Abstract
Background
Chronic myeloid leukaemia (CML) is one of the most well characterised human malignancies. Most patients have a cytogenetically visible translocation between chromosomes 9 and 22 which generates the pathognomonic BCR::ABL1 fusion gene. The derivative chromosome 22 (‘Philadelphia’ or Ph chromosome) usually harbours the fusion gene encoding a constitutively active ABL1 kinase domain.
A small subset of patients have no visible translocation. Historically, these ‘Philadelphia chromosome negative’ patients caused diagnostic confusion between CML and other myeloproliferative neoplasms; it is now well established that the BCR::ABL1 fusion gene can be generated via submicroscopic intrachromosomal insertion of ABL1 sequence into BCR, or, more rarely, of BCR into ABL1. The fusion genes arising from cryptic insertions are not detectable via G-banded chromosome analysis [karyotype] but can nevertheless always be detected using fluorescence in situ hybridisation (FISH) and/or qualitative reverse transcriptase PCR.
Case presentation
A 43-year-old female presented with suspected CML in 2007; however, contemporaneous gold standard laboratory investigations, G-banded chromosome analysis and FISH, were both negative. The reverse transcriptase quantitative PCR (RT-qPCR) assay available at the time, which was capable of detecting the common BCR::ABL1 transcripts (e13a2/e14a2), was also negative. Upon review in 2009, the newly recommended reverse transcriptase multiplex PCR (capable of detecting all BCR::ABL1 transcripts including the atypical ones) subsequently detected an e19a2 fusion. The patient then responded to tyrosine kinase inhibitor therapy. In contrast, FISH studies of both samples with three commercially available probes remained consistently negative.
Retrospective whole genome sequencing, undertaken as part of the 100,000 Genomes Project, has now revealed that the patient’s BCR::ABL1 fusion gene arose via a uniquely small insertion of 122 kb ABL1 sequences into BCR.
Conclusions
We present a patient with suspected chronic myeloid leukaemia whose genetic investigations were originally negative at the time of diagnosis despite the use of contemporaneous gold standard methods.
This is the first report of a FISH-negative, BCR::ABL1 positive CML which demonstrates that, even after sixty years of research into one of the most well understood human malignancies, whole genome sequencing can yield novel diagnostic findings in CML.
Similar content being viewed by others
Background
CML is one of the most well characterised human malignancies; the toponymous Philadelphia chromosome was discovered in 1960 using some of the earliest genetic techniques [1]. Using newly developed G-banded chromosome analysis in 1973, the Philadelphia chromosome was then established to be a derivative chromosome arising from a balanced translocation between chromosomes 9 and 22 [2]. The involvement of BCR and ABL1 in the fusion gene was established in 1984 and 1986, respectively [3, 4]. This early knowledge of the BCR::ABL1 fusion gene has resulted in CML being the target of many laboratory and clinical firsts, including bone marrow transplantation [5], international standardisation of molecular monitoring [6, 7] and one of the first targeted cancer therapies, imatinib, a tyrosine kinase inhibitor [8].
The BCR::ABL1 fusion gene can be found in four main variant forms (major, minor, micro and nano) defined by the breakpoint location within the BCR gene, and thus the size of the resulting fusion protein. Whilst the breakpoints in ABL1 are relatively tightly clustered in a region surrounding exons 1b to 2, breakpoints in BCR can occur within any of several relatively large regions of the gene. Those falling within the major breakpoint cluster region (M-bcr) yield the common 210 kDa fusion protein (P210BCR::ABL1) which is highly specific for chronic myeloid leukaemia [3]. The M-bcr region spans BCR exons 12 to 16 and is approximately 4 Kb. Philadelphia-positive acute lymphoblastic leukaemia (ALL) is also defined by the BCR::ABL1 fusion gene, however, the majority of fusion genes in ALL arise from rearrangements within the minor breakpoint cluster region (m-bcr). This 72 kb m-bcr region between BCR exons 1 and 2 gives rise to the P190BCR::ABL1 protein [9], found in CML only rarely [10]. The 2 kb micro breakpoint cluster region (µ-bcr) spans BCR introns 18 to 22; these rare transcripts were not identified until more recently [11] and have been associated with neutrophilia [12] and a less severe disease course. Lastly, a handful of patients have been reported with a breakpoint in the nano (ν-bcr) breakpoint cluster region around exon 6 [13].
