Abstract
Background
Human epidermal growth factor receptor 2 (HER2) positive breast carcinomas due to HER2 amplification are associated with aggressive behavior and a poor prognosis. Anti-HER2-targeted therapies are widely used to treat HER2-positive breast carcinomas with excellent outcomes. Accurate identification of HER2 amplification status in breast carcinomas is of important diagnostic and treatment value. Currently, HER2 amplification status is routinely determined by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) testing. This study will review our past HER2 data to determine and characterize discordant results between HER2 IHC and FISH. It will also determine a potential impact of HER2 amplification status by next-generation sequencing (NGS) on these patients.
Methods
We reviewed a total of 4884 breast carcinomas with coexisting HER2 IHC and HER2 FISH performed at our institution from 2010 to 2022. 57 cases also had a Next-Generation-Sequencing-based (NGS) gene panel performed. Given the advances in biostatic analysis pipelines, NGS methods were utilized to provide results on HER2 amplification status along with somatic mutations.
Results
While the majority (ranging from 98.5% with IHC score of 0 and 93.1% with IHC score of 1 +) of 4884 breast carcinomas had concordant results from HER2 IHC and HER2 FISH testing, a small percentage of patients (ranging from 1.5% in those with IHC score of 0, to 6.9% with IHC score of 1 +) had discordant results, with negative HER2 IHC and positive HER2 FISH results. These patients could be reported as HER2-negative breast carcinomas if only HER2 IHC testing has been performed according to a current cost-effective HER2 test strategy. 57 patients had HER2 amplification status determined by NGS, and all patients had concordant results between HER2 NGS and FISH tests. A HER2-amplified breast carcinoma by NGS had a negative IHC and a positive HER2 FISH result. This case was classified as a HER2-positive breast carcinoma, had anti-HER2-targeted therapy, and achieved a complete clinical response.
Conclusions
A small percentage of HER2-positive breast carcinomas are unidentified because of a negative HER2 IHC based on our current cost-effective HER2 test strategy. It is not feasible and affordable in routine clinical practice to perform HER2 FISH for the cases with negative HER2 IHC (IHC score 0 and 1 +). Therefore, NGS assays capable of simultaneously detecting both somatic mutations and HER2 amplification could provide a more comprehensive genetic profiling for breast carcinomas in a clinical setting. Identification of HER2 amplification by NGS in HER2-positive breast carcinomas with negative HER2 IHC results is important since these cases are concealed by our current cost-effective HER2 test strategy with IHC first (for all cases) and FISH reflex (only for cases with IHC score of 2 +), and would offer the opportunity for potentially beneficial anti-HER2-targeted therapies for these patients.
Similar content being viewed by others
Background
Breast cancer is the most common cancer and second leading cause of death among all cancers in women [1] and is a heterogeneous disease [2]. Human epidermal growth factor receptor 2 (HER2) due to amplification of its coding gene has been described in approximately one fifth of primary invasive breast carcinomas [3,4,5]. HER2 amplified (HER2-positive) breast carcinomas are associated with aggressive behavior and a poor prognosis compared with those in which HER2 is not amplified [4]. Anti-HER2-targeted therapies are widely used to treat HER2-positive breast carcinomas with excellent outcomes and have no role in the treatment of HER2-negative breast carcinomas [6,7,8,9].
