Abstract
ETV6-ABL1 is a rare gene fusion with oncogenic properties, reported so far in 28 patients presenting a variety of haematological malignancies associated with clinical outcome, including chronic myeloid leukaemia (CML), acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL) and chronic myeloproliferative neoplasm (cMPN). Here we report on a 46-year-old female who presented with Philadelphia negative CML, positive for the ETV6-ABL1 fusion. Whole genome screening carried out with oligonucleotide arrays showed a subtle loss at 12p13 and cryptic imbalances within the 9q34.3 region in a highly unstable genome. FISH mapping with custom BAC probes identified two breakpoints 5 Mb apart within the 9q34 region, together with a break at 12p13. While FISH with commercial BCR-ABL1 probes failed to detect any ABL1 changes, the ETV6 break-apart probe conclusively identified the ETV6-ABL1 fusion thus determining the probe’s role as the primary diagnostic FISH test for this chimeric oncogene. In addition, we confirm the association of the ETV6-ABL1 fusion with imatinib resistance reported so far in three other patients, while recording excellent response to the 2nd generation tyrosine kinase inhibitor (TKI) nilotinib. In summary, we highlight the value of ETV6 FISH as a diagnostic test and the therapy resistance of ETV6-ABL1 positive disorders to imatinib.
Similar content being viewed by others
Introduction
CML is one of the most extensively studied human malignancies and was the first example of a disease, where the underlying molecular basis was a consistent chromosomal abnormality, the Philadelphia chromosome (Ph), that is the product of a balanced reciprocal translocation involving chromosomes 9 and 22. Over 95% of CML patients are found to carry the t(9;22)(q34;q11) and the resultant BCR/ABL1 fusion gene as postulated by WHO classification (2008) [1]. Six other genes in addition to BCR can form a fusion product with ABL1. The chimeric proteins contain the kinase domain of ABL1 and are composed of the N-terminal part of the partner protein that includes a coiled-coil or a helix-loop-helix domain. Fusion genes with a break within intron 1 or 2 of ABL1, such as BCR-ABL1, ZMIZ1-ABL1, EML1-ABL1 and ETV6-ABL1, carry transforming activity, while the NUP214-ABL1 requires amplification to be efficient [2].
The ETV6-ABL1 fusion was reported for the first time in a 22-month-old girl with ALL by Papadopoulos et al. in 1995 [3] and to date, this fusion gene has been reported in 28 cases of haematological malignancy [4–6]. Common characteristics of the ETV6-ABL1 translocation appears to be eosinophilia, seen in 16 out of the 21 patients for which data was available, a 2:1 male predominance and seen in patients with ages varying between 8 months and 81 years. The ETV6-ABL1 fusion is seen in a wide range of haematological malignancies: 5 patients had AML, 10 ALL and 3 with an MPN while the remaining 11 were described as having Ph negative CML, of which 3 presented in a blast crisis, summarised in Table 1[3, 4, 7–24].
The structure of the ETV6-ABL1 oncoprotein is similar to that of BCR-ABL1, as evidenced by the fact that both fusion products lead to activation of the non-receptor tyrosine kinase ABL1 with initiation of similar downstream pathways effecting cellular survival, growth rate and independence as well as transforming capacity [25].
The existence of two different transcripts, type A and B, is evidence for alternative splicing. Type A transcript includes the first four exons of ETV6, fused to exon 2 of ABL1, while type B includes exons 1 to 5 of ETV6 fused to ABL1 exon 2. The difference between the two transcripts at protein level is the presence or absence of a direct binding site for the SH2 domain of the GRB2. Million et al [26] showed that the GRB2 binding site in the ETV6-ABL1 product has several functions - from activation of the GAB2, PI3-kinase, and ERK-MAPK pathways to transformation of fibroblasts and B-lymphoid cells all of which are required for efficient induction of CML-like MPN. Furthermore, they demonstrated that the absence of ETV6 exon 5 leads to a slightly lower tyrosine kinase activity of the type A ETV6-ABL1 protein, although both kinases are as catalytically active as is the BCR-ABL1 product.