Approximately 1–2% of CML patients have an apparently normal karyotype. In these patients, the BCR::ABL1 fusion gene arises as a consequence of an insertion of ABL1 sequences into BCR, or vice versa [14]. Such submicroscopic rearrangements are termed ‘masked Ph’ or cryptic insertions. Although it can be inferred that cryptic insertions arise from a minimum of three breakage events, as opposed to the two required for a standard translocation, the presence of a simple, balanced cryptic insertion does not appear to have a prognostic impact [15].
Whilst undetectable by karyotype analysis, cryptic insertions can be detected using FISH [16], reverse transcriptase multiplex PCR and/or the appropriate RT-qPCR assay for the transcript type. Hence, the European Leukaemia Net guidelines in 2013 [17] recommended multiplex PCR at diagnosis to identify the fusion transcript type, and the 2020 guidelines now state that this is mandatory [18]. This recommendation was introduced based on the need to avoid a false impression that a patient with a rare transcript is in complete molecular response after TKI treatment, if tested with the incorrect RT-qPCR assay.
Case presentation
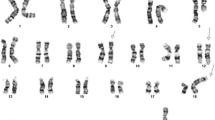
A 43-year old female presented with thrombocytosis of 1453 × 109/L and a mild leucocytosis of 23.2 × 109/L, with left shifted myelopoiesis and neutrophils of 16 × 109/L, eosinophils of 1 × 109/L and basophils of 0.4 × 109/L suspicious of CML. However laboratory investigations performed according to the contemporaneous best practice guidelines [19] did not support this: G-banded chromosome analysis showed a normal female karyotype in all metaphases examined (46,XX[20]) and FISH for BCR::ABL1 was negative (see Fig. 1). Her marrow was hypercellular with increased megakaryocytes and reticulin. On account of the clinical picture and absence of a BCR::ABL1 fusion, she was diagnosed with a Philadelphia-negative myeloproliferative neoplasm and treated accordingly with hydroxycarbamide.
fluorescence in situ hybridisation using dual colour dual fusion probe for BCR (green) and ABL1 (red) showing (top) dual channel image of a derivative chromosome 22 arising from a standard cryptic insertion, (middle) dual channel image of the current patient’s derivative chromosome 22 and (bottom) as above, showing only the single Texas Rad channel. No green signal is detectable
Upon the development of progressive basophilia, characteristic of CML, laboratory investigations were repeated two years later. The newly introduced qualitative PCR, designed to identify all known BCR:ABL1 transcripts [13], detected an e19a2 (µ-bcr) fusion transcript. Retrospective re-examination of cDNA from her first sample confirmed this transcript had been present, however, re-examination of fixed cell suspensions from the same two dates using three different commercially available FISH probes were consistently negative. There were no robust reports of FISH negative CML but a revised diagnosis of CML was made based on the qualitative PCR results. The patient was started on imatinib and achieved complete haematological response. The cryptic nature of the BCR::ABL1 fusion made cytogenetic monitoring impossible, but there was no molecular response. She was subsequently changed to a second generation TKI, dasatinib, and achieved a deep molecular response, with eventually undetectable BCR::ABL1 by RT-qPCR. Treatment free remission was attempted in 2018, but this resulted in loss of molecular response, and dasatinib was restarted. She subsequently re-achieved and has maintained a deep molecular response to date (Fig. 2).
Recently whole genome sequencing (WGS) of DNA derived from the patient’s diagnostic peripheral blood sample, and undertaken as part of the 100,000 Genomes Project, has revealed a 121,681 bp insertion of ABL1 sequence (chr9:130,813,850–130,935,531) into intron 19 of BCR at chr22:23,312,158 (all coordinates refer to ENST00000318560.6/NM_005157.6 and GRCh38; see Fig. 3). This is, to our knowledge, the smallest reported ABL1 insertion giving rise to a functional BCR::ABL1 fusion protein in a CML patient.
Discussion and conclusions
Cryptic insertions of ABL1 sequences into BCR, or vice versa, are not unusual. Approximately 1–2% of CML patients have no apparent Ph chromosome; undertaking FISH in suspected CML referrals with a normal karyotype has thus long been routine. When qualitative reverse transcriptase PCR testing at diagnosis of CML was first recommended by European LeukemiaNet in 2013, this was principally to determine the appropriate transcript for subsequent monitoring in order to avoid falsely assuming a patient had achieved a complete molecular response. This case is unique in that the patient presented with a rare e19a2 fusion and a uniquely small submicroscopic insertion.