Adjuvant Anti-HER2-targeted therapies significantly improve outcomes for patients with HER2-positive early breast cancer. In HER2-positive early breast cancer, anti-HER2 therapy together with neoadjuvant chemotherapy has become the standard of care as achievement of pathological complete response is correlated with improved progression-free survival and disease-free survival [10]. In the neoadjuvant setting, dual HER2-blockade with trastuzumab and pertuzumab together with chemotherapy improves rates of pathological complete response and is, therefore, considered standard of care [11]. In patients with HER2-positive metastatic breast cancer, progression-free survival and overall survival were significantly improved and maintained after first-line therapy with anti-HER2 monoclonal antibodies pertuzumab and trastuzumab in combination together with docetaxel (pertuzumab group) [12, 13]. HER2-targeted therapy has dramatically changed the natural history of HER2-positive metastatic breast cancer, and the recommended first-line therapy for HER2-positive metastatic breast cancer consists of the anti-HER2 monoclonal antibodies trastuzumab and pertuzumab [14]. Therefore, accurate identification of HER2 amplification status in breast carcinomas is of important diagnostic and treatment value and is necessary to ensure adequate patient treatment management, better treatment planning, and avoid patient exposure to unnecessary and potentially harmful treatments.
All patients undergo HER2 testing upon primary breast cancer diagnosis, relapsed and metastatic setting to inform treatment decisions [15]. HER2 amplification status is routinely determined by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) testing [16]. The HER2 IHC testing indirectly measures overexpression of HER2 receptors on the surface of breast cancer cells, based on the intensity of the color reaction. HER2 protein expression status by IHC ranges from 0 to 3 + , with HER2 IHC scores of 0 reported as HER2-negative breast carcinomas, and HER2 IHC score of 3 + reported as HER2-positive breast carcinomas [17]. Currently, breast cancer with HER2 IHC score of 1 + or 2 + and negative FISH result defines as HER2-low breast cancer [18, 19]. HER2 FISH testing measures the exact number of copies of the HER2 gene per nucleus and the ratio between the HER2 gene and a control probe to determine HER2 amplification status. Both the HER2 IHC and FISH testing are US Food and Drug Administration (FDA) approved methods for the determination of HER2 amplification status. As a routine practice for all newly diagnosed breast cancer in pathology, the HER2 test strategy with a low cost-effectiveness ratio involves screening all newly diagnosed breast cancers with IHC testing (a screening test), and reflexing HER2 IHC score of 2 + for evaluation by FISH testing (as a confirmation/follow-up test) [17, 20]. Interpretation of HER2 IHC and HER2 FISH test results include five different groups for breast carcinomas with HER2 IHC score of 2 + further evaluated by HER2 FISH testing [21,22,23]. HER2 FISH testing is usually not performed for invasive breast carcinomas with IHC score of 0 or 1 + and positive (IHC 3 +) HER2 IHC testing results [21,22,23]. Although HER2 IHC and FISH testing are mostly concordant, a small percentage of patients (~ 1.5%) have been described to have discordant HER2 IHC and FISH results with negative HER2 IHC and positive HER2 FISH results [24]. These patients showed either complete remission or partial remission after anti-HER2-targeted therapies [24]. Somatic DNA mutations in invasive breast carcinomas are important [25] and could be commonly detected by next-generation sequencing (NGS) methods. Given the advances in biostatic analysis pipelines, NGS DNA data could be utilized to provide results on both somatic mutations and copy number alterations (such as gain, amplification, loss) in the same assay [26, 27]. NGS data could be used to confirm HER2 amplification in HER2-positive invasive breast carcinomas with positive HER2 IHC results and to reveal HER2 amplification in HER2-positive invasive breast carcinomas with negative HER2 IHC results.
Here, we performed a retrospective review of concurrent HER2 IHC and HER2 FISH performed at our institution over the past 12 years to determine the frequency of breast carcinomas with discordant results between HER2 IHC and FISH, such as negative HER2 IHC and positive HER2 FISH results. These breast carcinomas would be reported as HER2-negative breast carcinomas according to negative HER2 IHC results if only IHC had been performed. Given the advances in biostatic analysis pipelines, NGS methods detect not only somatic mutations, but also have been utilized to evaluate HER2 amplification status. 57 breast carcinoma specimens had NGS data for somatic mutations and HER2 amplification status determined using NGS copy number alteration pipelines [28]. HER2 amplification status was further compared between HER2 NGS and FISH testing to determine whether HER2 amplification status by NGS could be useful to reveal a subset of HER2-positive breast carcinomas with a negative IHC and a positive HER2 FISH result. Identification of HER2 amplification by NGS in HER2-positive breast carcinomas with negative HER2 IHC results is important to reveal those cases that are hidden by our current HER2 test strategy with IHC first (for all cases) and FISH reflex (only for cases with IHC score of 2 +).