Since the ETV6 and ABL1 genes have an opposite orientation to the chromosome centromere, the formation of a fusion requires at least three chromosomal breaks to be generated [2]. This could be the reason for the well-documented low incidence of ETV6-ABL1 fusion cases. Furthermore little is known regarding the effect of treatment with tyrosine kinase inhibitors on ETV6-ABL1 fusion positive haematological conditions.
Here we report a female patient, who presented with Philadelphia negative CML and t(9;12)(q34;p13) as a sole bone marrow karyotype abnormality leading to ETV6-ABL1 fusion formation. Located on der(9)t(9;12), this fusion oncogene is shown to result from multiple events within a 5.6 Mb region at 9q34.12-q34.3, which escapes detection by commercial BCR-ABL1 FISH probes. Importantly, full cytogenetic and molecular remission was achieved only after second generation TKI treatment thus associating the ETV6-ABL1 fusion with TKI resistance.
Case report
In August 2011 a 46-year-old female presented with fatigue, a leucocytosis and thrombocytosis. Blood film examination was suggestive for CML with a neutrophilia, myelocyte peak, basophilia and also of note a eosinophilia of 2.5 × 109/l. The aspirate morphology reported myeloid hyperplasia with reduced erythropoiesis, and increased eosinophilic and basophilic precursors. Histopathology reported a myeloproliferative neoplasm suggestive for CML chronic phase, with a markedly hypercellular bone marrow and myeloid hyperplasia with a left shift and loss of normal architecture. Erythroid activity was markedly reduced - only scattered CD71 positive cells were present. CD34 and CD117 show less than 5% blasts. G-banding identified a balanced t(9;12)(q34;p13) in 10/10 metaphases and interphase FISH was positive in 92% of cells analysed. RT-PCR revealed the presence of ETV6-ABL1 fusion mRNA, while BCR-ABL1 was not detected. Such cases are not adequately covered by the WHO classification of myeloproliferative disorders and could be categorized as a ? CML variant.
Imatinib therapy achieved a complete haematological response (CHR) after 4 weeks and at 3 months, interphase FISH on the peripheral blood was positive in 37/200 cell (18.5%). At 5 months thrombocytosis had recurred with the reappearance of a mild neutrophilia, basophilia and eosinophilia at 6 months. FISH analysis was positive in both interphase (50/200) and metaphase (6/20) cells and residual disease was confirmed by nested RT-PCR. She was switched to nilotinib, once again achieving a CHR after 4 weeks, and a complete cytogenetic response (CCyR) at 3 months. Major molecular response (MMR) was also achieved and in the subsequent specimens taken at 10, 12 and 18 months, ETV6-ABL1 mRNA was no longer detectable with nested RT-PCR. Both CCyR and MMR are sustained to date 22 months from diagnosis.
Methods
(i) Cell Culture and chromosome studies
Bone marrow (BM) and peripheral blood (PB) cell samples were cultured in RPMI medium following routine protocols as previously described [11]. High-resolution chromosome banding analysis was carried out and ISCN 2013 nomenclature was used to describe chromosome abnormalities [18].
(ii) FISH and molecular cytogenetic analysis
Fluorescence in situ hybridization (FISH) investigations were performed using commercially available BCR-ABL1 probes (dual fusion probe, Vysis, USA) and ETV6 (break-apart probe Vysis, USA). Chromosome mapping of the 9q34 regions was carried out with a range of BAC clones (BACPAC Resources, USA). For disease monitoring a double fusion dual colour FISH probe was made with BAC clones covering the regions of ABL1 (RP11-57C19 and RP11-83 J21), ETV6 (RP11-356 K6, RP11-418C2 and RP11-639O1) and NOTCH1 (RP11-707O3 and RP11-678D10) genes.