The insertion was not detectable using any of three commercially available probes at the time (Abbott Molecular, Illinois; Kreatech Diagnostics, Amsterdam; and Cytocell, Cambridge) despite all three manufacturers’ probe maps indicating they had full coverage of the entire ABL1 coding region and at least 300 kb of sequence up or downstream (Fig. 4). Analysis included dual and single channel examination of at least 200 interphases and/or metaphases, single channel inspection of both fluorochromes and digital enhancement (i.e. artificially boosting the signal to enable visualisation) of both fluorochromes; no ectopic signals could be identified contemporaneously or retrospectively Fig. 1. Until recently, FISH probes were manufactured by nick translation labelling of bacterial artificial chromosome (BAC) clones, or fragmented subclones thereof. Unfortunately, repetitive sequences can cause background fluorescence and it is thus sometimes necessary to exclude certain clones from the target probe mix, which can lead to gaps in the coverage; this may explain the inability of the three probe systems to detect the ABL1 insertion seen in this patient, which, though relatively small nevertheless included the majority of the ABL1 gene and encompassed a region well within the detection limit of FISH. In recognition that FISH probes may have gaps within the probe, FISH probe manufacturers often caveat the probe specification, for example, “breakpoints outside this region, or variant rearrangements wholly contained within this region, may not be detected”. In support of this finding, it is worth noting that a small number of FISH-negative PML::RARA-positive cryptic insertions have been reported in acute promyelocytic leukaemia [20]. Unfortunately, due to more than a decade passing between the initial presentation and the whole genome sequencing results, it was not possible to undertake further FISH testing; it is possible that an ABL1 break apart probe or whole chromosome painting may have sufficiently improved coverage for detection of the insertion.
Schematic showing region of interest from UCSC genome browser with GENCODE v37 transcripts indicated. ABL1 is highlighted in light blue and the size of the current patient’s cryptic insertion is indicated with an asterisk (*). Approximate locations (according to package inserts) of commercial FISH probes are indicated with red bars
The 122 kb insertion included almost 40 kb intronic sequence upstream of the second ABL1 exon (the first exon translated in the BCR::ABL1 fusion protein) and 50 kb downstream (including an intergenic region, the entire QFRFP gene and the majority of the downstream gene FIBCD1), all of which are superfluous for the formation of the fusion gene. Hypothetically, an insertion of just 40 kb could contain all the necessary coding sequences for an in-frame BCR::ABL1 fusion; the smallest possible insertion resulting in a functional BCR::ABL1 fusion gene could be considerably smaller than the one reported here. Conversely, a reciprocal insertion of BCR into ABL1 would require a minimal insertion of just BCR exon 1 to form P190BCR::ABL1. BCR-into-ABL1 insertions are documented, however all those reported to date have all been large enough to be detected by FISH [21].
In summary, we report the first FISH-negative BCR::ABL1 positive chronic myeloid leukaemia patient and have been able to fully characterise the fusion. Whole genome sequencing has demonstrated insertion of 122 Kb of coding ABL1 sequence into intron 19 of BCR resulting in a rare in-frame e19a2 fusion transcript, the smallest cryptic insertion reported to date. Despite over 60 years of research into CML and our advanced understanding of its genetics making it the paradigm for the precision medicine era, whole genome sequencing has only now explained a 15-year diagnostic mystery.
Availability of data and materials
The data that support the findings of this study are available from 100,000 Genomes Project but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from Genomics England Limited upon reasonable request.
Abbreviations
- ALL:
-
Acute lymphoblastic leukaemia
- CML:
-
Chronic myeloid leukaemia
- FISH:
-
Fluorescence in situ hybridisation
- PCR:
-
Polymerase chain reaction
References
Nowell PC, Hungerford. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497–501.
Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–3.
Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36(1):93–9.
Deklein A, Hagemeijer A, Bartram CR, Houwen R, Hoefsloot L, Carbonell F, et al. BCR rearrangement and translocation of the C-ABL Oncogene in Philadelphia positive acute lymphoblastic-leukemia. Blood. 1986;68(6):1369–75.
Fefer A, Cheever MA, Thomas ED, Boyd C, Ramberg R, Glucksberg H, et al. Disappearance of Ph1-positive cells in four patients with chronic granulocytic leukemia after chemotherapy, irradiation and marrow transplantation from an identical twin. N Engl J Med. 1979;300(7):333–7.