Methods
Patients and samples
A clinical database was maintained for breast carcinomas that had coexisting HER2 IHC and HER2 FISH results as part of routine clinical testing from January 1, 2010 to April 30, 2022. We identified a study cohort (n = 4884 cases) with breast carcinoma as a general diagnosis. A subset (n = 57) of breast carcinoma specimens in this study cohort also had HER2 amplification status determined by NGS. The institutional review board of the hospital approved this study (JHIRB00339499).
HER2 immunohistochemistry (HER2 IHC)
HER2 immunohistochemistry was performed on the formalin fixed paraffin embedded (FFPE) breast carcinoma specimens and was analyzed with the Ventana PATHWAY system, using an anti-HER2/neu (4B5) rabbit monoclonal primary antibody and Ventana iVIEW DAB Detection Kit (Ventana, Tucson, Arizona, USA). Positive HER2 (IHC score of 3 +) was defined as intense, complete, and circumferential membrane staining in more than 10% of invasive tumor cells. Equivocal HER2 expression (IHC score of 2 +) was defined as weak to moderate complete membrane staining observed in more than 10% of tumor cells, and/or intense complete membrane staining in less than 10% of tumor cells. IHC score of 2–3 + focal was defined as heterogeneous tumor population with some IHC 2 + regions and some IHC 3 + regions. HER2-negative status was defined as either incomplete/faint membrane staining in more than 10% of invasive tumor cells (IHC score of 1 +) or no staining or incomplete/faint membrane staining in less than 10% of invasive tumor cells (IHC score of 0). IHC score of 1–2 + focal was defined as heterogeneous tumor population with some IHC 1 + regions and some IHC 2 + regions. For our institutional standard practice, HER2 IHC was performed on all breast carcinoma cases and primarily the equivocal IHC (sore of 2 +) cases were reflexed to perform HER2 FISH. Occasionally, a subset of breast carcinomas had concurrent HER2 IHC and HER2 FISH tests per clinical requests.
HER2 fluorescence in situ hybridization (HER2 FISH)
HER2 gene amplification by FISH was tested using a HER2 FISH probe set according to the manufacturer’s recommendations (PathVysion HER2 DNA Probe Kit, Vysis, Abbott Molecular, Des Plaines, Illinois, USA). A total of 60 nuclei were visually evaluated with fluorescence microscopy by two technologists scoring blinded from each other using a Zeiss Axioscope system (Carl Zeiss Microscopy, LLC, White Plains, NY, USA). The analysis was performed using Cytovision software version 7.7 (Leica Inc., Buffalo Grove, IL, USA). HER2 FISH results were classified according to the 2018 ASCO/CAP guideline into 5 groups/categories. Groups 1 and 3 are reported as HER2-positive breast carcinomas, and group 5 is reported as HER2-negative breast carcinoma [23]. For groups 2 and 4, an additional 20 nuclei were evaluated by a third blinded technologist and were reported as HER2-negative breast carcinomas for HER2 IHC scores of 2 + [23]. Depending on the date of testing, HER2 FISH results were reclassified according to the 2018 ASCO/CAP guideline [23]. HER2 IHC and HER2 FISH tests were performed on the same tumor block(s) from the same specimen.