(iii) RT-PCR
Total RNA was extracted from the cell culture using Trizol reagent (Life Technologies, USA). Purified RNA was reverse transcribed, quality controlled and tested for BCR-ABL1 as described previously [27]ETV6-ABL1 was amplified in pretreatment samples by single step PCR using primers Tel3F2 (5’-CGCTATCGATCTCCTCATTCA-3’) and CA3-b: (5’-ACACCATTCCCCATTGTGAT-3’). Two products were amplified, corresponding to a fusion ETV6 exon 4 to ABL1 exon 2 and ETV6 exon 5 to ABL1 exon 2. Remission samples were tested by nested PCR using primers TEL3F: (5’-CTGCTGACCAAAGAGGACTT-3’) and CA3-: (5’-TGTTGACTGGCGTGATGTAGTTGCTTGG-3’) in the first step and Tel32F2 + CA3-b in the second step. Dilution of pretreatment cDNA into negative control DNA indicated a sensitivity of detection of 10-4.
(iv) Array Comparative Genomic Hybridisation (aCGH)
Array CGH analysis was performed with a high-density customised 400 K oligonucleotide platform enriched with probes covering the coding regions of oncogenes (ID 027193, Agilent technologies, USA) following the manufacturer’s protocol. In brief, 1500 ng genomic test DNA was hybridised against a reference of a pooled DNA samples collected from PBMC of 6 - 8 disease free individuals (Promega, UK). We used Genomic Workbench v.6 (Agilent technologies, USA) for data analysis as previously [28].
Results
(i) Philadelphia chromosome and BCR/ABL1 fusion are absent
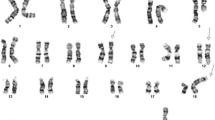
G banding analysis of bone marrow cultures found both homologues of chromosome 22 intact, but identified a translocation of the long arm of chromosome 9 due to t(9;12)(q34;p13) (Figure 1a). FISH analysis using a dual fusion, dual colour BCR-ABL1 probe (Vysis) confirmed the absence of a fusion gene and hence the Philadelphia chromosome. However, some 21% of the interphase BM cells were found to harbour a third, albeit significantly smaller, ABL1 signal found to be absent from the metaphases. The ABL1 part of the D-FISH probe is 650 Kb long and any break upstream of exon 2 would result in two different in size segments. The proximal fragment covering the ASS – ABL1 exon 2 region is larger (~500 Kb) and will remain on der(9)t(9;12). However, the FISH signal from the smaller distal segment (~150 Kb) encompassing ABL1 exon 2 to 11 was not found on the der(12)t(9;12) chromosome as expected, although detected in some of the interphase cells (Figure 1b).
Representative Philadelphia negative metaphase bone marrow cell with a t(9;12)(q34;p13) translocation. (a) G-banded karyotype; (b) FISH analysis demonstrates lack of BCR-ABL1 fusion but reveals a small third signal from the ABL1 probe (arrow) and; (c) The ETV6 split signal (arrowed in red) on der(9)t(9;12) from the break-apart FISH probe shows the gene rearrangement and confirms G banding results. The red/green fusion signal marks the normal gene.
