Abstract
N-doped mesoporous TiO2 nanorods were fabricated by a modified and facile sol–gel approach without any templates. Ammonium nitrate was used as a raw source of N dopants, which could produce a lot of gasses such as N2, NO2, and H2O in the process of heating samples. These gasses were proved to be vitally important to form the special mesoporous structure. The samples were characterized by the powder X-ray diffraction, X-ray photoelectron spectrometer, nitrogen adsorption isotherms, scanning electron microscopy, transmission electron microscopy, and UV-visible absorption spectra. The average length and the cross section diameter of the as-prepared samples were ca. 1.5 μm and ca. 80 nm, respectively. The photocatalytic activity was evaluated by photodegradation of methylene blue (MB) in aqueous solution. The N-doped mesoporous TiO2 nanorods showed an excellent photocatalytic activity, which may be attributed to the enlarged surface area (106.4 m2 g-1) and the narrowed band gap (2.05 eV). Besides, the rod-like photocatalyst was found to be easy to recycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Since the exciting discovery of the synthesis of TiO2 - xN x film with an enhanced visible light absorption[1], N-doped TiO2 nanoparticles have been widely studied in the fields of degrading recalcitrant organic contaminants under visible light in recent years[2, 3]. However, practical applications of N-doped TiO2 nanoparticles are greatly limited due to their low recycle rate. To solve this problem, N-doped TiO2 with different morphologies such as nanowires[4], nanotubes[5], hollow spheres[6], and nanorods were prepared[7, 8]. It is well known that N-doped TiO2 nanorods can be fabricated by chemically nitriding TiO2 nanorods. However, with this route, the nitridation is limited in the surface of the nanorods at a very low level, and thin nitridation layer can be easily removed during the photocatalytic reaction[9]. Besides, the rod-like structure leads to the formation of small surface areas in many cases due to the accumulation of the nanoparticles.
In this work, N-doped TiO2 nanorods with mesoporous structure were fabricated by a modified and facile sol–gel approach without any templates. The photocatalytic activity was evaluated by photodegradation of methylene blue (MB) in aqueous solution. The reasons why the N-doped mesoporous TiO2 nanorods showed an excellent photocatalytic activity and photochemical stability had been investigated.
Methods
Materials
In the experiments, deionized water was used. All of the chemicals were analytical grade. TiO2 used for comparison was Degussa P25 (Frankfurt, Germany), whose surface area and particle size were reported as 50 m2 g-1 and 21 nm, respectively[10].
Preparation of N-doped mesoporous TiO2 nanorods
Typically, 5 mL of tetrabutyl titanate (TBOT), 30 mL of ethanol, and certain ammonium nitrate were mixed together in the reaction flask of the rotary evaporator, and ten agate granules with a diameter of about 1 cm were added into the system for better stirring. The rotary evaporator was turned on and the system was maintained at 25°C. In the mean time, an air blower connected with a round bottom flask containing some deionized water was turned on to transport air at a rate of 40 L min-1. A small amount of water vapor was carried into the reaction flask with air to react with the TBOT. The TBOT solution was hydrolyzed slowly to form a cream color emulsion. Reaction stopped after 3 h and then the emulsion was distillated at 50°C for 15 min under vacuum. Finally, the samples were annealed at different temperatures for 2 h to obtain the N-doped mesoporous TiO2 nanorods, designated as NMTNR-x-y, where x represents the theoretical molar ratio of N (%) and y represents the calcination temperature (°C).
Characterization of the samples
The crystalline phase identification and structural analysis were carried out by X-ray diffraction (XRD) instrument with Cu Kα radiation. A Japan ULVAC-PHI PHI 5000 VersaProbe X-ray photoelectron spectrometer (XPS; Kanagawa, Japan) was applied to analyze the elemental composition and state of the samples. The microstructures were analyzed by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and high-resolution transmission electron microscopy (HRTEM). N2 adsorption-desorption isotherms were measured at 77 K on a Micromeritics Tristar 3020 system (Norcross, GA, USA). The UV-visible (UV–vis) absorbance spectra of the samples were characterized using a Japan Shimadzu UV240 UV–vis spectrophotometer (Kyoto, Japan).