HER2 copy number estimation by a NGS assay
The target NGS assay was described previously [28, 29]. Briefly, DNA was extracted from FFPE specimens with the Siemens tissue preparation automated method (Siemens Healthineers, Munich, Germany). DNA concentration was assessed by the Qubit fluorometer according to vendor specification (Thermo Fisher Scientific, Waltham, MA, USA). Library preparation was performed using Kapa Roche HyperPrep reagents (Roche Diagnostics, Inc., Wilmington, MA, USA), and hybrid capture was executed using 40,670 Integrated DNA Technologies probes (Integrated DNA Technologies, Inc., Coralville, IA, USA). Copy number alterations from the NGS data generated using bioinformatics pipelines were described previously [28, 30]. NGS coverage-based copy number estimation based on the sequencing coverage depth was used to calculate Log2 fold change for copy number detection [28]. Log2 ratio thresholds were set at ≥ 1.3 as a positive result for HER2 amplification and at < 1.3 as a negative result for HER2 amplification. HER2 amplification status by NGS has been adopted clinically since last year.
Statistical calculators
Comparison of numerical variables was performed by the Chi-square calculator and Fisher exact test calculator (Social Science Statistics, https://www.socscistatistics.com, last accessed on July 6, 2022). P ≤ 0.05 was considered statistically significant. Comparison of sensitivity, specificity, positive predictive value, negative predictive value, and the accuracy of the HER2 amplification status by NGS and FISH methods was performed using MEDCALC statistical software (https://www.medcalc.org/calc/diagnostic_test.php, last accessed July 6, 2022).
Results
Retrospective review of concurrent HER2 IHC and HER2 FISH
Among 4884 breast carcinomas that had concurrent HER2 IHC and HER2 FISH, over one-fifth of cases (n = 1059, 21.7%) had a negative HER2 IHC result (IHC score of 0 and 1 +) and approximate three-fourth of cases (n = 3563, 73.0%) had an equivocal IHC result (IHC score of 2 +) (Fig. 1). Positive HER2 FISH was detected at 1.5, 6.9, 20.4, 20.9, 44.4, and 91.4% in cases with IHC scores of 0, 1 + , 1–2 + focal, 2 + , 2–3 + focal, and 3 + , respectively (Fig. 1). FISH positivity in the IHC score of the 1 + group was significantly higher than in the IHC score of the 0 group (P < 0.001) (Fig. 1). The IHC 2 + group had a significantly higher FISH positivity compared to negative IHC (score of 0 and 1 +) groups (P < 0.001) (Fig. 1). No significantly different positivity of FISH was observed among IHC scores of 1–2 + focal, 2 + , and 2–3 + focal groups (Fig. 1).
HER2 amplification status by NGS, FISH, and IHC
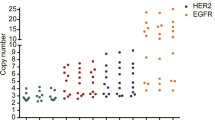
Among 57 breast carcinomas analyzed by NGS, HER2 FISH, and IHC, 3 (5.3%) had HER2 amplification by NGS, all of which had positive HER2 FISH results. 54 (94.7%) were negative for HER2 amplification by NGS, all of which had negative HER2 FISH results. Therefore, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the HER2 amplification status by NGS were 100% compared with HER2 FISH.
Of three breast carcinomas with HER2 amplification by NGS, one (33.3%) had a negative HER2 IHC (score of 1 +) and two (66.7%) had a positive HER2 IHC (score of 3 +). The remaining 54 breast carcinomas had either negative or equivocal HER2 IHC (score of 0–2 +). Therefore, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the HER2 amplification status by NGS were 100%, 98.2%, 66.7%, 100%, 98.3%, respectively, compared with HER2 IHC.