Branford S, Fletcher L, Cross NCP, Mueller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–8.
White HE, Matejtschuk P, Rigsby P, Gabert J, Lin F, Wang YL, et al. Establishment of the first world health organization international genetic reference panel for quantitation of BCR-ABL mRNA. Blood. 2010;116(22):E111–7.
Druker BJ, Ohno S, Buchdunger E, Tamura S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–6.
Fainstein E, Marcelle C, Rosner A, Canaani E, Gale R, Dreazen O, et al. A new fused transcript in Philadelphia chromosome positive acute lymphocytic leukaemia. Nature. 1987;330(6146):386–8.
Melo JV, Myint H, Galton DA, Goldman JM. P190BCR-ABL chronic myeloid leukaemia: the missing link with chronic myelomonocytic leukaemia? Leukemia. 1994;8(1):208–11.
Saglio G, Guerrasio A, Rosso C, Zaccaria A, Tassinari A, Serra A, et al. New type of Bcr/Abl junction in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1990;76(9):1819–24.
Pane F, Frigeri F, Sindona M, Luciano L, Ferrara F, Cimino R, et al. Neutrophilic-chronic myeloid leukemia: a distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction). Blood.1996;88(7):2410–4.
Burmeister T, Reinhardt R. A multiplex PCR for improved detection of typical and atypical BCR–ABL fusion transcripts. Leuk Res. 2008;32(4):579–85.
Hagemeijer A, Buijs A, Smit E, Janssen B, Creemers GJ, Plas DVD, et al. Translocation of BCR to chromosome 9: A new cytogenetic variant detected by FISH in two Ph-negative, BCR-positive patients with chronic myeloid leukemia. Genes Chromosom Cancer. 1993;8(4):237–45.
Luatti S, Baldazzi C, Marzocchi G, Ameli G, Bochicchio MT, Soverini S, et al. Cryptic BCR-ABL fusion gene as variant rearrangement in chronic myeloid leukemia: molecular cytogenetic characterization and influence on TKIs therapy. Oncotarget. 2017;8(18):29906.
Sessarego M, Fugazza G, Canepa L, Bacigalupo A, Bruzzone R, Patrone F. Fluorescence in situ hybridization provides evidence for two-step rearrangement in a masked Ph chromosome formation. Leuk Res. 1995;19(12):921–5.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84.
Hochhaus A, Baccarani M, Silver R, Schiffer C, Apperley J, Cervantes F, LeukemiaNet E, et al. recommendations for treating chronic myeloid leukemia. Leukemia. 2020;2020:1–19.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20.
Kim MJ, Cho SY, Kim M-H, Lee JJ, Kang SY, Cho EH, et al. FISH-negative cryptic PML–RARA rearrangement detected by long-distance polymerase chain reaction and sequencing analyses: a case study and review of the literature. Cancer Genet Cytogenet. 2010;203(2):278–83.
Virgili A, Brazma D, Reid AG, Howard-Reeves J, Valgañón M, Chanalaris A, et al. FISH mapping of Philadelphia negative BCR/ABL1 positive CML. Mol Cytogenet. 2008;1(1):14.
Acknowledgements
This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support. The authors acknowledge the contributions made by all molecular pathology staff at Imperial College Healthcare NHS Trust during this patient’s monitoring. JFA, JSK, DM and AJI acknowledge the support of the Imperial College NIHR Biomedical Research Centre. JFA is a NIHR Senior Investigator.
Funding
Not applicable.
Author information
Authors and Affiliations
Consortia
Contributions
The authors confirm contribution to the paper as follows: study conception and design: PCM, AGR & AJI; data collection: PCM & Genomics England; analysis and interpretation of results: PCM, AGR, MER JSK, AJI & Genomics England; draft manuscript preparation: PCM, JFA & AJI. All authors reviewed the results and approved the final version of the manuscript.
Authors’ informations
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All 100,000 Genome Project participants give consent to Genomics England Limited.
Consent for publication
The patient has provided written informed consent for publication.
Competing interests
PCM, AGR, MER, JSK, SC, AGR, AJI: none.
DM: Honoraria from Incyte, BMS, Novartis and Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
May, P.C., Reid, A.G., Robinson, M.E. et al. FISH-negative BCR::ABL1-positive e19a2 chronic myeloid leukaemia: the most cryptic of insertions. BMC Med Genomics 16, 172 (2023). https://doi.org/10.1186/s12920-023-01607-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01607-7