(ii) Formation of the ETV6-ABL fusion at der (9)t(9;12)(q34;p13)
A commercial ETV6 break-apart probe (Vysis) yielded a split signal located on der(9)t(9;12) in all dividing and 92% of the interphase BM cells demonstrating the rearrangement (Figure 1c). RT-PCR using published primers identified both type A and B transcripts of the ETV6-ABL1 fusion thus confirming ABL1 involvement [10]. However, such transcripts are unlikely to be a direct result of the t(9;12)(q34;p13) because of the different orientation of the ETV6 and ABL1 genes. Furthermore, extensive research has already shown [4, 10] that the formation of a functional ETV6-ABL1 fusion gene requires additional genome aberrations. Our failure to detect ABL1 signals on the der(12)t(9;12) in metaphase cells prompted us to search the 9q34 region by FISH 'walking’ for multiple events that could facilitate the formation of the ETV6-ABL1 fusion (Figure 2). We detected a single break within 12p13 region and translocation of the 5’ part of the ETV6 gene (exon 1-5) some 40 Kb distal of the NOTCH1 gene (Figure 1c). This was shown by the presence of the BAC RP11-707O3 on der(9)t(9;12), while the distally located RP11-678D10 is moved to der(12)t(9;12). This FISH signal pattern suggests that ETV6 exons 1 - 5 sequences are located distally to ABL1 (Figure 2, Additional files 1 & 2). Indeed the ABL1 gene appears unaffected since FISH analysis of dividing cells shows both BAC clones that encompass the entire gene (i.e. RP11-57C19 and RP11-83 J21) within the 9q34 region of der(9)t(9;12). In contrast, FISH using either custom 9q34 BAC clones or commercial BCR-ABL1 probes on interphase cells where the chromatin is less condensed allowing the discrimination of closely spaced DNA probes, revealed a third ABL1 signal indicating breakage. The application of a cocktail of BAC clones in a three colour FISH experiment covering all relevant breakpoints in 12p13, 9q34.12 and 9q34.3, showed that in interphase cells the ETV6-ABL1 fusion is a considerable distance away from the proximal part of ABL1 (exon 1b-2) and the neighbouring NOTCH1 gene (Figure 3, arrows), while in metaphase cells their discrimination is impossible. The ETV6-ABL1 fusion (both type A and type B transcripts) was confirmed by the RT-PCR and shown to occur after the break in the exon 1a-2 region of ABL1 and exon 5 of ETV6 as expected. Whole genome scanning using oligonucleotide arrays revealed a total of 37 cryptic copy number aberrations (CNAs), mostly (24/37) gains ( Additional file 3). A cluster of CNAs was found within 9q33-qter including extra copy of the DAB2IP (a tumour suppressor gene with a recognised role in breast and prostate cancer) and within the regions flanking NOTCH1. A cryptic 140 bp loss at chr12: 12,045,340 – 12,045,484 was found at the breakpoint in the ETV6 gene (arrowed in Additional file 3). Other affected genome sites include the TNK2 gene at 3q29, ~3 Mb gain at 8q24.3 that involves TOP1MT and MAPK15 genes among others and extra copies of the MLL gene at 11q23 as well as MLLT1 at 19p13. Among the loci found deleted are transcription co-activator gene CREBBP at 16p13.3 and CBFb at 16q22 (Additional file 3). It is noteworthy that only 2 of the 37 CNAs in this molecular karyotype are reported polymorphic imbalances, the rest represent cryptic secondary changes indicative of genome instability.
FISH mapping of 9q34 and 12p13 regions in t (9;12)(q34;p13). (a) Diagram of 9q34 (top) and 12p13 chromosome regions with a map of the BAC clones used for FISH analysis. Red arrows indicate breakpoint positions in 9q34.12, 9q34.3 and 12p13.2; (b) Exons 2-11 of the ABL1 (RP11-83 J21 in red) on der(9)t(9;12) co-localizing with the 5’ part of the ETV6 gene (RP11-418C2 in green) and (c) The break at 9q34.3, 5.63 Mb distal to ABL1, is flanked proximally by RP11-707O3 (green) which houses the NOTCH1 gene and remains in der(9)t(9;12) and distally by RP11-678D10 (red) which has moved to der(12)t(9;12) (arrowed).
Representative cells with FISH signals showing the cryptic three way rearrangement of ABL1, NOTCH1 and ETV6 associated with t(9;12)(q34;p13). BAC clones covering the regions of interest are as follows: ABL1 (RP11-83 J21 & RP11-57C19, in red), NOTCH1 (RP11-707O3&RP11-678D10, in blue) and ETV6 (RP11-418C2 & RP11-36 K5, in green) (a) FISH signals from all three genes - ETV6, ABL1 and NOTCH1 - cluster at der(9)t(9;12) in a metaphase cell (arrows) (b) in a non-dividing cell the ETV6 (exons 1-5)/ABL1 (exons 2-11) fusion(green/red arrow) is separated from the co-localized ABL1 (exons 1b-2) and NOTCH1 signals (red/blue arrows); while ABL1 and NOTCH1 signals (~5.6 Mb apart) mark the normal 9 homologue and signals from ETV6 (green) and RP11-678D10 (downstream of NOTCH1, blue) co-hybridize at der(12)t(9;12).