A HER2-positive breast carcinoma by NGS with negative HER2 IHC
A brief clinical presentation of a HER2 amplified breast carcinoma by NGS with negative HER2 IHC follows. A 33 year-old female patient had left breast invasive ductal carcinoma. HER2 IHC testing was negative (score of 1 +) and reported as a HER2-negative breast carcinoma (Fig. 2A, B). IHC also revealed positive estrogen (ER) and negative progesterone (PR) results (data not shown). NGS revealed a pathogenic GATA3 frame-shift mutation, which occurs in approximately 15% of primary ER positive breast carcinomas [25, 31]. No other mutations such as BRCA1, BRCA2, PIK3CA, and ESR1 genes were observed. However, HER2 amplification was detected by NGS based on Log2-R ratio (Fig. 2C). HER2 FISH confirmed the HER2 amplification (Fig. 2D) in this specimen as a group 1 positive HER2 FISH result per 2018 ASCO/CAP breast cancer guideline for dual color probes when the HER2/CEP17 ratio is ≥ 2 and the average HER2 copy number is ≥ 4.0 signals per cell [23]. Positive HER2 FISH results with or without HER2 proteins by IHC are considered HER2-positive breast carcinomas. The patient was then treated with anti-HER2-targeted therapy and showed a complete clinical response.
HER2 amplification by NGS in a breast carcinoma with a negative HER2 IHC result. A, B Microscopy image of HER2 IHC 1 + in a surgical specimen. A is hematoxylin and eosin (H & E) staining and B is HER2 IHC staining. C Amplification of all probes of the HER2 gene by next-generation sequencing based on Log2-R ratio (> 1.3). D HER2 amplification by FISH. Dual-color HER2 FISH revealed ratio of the HER2 to D17Z1 (a centromere control probe) = 4.0, average number of HER2 signals per nucleus = 8.4, and average number of CEP17 signals per nucleus = 2.1
Discussion
In our current laboratory practice, testing for HER2 expression by immunochemistry is routinely performed for all breast carcinomas. HER2 IHC is one of the most rigorously controlled techniques, and guidelines for testing standardization, specimen handling, and reporting were established to guarantee accuracy and decrease laboratorial variability. Although the concordance rate between HER2 IHC and FISH is very high, a small percentage of patients had discordant results with negative HER2 IHC and positive HER2 FISH results: 1.5% positive HER2 FISH in patients with HER2 IHC score of 0, and 6.9% positive HER2 FISH in patients with HER2 IHC score of 1 in this study cohort of 4884 breast carcinomas. Given the significantly high positivity of FISH in the IHC score of 1 + group compared to the IHC score of 0 group in this study, it is important to distinguish between these 2 groups. Although IHC 1 + and IHC 0 groups may have different pathological and clinical features [32, 33], there could be challenges to achieve a good concordance rate by IHC among US laboratories [34].
Several technical and non-technical factors may contribute to the negative IHC and positive FISH breast carcinoma phenomenon. Besides pre-analytic technical issues, such as HER2 IHC signal intensity decreasing over time [35], and IHC performed on old sections stored for more than 6 weeks [23], other biologic factors including intra-tumor genomic heterogeneity, co-amplification/polysomy 17 and monosomy 17 may contribute to this phenomenon [32, 36,37,38,39,40,41,42,43]. HER2 intra-tumor genomic heterogeneity is the co-existence of multiple tumor cell populations with discernibly different levels of HER2 expression within the same tumor, which has been reported in up to half of breast cancers [32, 41,42,43]. Aneuploidy of chromosome 17, including gain (trisomy/polysomy 17) and loss (monosomy 17), has been reported in breast carcinomas [36,37,38,39]. HER2 intra-tumor genomic heterogeneity along with aneuploidy chromosome 17 may further lead to skewing IHC results [41]. Chromosomal microarray or alternative probes on chromosome 17 are required to distinguish between true polysomy 17 and co-amplification/focal amplification [44, 45].