Photocatalytic activity
The photocatalytic activity of the samples was estimated by MB degradation performed in a 500-mL cylindrical glass photocatalytic reactor, and a 500-W xenon lamp was selected as the visible light source. Between the xenon lamp and reactor, a cut filter was inserted to eliminate ultraviolet light. In a typical experiment, 0.08 g of photocatalyst was dispersed into 250 mL of MB solution (10 mg L-1). The actual effect of photocatalytic activity by chemical reaction was studied by maintaining the solutions in the dark for 1 h before irradiation. The MB solution (5 mL) was taken out every 5 min and analyzed using UV–vis spectrophotometer. The degradation of MB can be calculated via the formula η = (1 – A i /A0) × 100%, where A0 is the absorbance of the original MB solution before irradiation and Ai is the absorbance of MB solution measured every 5 min. The photodegradation of MB follows pseudo-first-order kinetics. Its kinetics can be expressed as ln(C0/C) = kt, where k (per minute) is the degradation rate constant.
The stability of photocatalyst was evaluated by the degradation of MB with reused photocatalyst, and 250 mL of new MB solution (10 mg L-1) was added into the reactor each time.
Discussion
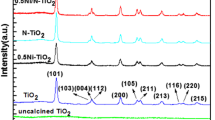
Figure 1 shows the typical XRD patterns of N-doped mesoporous TiO2 nanorods. It is obvious that the samples except NMTNR-4-600 were in anatase phase according to the identified diffraction peaks (JCPDS no. 21–1272). The weaker peak of NMTNR-4-400 indicates the lower crystallinity of the sample. The average crystal sizes of the samples were calculated with the Scherrer formula and were listed in Table 1. In addition, no nitrogen-derived peaks can be detected in the samples. This is because of the low dosage of the dopant well dispersed in mesoporous TiO2 nanorods[11, 12].
XPS analysis of the sample NMTNR-4-500 was shown in Figure 2a. The binding energies were corrected for specimen charging by referencing C ls to 285 eV. The peaks observed in this spectrum were assigned to C, O, Ti, and N. Figure 2b displays the high-resolution N 1 s spectra, which reveals a major N 1 s peak at around 400 eV due to the adsorbed NO or N in Ti-O-N and O-Ti-N bonds[2, 13, 14]. The N contents of different samples estimated from XPS spectra were listed in Table 1. It is obvious that the N peaks become stronger and stronger with the increase of the N content.
Figure 3 depicts the N2 adsorption-desorption isotherms of N-doped mesoporous TiO2 nanorods. The isotherms belong to the type IV with H2 hysteresis loop, indicating the existence of the porous structure[15]. According to the Brunauer-Emmett-Teller (BET) method, the specific surface areas for these samples (Table 1) are remarkably higher (76.1 to 106.4 m2 g-1) than that of Degussa P25 (50 m2 g-1). The Barrett-Joyner-Halenda (BJH) adsorption average pore diameters (4 V/A) and the pore volumes of the samples were also given in Table 1. It could be observed that with the increase of N proportion, the specific surface area and the pore volume was increased. The BJH adsorption average pore diameters were in the range of 5 to 10 nm.
SEM, TEM, and HRTEM images of the sample NMTNR-4-500 are shown in Figure 4. It can be observed that the sample is made up of several nanorods with an average length of ca. 1.5 μm and a cross section diameter of ca. 80 nm. As shown in Figure 4b,c, the N-doped TiO2 nanorods are mesoporous structure. The corresponding HRTEM image is displayed in Figure 4d which proves the coexistence of mesoporous structure and a high crystallinity. The pore diameter is in the range of 5 to 10 nm, which is consistent with the N2 adsorption-desorption results (Table 1). The spacing of two neighboring parallel fringes is around 0.35 nm, which matches well with the d spacing between adjacent (101) crystallographic planes of anatase phase[16].