Discussion
ETV6 is one of the six genes known to form fusion chimeric transcripts with ABL1. As a rule, the fusion gene results from joining the 3’sequences of ABL1 with the 5’ end of the partner genes. While most of these fusion genes are associated with a specific type of leukaemia, BCR-ABL1 and ETV6-ABL1 are found in a wide spectrum of clinically and morphologically different malignant blood conditions [2]. In spite of their heterogeneity these conditions are likely to share a common progenitor cell, the pluripotent stem cell, since both chimeric genes activate similar transduction pathways with similar transforming activity [29].
The ETV6-ABL1 fusion gene is a truly rare event. So far there are 29 cases including the present study published after the first report in 1995 by Papadopoulos et al. (summarised in Table 1). The rarity of this fusion is due to the opposite transcriptional orientation of the two genes relative to the centromeres, which would require at least two events to form an in-frame fusion transcript. It is therefore not a surprise that the chromosome rearrangement producing the fusion gene often remains hidden. Perhaps the ETV6-ABL1 fusion is not as rare as the literature suggests since there are no satisfactory commercial FISH probes available for the detection of the ETV6-ABL1 fusion. In dividing cells with t(9;12)(q34;p13), the dual colour/dual probe BCR/ABL1 set (D-FISH) should indicate an ABL1 rearrangement by producing a third ABL signal on der(12) while BCR remains intact (Figure 1b). Unfortunately this abnormal signal pattern was not seen in metaphase cells in nearly a third of cases (27%) where both parts of the rearranged ABL1 remain within the 9q34/qter (Table 1). The situation is worse in interphase cells where the third signal may remain unaccounted for due to its small size as illustrated in our case and seen in a further 6 reports with ETV6-ABL1 fusion (Table 1). Analysts using widely available BCR-ABL1 commercial assays on interphase cells may regard the disproportionately small third signal from the ABL1 probe as 'noise’. In contrast, FISH with the ETV6 break-apart (BA) probe will produce a split (third) signal at the der(9) chromosome that is easy to detect in interphase cells of fusion carriers (see Figure 2c). Therefore FISH screening with ETV6 (BA) probe of samples suspected for CML but negative for BCR-ABL1 rearrangement by FISH, provides a reliable way to reassess the 'rarity’ of this fusion gene. Indeed two recent studies [4, 5] have indicated that the incidence of ETV6-ABL1 fusion transcript may well be around 1%, thus confirming our concerns that the occurrence of this translocation in haematological diseases is underestimated.
There are few descriptions in the literature of in-frame fusion products involving genes with different orientations. As a rule, formation of these fusion genes is associated with more than one event and more than the two breaks that are necessary for a classical reciprocal translocation. For example, Van Limbergen and colleagues [10] suggested two alternative mechanism. Our findings support a complex model (see Figure 4) that incorporates some of the suggested changes and highlight the fact that the ETV6-ABL1 fusion resides on der(9)t(9;12) in nearly a third of the cases thus rendering FISH with ABL1 probes unfit for purpose.
Formation of a fusion between the ABL1 and ETV6 genes with opposite chromosomal orientation via two events: (i) firstly, a balanced t(9;12)(q34;p13) results in the juxtaposition of part of the ETV6 gene (exons 1-5) in the vicinity of NOTCH1 at 9q34.3 on der(9)t(9;12) while ABL1 remains intact followed by (ii) an inversion within the 9q34 segment of der(9)t(9;12) after breaks at 9q34.12 (within ABL1 , upstream of exon 2) and 9q34.3. This leads to formation of an ETV6-ABL1 fusion gene but leaves the NOTCH1 gene intact.