Because of low cost and advances in NGS technology, the NGS gene panel is clinically adopted to be used to identify actionable mutations for targeted therapy. Besides HER2-targeted therapy significantly improving the survival of HER2-positive breast cancer patients, other targeted therapies are also a powerful therapeutic strategy for breast cancer, such as poly (ADP-ribose) polymerase inhibitors (olaparib and talazoparib) for BRCA1/2 mutation carriers [46], PI3K-alpha inhibitors (alpelisib) and estrogen receptor antagonist (fulvestrant) for PIK3CA activating mutations [47], tumor-agnostic tropomyosin receptor kinase inhibitors (larotrectinib and entrectinib) for NTRK fusions [48], immune checkpoint inhibitors (pembrolizumab) for high tumor mutation burden [49], etc. Given progressive biostatic analysis pipelines, the NGS assay is utilized to simultaneously detect both copy number variants and somatic mutations, which could provide more comprehensive genetic profiling for cancer patients using a single assay in a clinical setting. In our current NGS cohort with breast carcinomas, HER2 amplification status by NGS achieves a concordant result compared to HER2 FISH. NGS also identified a HER2 amplified breast carcinoma with a negative HER2 IHC, which could go unidentified by the current HER2 IHC-first test approach. HER2 IHC negative cases are reported as HER2-negative breast carcinomas without follow-up FISH in the majority of US laboratories. Concurrent HER2 IHC and FISH tests are not commonly used because of the high cost and complexity requirements of the FISH testing. This cost-effective test approach will miss a small percentage of breast carcinomas that have discordant HER2 IHC and FISH results.
The current study suggests potential clinical utilities of HER2 amplification status by NGS in a cost-effective HER2 test strategy (Fig. 3). Currently, NGS gene panel is used commonly to identify targetable biomarkers. For breast carcinomas, HER2 IHC and NGS are commonly performed. We outlined an algorithm for the HER2 amplification workup in breast carcinomas based on a common HER2 IHC first approach (Fig. 3). Based on HER2 IHC results, breast carcinomas can be categorized as HER2-negative, equivocal, or positive breast carcinomas (the second decision branch in Fig. 3). Equivocal HER2 IHC (score of 2 +) will automatically reflex to HER2 FISH to further determine HER2 amplification status. For IHC negative and positive breast carcinomas, HER2 amplification status by NGS is recommended to further determine HER2 amplification categories (the third decision branch in Fig. 3). Follow-up HER2 FISH will only be performed for the cases with negative IHC/positive NGS and positive IHC/negative NGS (the fourth decision branch in Fig. 3). This approach dramatically reduces the follow-up HER2 FISH number, since discordant IHC/FISH is only present in a small percentage of IHC scores of 0–1 + and 3 + in this study. Using a combination of HER2 IHC and NGS could achieve the optimum balance of sensitivity and specificity to identify HER2 amplified breast carcinomas among discordant HER2 IHC and FISH results. Identification of HER2 amplified breast carcinomas with negative IHC results is important and might provide potentially beneficial anti-HER2-targeted therapies for these patients. Although NGS mutation pipelines have been widely adopted into clinical labs, NGS copy number alteration pipelines have not been commonly established across all NGS panels. This proposed algorithm has a crucial limitation since not all NGS panels provide information in copy number alterations. However, given further advances in NGS copy number alteration pipelines throughout all NGS panels, this algorithm might become more integrated into the routine diagnostic workflow of clinical labs.
Conclusion
HER2-positive invasive breast carcinomas with negative HER2 IHC results and positive HER2 FISH results are usually concealed by our current HER2 test strategy with HER2 IHC testing as a screening test for all newly diagnosed breast cancer. Given the advances in biostatic analysis pipelines, NGS-based methods could be utilized to provide results on both somatic mutations and copy number alterations (such as HER2 amplification) in the same assay. Identification of HER2 amplification by NGS in HER2-positive invasive breast carcinomas with negative HER2 IHC results would provide the opportunity for potentially beneficial anti–HER2-targeted therapies. Further research is needed to understand the mechanisms of HER2 amplification without detectable HER2 proteins, as well as the exact mechanism by which HER2 antibody–drug conjugates are the most effective in these patients.
Availability of data and materials
The dataset for the current study is available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2020;11:632079.
Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–7.
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–68.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Press MF, Finn RS, Cameron D, Di Leo A, Geyer CE, Villalobos IE, Santiago A, Guzman R, Gasparyan A, Ma Y, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14(23):7861–70.