Figure 5 shows a schematic illustration for the forming process of N-doped mesoporous TiO2 nanorods. This is based on the SEM observations of the N-doped mesoporous TiO2 nanorods at different periods and the existing mechanism of crystal growth[17]. In the experiment, vaporized molecules were transported with air into the reaction flask, resulting in the hydrolysis reaction of TBOT in the gas–liquid interface. Colloidal nucleus was formed in this process (Figure 5a). In addition, the rotation and the ball milling could improve the dispersion of colloidal nucleus in three-dimensional space. The colloidal nucleus rearranged to find a suitable place to reduce the surface energy (Figure 5b). Finally, TiO2 aggregates with rod-like structures were obtained (Figure 5c). When being annealed at 500°C, the ammonium nitrate attached on the surface of colloidal nucleus (see Additional file1: Figure S1) was decomposed into N2, NO2, and H2O, which may result in the formation of mesoporous structure. At the same time, N2 and NO2 may provide the N source of as-prepared N-doped mesoporous TiO2 nanorods (Figure 5d).
The UV–vis absorbance spectra of as-prepared samples were shown in Figure 6a. It can be seen that the N-doped mesoporous TiO2 nanorods present a significant absorption in the visible region between 400 and 550 nm, which is the typical absorption feature of nitrogen-doped TiO2[18, 19]. Kubelka-Munk function was used to estimate the band gap energy of the prepared samples. As TiO2 is an indirect transition semiconductor, plots of the (αhν)1/2 vs the energy of absorbed light afford the band gaps of the different samples (Figure 6b). The band gaps optically obtained in such a way were presented in Table 1. It reveals that the band gaps of N-doped mesoporous TiO2 nanorods are significantly narrower than that of P25, which is beneficial to the improvement of the photocatalytic efficiency.
Figure 7a displays the degradation efficiency of MB versus irradiation time over different samples. A blank study (absence of catalyst) was carried out as a background check. For a comparison, P25 was investigated under the same conditions. It could be observed that without catalysts, only 21% of MB was degraded within 60 min. In contrast, the degradation efficiency of MB enhanced greatly in the presence of catalysts. The photocatalytic activity of the N-doped mesoporous TiO2 nanorods was much higher than that of the C-N co-doped rod-like TiO2 photocatalyst in our previous work[11]. The best catalytic efficiency was found in the sample NMTNR-6-500, which takes 60 min to degrade 99.8% MB in the solution, while the P25 degraded only 54% MB in the solution during the same time. Figure 7b shows a linear relationship between ln(C0/C) and the reaction time, indicating that the photodegradation of MB follows the first-order kinetics. The order of rate constants was summarized as follows: blank < P25 < NMTNR-4-600 < NMTNR-4-400 < NMTNR-2-500 < NMTNR-4-500 < NMTNR-6-500, which is consistent with the conclusions of photocatalytic degradation curves presented in Figure 7a.
Based on the data in Table 1, the excellent photocatalytic performance of N-doped mesoporous TiO2 nanorods might be explained by the following factors. Firstly, N doping could extend the spectral response to visible light and greatly improve the utilization of visible light[1, 20]. Secondly, it is known that mesoporosity can improve surface adsorption capacity of the reactants due to the increased surface area[21, 22]. It is obvious that with the increase of N proportion, the photocatalytic efficiency was improved. This may be resulting from the narrowed band gap and the enlarged surface area of N-doped mesoporous TiO2 nanorods. In addition, the calcination temperature also plays an important role in the catalytic efficiency. On the one hand, with the increase of the temperature, the grain size and band gap increased and the specific surface area decreased, which are responsible for the depress of photocatalytic activity. On the other hand, under lower temperature, TiO2 had a lower crystallinity, which results in the lower photocatalytic activity.