Million et al. [30] demonstrated a striking similarity between the ETV6-ABL1 and BCR-ABL1 induced leukaemia in mouse models, the only difference being the latency period. They found that the ETV6-ABL1 fusion protein is significantly more active compared to the p210 BCR-ABL1. Importantly, both fusion proteins were shown to have similar helix-loop-helix domains, which is fused to the kinase domain of ABL1 and implicated in protein oligomerisation process [31]. Indeed ETV6-ABL1 positive patients respond to treatment with tyrosine kinase inhibitors (TKI) as expected. Kawamata et al., reported a favourable response to imatinib in chronic phase CML with normal karyotype and ETV6-ABL1[20]. However, the inhibitory effect of imatinib was short lived and unable to induce a complete remission in two further CML cases [13, 15]. Similarly, Nand et al., [21] reported an ETV6-ABL1 fusion positive patient with a myeloproliferative disorder who developed morphologic and cytogenetic relapse after 17 months on imatinib but achieved complete remission on a second second-generation TKI (nilotinib). After an initial haematological response to imatinib 400 mg daily our patient, who presented with typical chronic phase CML and t(9;12)(q34;p13) as sole karyotype abnormality, progressed at 3 months; whereas nilotinib achieved a prompt CCyR, followed by an MMR 16 months after diagnosis, which is sustained to date. The mechanism of the imatinib resistance reported here and by others is still unexplained. Although the long-term response to second generation TKIs remains to be determined, their implementation as first line therapy in ETV6-ABL1 (+) disorders is well supported.
Conclusion
In summary, we highlight two main features of ETV6-ABL1 positive disorders: firstly, the diagnostic value of FISH with the ETV6 (BA) probe and secondly, while this chimeric oncogene is associated with poor long term response to imatinib, our patient achieved a sustained response to nilotinib.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Al-Achkar W: A chronic myeloid leukemia case with a unique variant Philadelphia translocation: t(9;22;21)(q34;q11;p12). Oncol Lett 2012, 3(5):1027–1029. Epub 2012 Feb 28
De-Braekeleer E, Douet-Guilbert N, Rowe D, Bown N, Morel F, Berthou C, Férec C, De-Braekeleer M: ABL1 fusion genes in hematological malignancies: a review. Eur J Haematol 2011, 86: 361–371. 10.1111/j.1600-0609.2011.01586.x
Papadopoulos P, Ridge SA, Boucher CA, Stocking C, Wiedemann LM: The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res 1995, 55: 34–38.
Zuna J, Zaliova M, Muzikova K, Meyer C, Lizcova L, Zemanova Z, Brezinova J, Votava F, Marschalek R, Stary J, Trka J: Acute leukemias with ETV6/ABL1 (TEL/ABL) fusion: poor prognosis and prenatal origin. Genes Chromosomes Cancer 2010, 49: 873–884. 10.1002/gcc.20796
Zhou M-H, Gao L, Jing Y, Xu Y-Y, Ding Y, Wang N, Wang W, Li M-Y, Han X-P, Sun J-Z, Wang L-L, Yu L: Detection of ETV6 gene rearrangements in adult acute lymphoblastic leukemia. Ann Hematol 2012, 91: 1235–1243. 10.1007/s00277-012-1431-4
Malone A, Langabeer S, O’Marcaigh A, Storey L, Bacon CL, Smith OP: A doctor(s) dilemma: ETV6-ABL1 positive acute lymphoblastic leukaemia. Br J Haematol 2010, 151: 101–102. 10.1111/j.1365-2141.2010.08323.x
Golub TR, Goga A, Barker GF, Afar DE, McLaughlin J, Bohlander SK, Rowley JD, Witte ON: Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol 1996, 16: 4107–4116.