Gianni L, Llado A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA, Miles D, Salvagni S, Wardley A, Goeminne JC, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(7):1131–7.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, et al. 5 Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34.
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19.
Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–30.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49.
Varga Z, Noske A, Ramach C, Padberg B, Moch H. Assessment of HER2 status in breast cancer: overall positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: a quality control study. BMC Cancer. 2013;13:615.
Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, Provenzano E, Hanby A, Hales S, Lee AH, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–9.
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D, Cardoso F, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–62.
Venetis K, Crimini E, Sajjadi E, Corti C, Guerini-Rocco E, Viale G, Curigliano G, Criscitiello C, Fusco N. HER2 low, ultra-low, and novel complementary biomarkers: expanding the spectrum of HER2 positivity in breast cancer. Front Mol Biosci. 2022;9: 834651.
Dendukuri N, Khetani K, McIsaac M, Brophy J. Testing for HER2-positive breast cancer: a systematic review and cost-effectiveness analysis. CMAJ. 2007;176(10):1429–34.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–22.
Gibbons-Fideler IS, Nitta H, Murillo A, Tozbikian G, Banks P, Parwani AV, Li Z. Identification of HER2 immunohistochemistry-negative, FISH-amplified breast cancers and their response to anti-HER2 neoadjuvant chemotherapy. Am J Clin Pathol. 2019;151(2):176–84.
Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, Pugh M, Jones L, Russell R, Sammut SJ, et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479.
Ross DS, Zehir A, Cheng DT, Benayed R, Nafa K, Hechtman JF, Janjigian YY, Weigelt B, Razavi P, Hyman DM, et al. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn. 2017;19(2):244–54.
Roca I, Gonzalez-Castro L, Fernandez H, Couce ML, Fernandez-Marmiesse A. Free-access copy-number variant detection tools for targeted next-generation sequencing data. Mutat Res Rev Mutat Res. 2019;779:114–25.
Pallavajjala A, Haley L, Stinnett V, Adams E, Pallavajjala R, Huang J, Morsberger L, Hardy M, Long P, Gocke CD, et al. Utility of targeted next-generation sequencing assay to detect 1p/19q co-deletion in formalin-fixed paraffin-embedded glioma specimens. Hum Pathol. 2022;126:63–76.
Haley L, Parimi V, Jiang L, Pallavajjala A, Hardy M, Yonescu R, Morsberger L, Stinnett V, Long P, Zou YS, et al. Diagnostic utility of gene fusion panel to detect gene fusions in fresh and formalin-fixed paraffin-embedded cancer specimens. J Mol Diagn. 2021;23(10):1343–58.
Jiang L, Pallavajjala A, Huang J, Haley L, Morsberger L, Stinnett V, Hardy M, Park R, Ament C, Finch A, et al. Clinical utility of targeted next-generation sequencing assay to detect copy number variants associated with myelodysplastic syndrome in myeloid malignancies. J Mol Diagn. 2021;23(4):467–83.
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–19.
Schettini F, Chic N, Braso-Maristany F, Pare L, Pascual T, Conte B, Martinez-Saez O, Adamo B, Vidal M, Barnadas E, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1.
Marchio C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol. 2021;72:123–35.
Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K, Harigopal M, Jorns JM, Kuba MG, Ly A, et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8(4):1–4.
Mirlacher M, Kasper M, Storz M, Knecht Y, Durmuller U, Simon R, Mihatsch MJ, Sauter G. Influence of slide aging on results of translational research studies using immunohistochemistry. Mod Pathol. 2004;17(11):1414–20.
Vranic S, Teruya B, Repertinger S, Ulmer P, Hagenkord J, Gatalica Z. Assessment of HER2 gene status in breast carcinomas with polysomy of chromosome 17. Cancer. 2011;117(1):48–53.
