 10.1016/S0165-4608(01)00657-4
Mass RD, Press MF, Anderson S, et al.: Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 2005, 6: 240-246. 10.3816/CBC.2005.n.026
Nadler Y, Gonzalez AM, Camp RL, et al.: Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann Oncol 2010, 21: 466-473. 10.1093/annonc/mdp346
Neve RM, Chin K, Fridlyand J, et al.: A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10: 515-527. 10.1016/j.ccr.2006.10.008
Pauletti G, Godolphin W, Press MF, Slamon DJ: Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13: 63-72.
Pauletti G, Dandekar S, Rong H, et al.: Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000, 18: 3651-3664.
Perez EA, Dueck AC, McCullough AE, et al.: Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst 2012, 104: 159-162. 10.1093/jnci/djr490
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al.: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005, 353: 1659-1672. 10.1056/NEJMoa052306
Press MF, Slamon DJ, Flom KJ, et al.: Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol 2002, 20: 3095-3105.
Press MF, Sauter G, Bernstein L, et al.: Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 2005, 11: 6598-6607. 10.1158/1078-0432.CCR-05-0636
Press MF, Finn RS, Cameron D, et al.: HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 2008, 14: 7861-7870. 10.1158/1078-0432.CCR-08-1056
Ramsey B, Bai T, Hanlon Newell A, et al.: GRB7 protein over-expression and clinical outcome in breast cancer. Breast Cancer Res Treat 2011, 127: 659-669. 10.1007/s10549-010-1010-0
Romond EH, Perez EA, Bryant J, et al.: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005, 353: 1673-1684. 10.1056/NEJMoa052122
Ross JS, Fletcher JA, Linette GP, et al.: The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 2003, 8: 307-325. 10.1634/theoncologist.8-4-307
Saez R, Molina MA, Ramsey EE, et al.: p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res 2006, 12: 424-431. 10.1158/1078-0432.CCR-05-1807
Sauter G, Lee J, Bartlett JM, et al.: Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 2009, 27: 1323-1333. 10.1200/JCO.2007.14.8197
Shen TL, Guan JL: Grb7 in intracellular signaling and its role in cell regulation. Front Biosci 2004, 9: 192-200. 10.2741/1229
Slamon DJ, Clark GM, Wong SG, et al.: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235: 177-182. 10.1126/science.3798106
Slamon DJ, Godolphin W, Jones LA, et al.: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244: 707-712. 10.1126/science.2470152
Slamon DJ, Leyland-Jones B, Shak S, et al.: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001, 344: 783-792. 10.1056/NEJM200103153441101
Sparano JA, Goldstein LJ, Childs BH, et al.: Relationship between quantitative GRB7 RNA expression and recurrence after adjuvant anthracycline chemotherapy in triple-negative breast cancer. Clin Cancer Res 2011, 17: 7194-7203. 10.1158/1078-0432.CCR-10-3357
Staaf J, Jonsson G, Ringner M, et al.: High-resolution genomic and expression analyses of copy number alterations in HER2-amplified breast cancer. Breast Cancer Res 2010, 12: R25. 10.1186/bcr2568
Stein D, Wu J, Fuqua SA, et al.: The SH2 domain protein GRB-7 is co-amplified, overexpressed and in a tight complex with HER2 in breast cancer. Embo J 1994, 13: 1331-1340.
Tandon AK, Clark GM, Chamness GC, et al.: HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989, 7: 1120-1128.
Taylor SL, Platt-Higgins A, Rudland PS, et al.: Cytoplasmic staining of c-erbB-2 is not associated with the presence of detectable c-erbB-2 mRNA in breast cancer specimens. Int J Cancer 1998, 76: 459-463. 10.1002/(SICI)1097-0215(19980518)76:4<459::AID-IJC2>3.0.CO;2-Q
Troxell ML, Bangs CD, Lawce HJ, et al.: Evaluation of Her-2/neu status in carcinomas with amplified chromosome 17 centromere locus. Am J Clin Pathol 2006, 126: 709-716. 10.1309/9EYM6VE58F2YCD9F
Tse CH, Hwang HC, Goldstein LC, et al.: Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol 2011, 29: 4168-4174. 10.1200/JCO.2011.36.0107
Wolff AC, Hammond ME, Schwartz JN, et al.: American Society of Clinical Oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007, 131: 18.
Xia W, Mullin RJ, Keith BR, et al.: Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002, 21: 6255-6263. 10.1038/sj.onc.1205794
Acknowledgement
We would like to acknowledge Nichole Owen for assistance with FISH images.
Funding
This work is supported in part by Portland VA Research Foundation and OHSU Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SWL designed the experiments, participated in all aspects of the experiments and drafted the manuscript. BR is the guardian of the breast cancer tissue repository and processed the human breast tumor tissues and performed the Western and RT-PCR assays on the tumor samples. AHN and SO performed the FISH assays. MT performed IHC assays and histological analysis of the frozen breast tumor tissues. ZH performed the cell line Western analysis. KC performed the data analysis and prepared the manuscript. PS participated in the analysis of the TCGA data. EK provided the banked frozen tumor tissues for analysis, participated in all aspects of the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
40064_2013_544_MOESM1_ESM.tiff
Additional file 1: Densitometric analysis of Western blotting results of Figure 1a. (TIFF 1 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Luoh, SW., Ramsey, B., Hanlon Newell, A. et al. HER-2 gene amplification in human breast cancer without concurrent HER-2 over-expression. SpringerPlus 2, 386 (2013). https://doi.org/10.1186/2193-1801-2-386
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-2-386