Brunel V, Sainty D, Costello R, Mozziconacci M-J, Simonetti J, Arnoulet C, Coignet L, Bouabdallah R, Gastaut JA, Gabert J: Translocation of BCR to chromosome 9 in a Philadelphia-negative chronic myeloid leukemia. Cancer Genet Cytogenet 1995, 85: 82–84. 10.1016/0165-4608(95)00140-9
Andreasson P, Johansson B, Carlsson M, Jarlsfelt I, Fioretos T, Mitelman F, Höglund M: BCR/ABL-negative chronic myeloid leukemia with ETV6/ABL fusion. Genes Chromosomes Cancer 1997, 20: 299–304. 10.1002/(SICI)1098-2264(199711)20:3<299::AID-GCC11>3.0.CO;2-K
Van-Limbergen H, Beverloo HB, van-Drunen E, Janssens A, Hählen K, Poppe B, Van-Roy N, Marynen P, De-Paepe A, Slater R: Molecular cytogenetic and clinical findings in ETV6/ABL1positive leukemia. Genes Chromosomes Cancer 2001, 30: 274–282. 10.1002/1098-2264(2000)9999:9999<1::AID-GCC1089>3.0.CO;2-1
O’Brien SG: Transient response to imatinib mesylate (STI571) in a patient with the ETV6-ABL t (9;12) translocation. Blood 2002, 99: 3465–3467. 10.1182/blood.V99.9.3465
Lin H, Guo JQ, Andreeff M, Arlinghaus RB: Detection of dual TEL-ABL transcripts and a Tel-Abl protein containing phosphotyrosine in a chronic myeloid leukemia patient. Leukemia 2002, 16: 294. 10.1038/sj.leu.2402353
Keung YK, Beaty M, Steward W, Jackle B, Pettnati M: Chronic myelocytic leukemia with eosinophilia, t(9;12)(q34;p13), and ETV6-ABL gene rearrangement: case report and review of the literature. Cancer Genet Cytogenet 2002, 138: 139–142. 10.1016/S0165-4608(02)00609-X
La-Starza RR, Trubia MM, Testoni NN, Ottaviani EE, Belloni EE, Crescenzi BB, Martelli MM, Flandrin GG, Pelicci PGP, Mecucci CC: Clonal eosinophils are a morphologic hallmark of ETV6/ABL1 positive acute myeloid leukemia. Haematologica 2002, 87: 789–794.
Barbouti A, Ahlgren T, Johansson B, Hoglund M, Lassen C, Turesson I, Mitelman F, Fioretos T: Clinical and genetic studies of ETV6/ABL1-positive chronic myeloid leukaemia in blast crisis treated with imatinib mesylate. Br J Haematol 2003, 122: 85–93. 10.1046/j.1365-2141.2003.04391.x
Tirado C, Sebastian S, Moore J, Gong J, Goodman B: Molecular and cytogenetic characterization of a novel rearrangement involving chromosomes 9, 12, and 17 resulting in ETV6 (TEL) and ABL fusion. Cancer Genet Cytogenet 2005, 157: 74–77. 10.1016/j.cancergencyto.2004.06.001
Meyer-Monard S, Mühlematter D, Streit A, Chase AJ, Gratwohl A, Cross NCP, Jotterand M, Tichelli A: Broad molecular screening of an unclassifiable myeloproliferative disorder reveals an unexpected ETV6/ABL1 fusion transcript. Leukemia 2005, 19: 1096–1099. 10.1038/sj.leu.2403697
Mozziconacci M-J, Sainty D, Chabannon C: A fifteen-year cytogenetic remission following interferon treatment in a patient with an indolent ETV6-ABL positive myeloproliferative syndrome. Am J Hematol 2007, 82: 688–689.