To evaluate the stability of these photocatalysts, the repeated experiments for the degradation of MB were performed, and the results were shown in Figure 8. The reused N-doped mesoporous TiO2 nanorods maintained a higher catalytic activity than that of P25. Among all of the samples, NMTNR-4-500 showed the best photochemical stability, and it can still degrade 91.4% of MB within 60 min after five recycles. The rod-like structure takes many advantages, such as easy separation, recovery, and high recycle rate, which could enhance the stability of the photocatalyst[23, 24]. However, it was noticed that the sample with the best catalytic efficiency (NMTNR-6-500) did not perform the best photochemical stability. This may be attributed to the destroyed nanorod structure caused by the excessive pores during the repeated use.
Conclusions
In summary, the N-doped mesoporous TiO2 nanorods had been successfully fabricated by a template-free modified sol–gel approach. Ammonium nitrate was used to form the mesoporous structure and provided the source of N dopants. The average length and the cross section diameter of the as-prepared samples were ca. 1.5 μm and ca. 80 nm, respectively. The BJH adsorption average pore diameters were in the range of 5 to 10 nm. The mesoporous TiO2 nanorods doped with 6% theoretical molar ratio of N and annealed at 500°C showed the best photocatalytic performance. The photodegradation rate constant of this sample is 0.092 min-1, which is 7.6 times higher than that of P25. Furthermore, the rod-like photocatalyst can be easily separated and recycled, which could enhance the stability of the photocatalyst. The results provide useful insights for designing highly active photocatalyst.
References
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y: Visible-light photocatalysis in nitrogen-doped titanium oxides. Sci 2001, 293: 269–271. 10.1126/science.1061051
Harb M, Sautet P, Raybaud P: Anionic or cationic S-doping in bulk anatase TiO2: insights on optical absorption from first principles calculations. J Phys Chem C 2013, 117: 8892–8902. 10.1021/jp312197g
Wang DH, Jia L, Wu XL, Lu LQ, Xu AW: One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4: 576–584. 10.1039/c1nr11353d
Yu A, Wu G, Zhang F, Yang Y, Guan N: Synthesis and characterization of N-doped TiO2 nanowires with visible light response. Catal Lett 2009, 129: 507–512. 10.1007/s10562-008-9832-7
You H, Qi J, Ye L, Kang X, Hu LJ: Study on catalytic efficiency of Ag⁄ N co-doped TiO2 nanotube arrays under visible light irradiation. Adv Mater Res 2013, 690: 511–517.
Lu J, Su F, Huang Z, Zhang C, Liu Y, Ma X, Gong J: N-doped Ag/TiO2 hollow spheres for highly efficient photocatalysis under visible-light irradiation. Rsc Adv 2013, 3: 720–724. 10.1039/c2ra22713d
Zhang Q, Su J, Zhang X, Li J, Zhang A: Chemical vapor deposition of a PbSe/CdS/nitrogen-doped TiO2 nanorod array photoelectrode and its band-edge level structure. New J Chem 2012, 36: 2302–2307. 10.1039/c2nj40509a
Wang J, Huang B, Wang Z, Qin X, Zhang X: Synthesis and characterization of C, N-codoped TiO2 nanotubes/nanorods with visible-light activity. Rare Met 2011, 30: 161–165. 10.1007/s12598-011-0261-1
He Z, He HY: Synthesis and photocatalytic property of N-doped TiO2 nanorods and nanotubes with high nitrogen content. Appl Surf Sci 2011, 258: 972–976. 10.1016/j.apsusc.2011.09.051
Lydakis–Simantiris N, Riga D, Katsivela E, Mantzavinos D, Xekoukoulotakis NP: Disinfection of spring water and secondary treated municipal wastewater by TiO2 photocatalysis. Desalination 2010, 250: 351–355. 10.1016/j.desal.2009.09.055
Li L, Lu J, Wang Z, Yang L, Zhou X, Han L: Fabrication of the C-N co-doped rod-like TiO2 photocatalyst with visible-light responsive photocatalytic activity. Mater Res Bull 2012, 47: 1508–1512. 10.1016/j.materresbull.2012.02.032
Lu J, Li LH, Wang ZS, Wen B, Cao JL: Synthesis of visible-light-active TiO2-based photo-catalysts by a modified sol–gel method. Mater Lett 2013, 94: 147–149.