Brunelli M, Nottegar A, Bogina G, Calio A, Cima L, Eccher A, Vicentini C, Marcolini L, Scarpa A, Pedron S, et al. Monosomy of chromosome 17 in breast cancer during interpretation of HER2 gene amplification. Am J Cancer Res. 2015;5(7):2212–21.
Ballard M, Jalikis F, Krings G, Schmidt RA, Chen YY, Rendi MH, Dintzis SM, Jensen KC, West RB, Sibley RK, et al. ‘Non-classical’ HER2 FISH results in breast cancer: a multi-institutional study. Mod Pathol. 2017;30(2):227–35.
Reinholz MM, Bruzek AK, Visscher DW, Lingle WL, Schroeder MJ, Perez EA, Jenkins RB. Breast cancer and aneusomy 17: implications for carcinogenesis and therapeutic response. Lancet Oncol. 2009;10(3):267–77.
Fumagalli C, Ranghiero A, Gandini S, Corso F, Taormina S, De Camilli E, Rappa A, Vacirca D, Viale G, Guerini-Rocco E, et al. Inter-tumor genomic heterogeneity of breast cancers: comprehensive genomic profile of primary early breast cancers and relapses. Breast Cancer Res. 2020;22(1):107.
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol. 2014;27(1):4–18.
Allott EH, Geradts J, Sun X, Cohen SM, Zirpoli GR, Khoury T, Bshara W, Chen M, Sherman ME, Palmer JR, et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast Cancer Res. 2016;18(1):68.
Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, Li Z. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166(2):447–57.
Tse CH, Hwang HC, Goldstein LC, Kandalaft PL, Wiley JC, Kussick SJ, Gown AM. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol. 2011;29(31):4168–74.
Gunn S, Yeh IT, Lytvak I, Tirtorahardjo B, Dzidic N, Zadeh S, Kim J, McCaskill C, Lim L, Gorre M, et al. Clinical array-based karyotyping of breast cancer with equivocal HER2 status resolves gene copy number and reveals chromosome 17 complexity. BMC Cancer. 2010;10:396.
Cortesi L, Rugo HS, Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol. 2021;16(3):255–82.
Narayan P, Prowell TM, Gao JJ, Fernandes LL, Li E, Jiang X, Qiu J, Fan J, Song P, Yu J, et al. FDA approval summary: alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin Cancer Res. 2021;27(7):1842–9.
Chu P, Batson S, Hodgson M, Mitchell CR, Steenrod A. Systematic review of neurotrophic tropomyosin-related kinase inhibition as a tumor-agnostic management strategy. Future Oncol. 2020;16(4):61–74.
Alva AS, Mangat PK, Garrett-Mayer E, Halabi S, Hansra D, Calfa CJ, Khalil MF, Ahn ER, Cannon TL, Crilley P, et al. Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the targeted agent and profiling utilization registry (TAPUR) study. J Clin Oncol. 2021;39(22):2443–51.
Acknowledgements
We thank all personnel of the Johns Hopkins cytogenomic laboratory involved in FISH, Drs. Guilin Tang and Yun Wu (MD Anderson Cancer Center, Houston, TX), Dr. Yi Zhou (Bio-Reference Laboratories Inc., Elmwood Park, NJ), and Dr. Zeba Singh (University of Maryland, Baltimore, MD) for assistance in HER2 IHC images and troubleshooting.
Funding
The Johns Hopkins Hospital Cytogenomics Laboratory is an academic laboratory supported by the Johns Hopkins School of Medicine Department of Pathology.
Author information
Authors and Affiliations
Contributions
Study concept: LM, AP, and YZ. Data collection: LM, RP, and PL. Manuscript preparation: LM and YZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Johns Hopkins Institutional Review Boards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morsberger, L., Pallavajjala, A., Long, P. et al. HER2 amplification by next-generation sequencing to identify HER2-positive invasive breast cancer with negative HER2 immunohistochemistry. Cancer Cell Int 22, 350 (2022). https://doi.org/10.1186/s12935-022-02761-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02761-1