Baeumler J, Szuhai K, Falkenburg JHF, van-Schie MLJ, Ottmann OG, Nijmeijer BA: Establishment and cytogenetic characterization of a human acute lymphoblastic leukemia cell line (ALL-VG) with ETV6/ABL1 rearrangement. Cancer Genet Cytogenet 2008, 185: 37–42. 10.1016/j.cancergencyto.2008.05.001
Kawamata N, Dashti A, Lu D, Miller B, Koeffler HP, Schreck R, Moore S, Ogawa S: Chronic phase of ETV6-ABL1positive CML responds to imatinib. Genes Chromosomes Cancer 2008, 47: 919–921. 10.1002/gcc.20593
Nand R, Bryke C, Kroft SH, Divgi A, Bredeson C, Atallah E: Myeloproliferative disorder with eosinophilia and ETV6–ABL gene rearrangement: Efficacy of second-generation tyrosine kinase inhibitors. Leuk Res 2009, 33: 1144–1146. 10.1016/j.leukres.2009.03.011
Kelly JC, Shahbazi N, Scheerle J, Jahn J, Suchen S, Christacos NC, Mowrey PN, Witt MH, Hostetter A, Meloni-Ehrig AM: Insertion (12;9)(p13;q34q34): a cryptic rearrangement involving ABL1/ETV6 fusion in a patient with Philadelphia-negative chronic myeloid leukemia. Cancer Genet Cytogenet 2009, 192: 36–39. 10.1016/j.cancergencyto.2009.02.012
Park J, Kim M, Lim J, Kim Y, Han K, Kim JS, Lee S, Kim H-J, Min WS: Variant of ETV6/ABL1 gene is associated with leukemia phenotype. Acta Haematol 2013, 129: 78–82. 10.1159/000342490
Perna F, Abdel-Wahab O, Jhanwar SC, Imada K, Nimer SD: ETV6-ABL1-positive “chronic myeloid leukemia”: clinical and molecular response to tyrosine kinase inhibition. Haematol 2011, 96: 342–343. 10.3324/haematol.2010.036673
Malinge S, Monni R, Bernard O, Penard-Lacronique V: Activation of the NF-κB pathway by the leukemogenic TEL-Jak2 and TEL-Abl fusion proteins leads to the accumulation of antiapoptotic IAP proteins and involves IKKα. Oncogene 2006, 25: 3589–3597. 10.1038/sj.onc.1209390
Million RP, Harakawa N, Roumiantsev S, Varticovski L, Van-Etten RA: A Direct Binding Site for Grb2 Contributes to Transformation and Leukemogenesis by the Tel-Abl (ETV6-Abl) Tyrosine Kinase. Mol Cell Biol 2004, 24: 4685–4695. 10.1128/MCB.24.11.4685-4695.2004
Cross NC, Melo JV, Feng L, Goldman JM: An Optimized Multiplex Polymerase Chain Reaction (PCR) for Detection of BCR-ABL Fusion mRNAs in Haematological Disorders. Leukemia 1994, 8: 186–189.
Nacheva EP, Brazma D, Virgili A, Howard-Reeves J, Chanalaris A, Gancheva K, Apostolova M, Valgañon M, Mazzullo H, Grace C: Deletions of immunoglobulin heavy chain and T cell receptor gene regions are uniquely associated with lymphoid blast transformation of chronic myeloid leukemia. BMC Genomics 2010, 11: 41. 10.1186/1471-2164-11-41
Okuda K: ARG tyrosine kinase activity is inhibited by STI571. Blood 2001, 97: 2440–2448. 10.1182/blood.V97.8.2440
Million RP: The Tel-Abl (ETV6-Abl) tyrosine kinase, product of complex (9;12) translocations in human leukemia, induces distinct myeloproliferative disease in mice. Blood 2002, 99: 4568–4577. 10.1182/blood-2001-12-0244
Hannemann JRJ, McManus DMD, Kabarowski JHJ, Wiedemann LML: Haemopoietic transformation by the TEL/ABL oncogene. Br J Haematol 1998, 102: 475–485. 10.1046/j.1365-2141.1998.00803.x
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KG carried out the molecular genetic studies, participated in the design of the study and drafted the manuscript. AV provided the clinical care and treatment and edited the manuscript. NC carried out the diagnostic RT-PCR analysis and edited the manuscript; JHR, DB, PK and FP carried out the diagnostic G-band and FISH analysis together with response to treatment. CG coordinated the array analysis and edited the manuscript. EN conceived, designed and coordinated the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gancheva, K., Virchis, A., Howard-Reeves, J. et al. Myeloproliferative neoplasm with ETV6-ABL1 fusion: a case report and literature review. Mol Cytogenet 6, 39 (2013). https://doi.org/10.1186/1755-8166-6-39
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-6-39