Ananpattarachai J, Kajitvichyanukul P, Seraphin S: Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J Hazard Mater 2009, 168: 253–261. 10.1016/j.jhazmat.2009.02.036
Sato S, Nakamura R, Abe S: Visible-light sensitization of TiO2 photocatalysts by wet-method N doping. Appl Catal A 2005, 284: 131–137. 10.1016/j.apcata.2005.01.028
Xie J, Bian L, Yao L, Hao YJ, Wei Y: Simple fabrication of mesoporous TiO2 microspheres for photocatalytic degradation of pentachlorophenol. Mater Lett 2013, 91: 213–216.
Wang DS, Duan YD, Luo QZ, Li XY, An J, Bao LL, Shi L: Novel preparation method for a new visible light photocatalyst: mesoporous TiO2 supported Ag/AgBr. J Mater Chem 2012, 22: 4847–4854. 10.1039/c2jm14628b
Huang XP, Pan CX: Large-scale synthesis of single-crystalline rutile TiO2 nanorods via a one-step solution route. J Cryst Growth 2007, 306: 117–122. 10.1016/j.jcrysgro.2007.04.018
Santos RS, Faria GA, Giles C, Leite CA, Barbosa HDS, Arruda MA, Longo C: Iron insertion and hematite segregation on Fe-doped TiO2 nanoparticles obtained from sol–gel and hydrothermal methods. ACS Appl Mater Inter 2012, 4: 5555–5561. 10.1021/am301444k
Jia HM, Zheng Z, Zhao HX, Zhang LZ, Zou ZG: Nonaqueous sol–gel synthesis and growth mechanism of single crystalline TiO2 nanorods with high photocatalytic activity. Mater Res Bull 2009, 44: 1312–1316. 10.1016/j.materresbull.2008.12.016
Hu ZY, Xu LB, Chen JF: Ordered arrays of N-doped mesoporous titania spheres with high visible light photocatalytic activity. Mater Lett 2013, 106: 421–424.
He ZL, Zhu ZF, Li JQ, Wei N, Zhou JQ: Characterization and activity of mesoporous titanium dioxide beads with high surface areas and controllable pore sizes. J Hazard Mater 2011, 190: 133–139. 10.1016/j.jhazmat.2011.03.011
Song F, Su HL, Han J, Lau WM, Moon WJ, Zhang D: Bioinspired hierarchical tin oxide scaffolds for enhanced gas sensing properties. J Phys Chem C 2012, 116: 10274–10281. 10.1021/jp2118136
Wu Z, Dong F, Zhao W, Wang H, Liu Y, Guan B: The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity. Nanotechnology 2009, 20: 235701–235709. 10.1088/0957-4484/20/23/235701
Xiong C, Deng X, Li J: Preparation and photodegradation activity of high aspect ratio rutile TiO2 single crystal nanorods. Appl Catal B–Environ 2010, 94: 234–240. 10.1016/j.apcatb.2009.11.013
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF- 2013-R1A1A2009154), the fund from a key project for Industry-Academia-Research in Jiangsu Province (BY2013030-04), and the fund from Colleges and Universities in Jiangsu Province Plans to Graduate Research and Innovation (CXLX13-812).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The experiments and characterization presented in this work were carried out by XZ, ML, and GY. The experiments were designed by XZ, ZW, JL, and HJS. XZ, XL, and JJ analyzed and discussed the results obtained from the experiments. The manuscript was prepared by XZ. JL, HJS, and MZ helped with the draft editing. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhou, X., Lu, J., Jiang, J. et al. Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity. Nanoscale Res Lett 9, 34 (2014). https://doi.org/10.1186/1556-276X-9-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1556-276X-9-34