Abstract
Background
Open-label, randomized controlled trials (RCTs) are subject to observer bias. If patient management is conducted without blinding, a difference between groups may be explained by other factors than study treatment. One factor may come from taking concomitant treatments with an efficacy on the studied outcomes. In type 2 diabetes, some antihypertensive or lipid-lowering drugs are effective against diabetic complications. We wanted to determine if these concomitant treatments were correctly reported in articles of RCTs on type 2 diabetes and if they might have influenced the outcome.
Methods
We performed a systematic review using Medline, Embase, and the Cochrane Library (from January 1950 to July 2010). Open-label RCTs assessing the effectiveness of intensive blood-glucose control in type 2 diabetes were included. We chose five therapeutic classes with proven efficacy against diabetes complications: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor antagonists (AIIRAs), fibrates, statins, and aspirin. Differences between concomitant treatments were considered statistically significant when p < 0.05.
Results
A total of eight open-label RCTs were included, but only three (37.5%) of them published concomitant treatments. In two studies (ACCORD and ADVANCE), a statistically significant difference was observed between the two groups for aspirin (p = 0.02) and ACEIs (p = 0.02).
Conclusions
Few concomitant treatments were published in this sample of open-label RCTs. We cannot completely eliminate an observer bias for these studies. This bias probably influenced the results to an extent that has yet to be determined.
Similar content being viewed by others
Background
In patients with type 2 diabetes (T2D), the efficacy of blood-glucose control is generally based on the UKPDS study [1]. The main results of this randomized study were published in 1998 and led to international guidelines on the treatment of type 2 diabetes [2]. It showed the efficacy of intensive blood-glucose control on the onset of microvascular complications. And also showed that metformin was efficacious against macrovascular complications and overall mortality in overweight patients [3]. However, even though UKPDS was randomized, this study is controversial because of its methodology and the publication of its results [4–6]. There was a risk of observer bias because the open-label study did not have any placebo group. The first potential problem lies in differences in the care management between the two groups throughout the study, combined with an imbalance in the prescription of concomitant treatments that may have influenced outcome measures [7]. This risk of bias, which is particularly high in open-label studies, can also occur in placebo-controlled, double-blind RCTs. For example in the FIELD study [8], the intake of statins is much bigger in the placebo group (36% vs 19%, p < 0.0001), which could partly explain why there is no significant difference for the primary endpoint. The consequences of such an imbalance in concomitant treatments between study groups may be particularly important since the studied outcomes are influenced by these treatments. In T2D, some antihypertensive and cholesterol-lowering drugs are effective against microvascular complications [9, 10] and/or cardiovascular mortality [11, 12]. Similarly, aspirin has a proven efficacy against the risk of having a coronary event in high-risk cardiovascular patients [13]. Because of this, we wondered how these concomitant treatments were reported in clinical trials on intensive blood-glucose control treatments in T2D. Our objective was also to compare concomitant treatments prescribed in each group in order to assess the possible confounding effect they may have had.
Methods
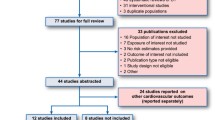
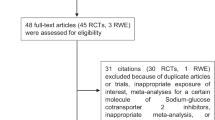
We previously performed a systematic review using Medline, Embase, and the Cochrane Library (from January 1950 to July 2010). RCTs which were randomized, assessing the efficacy of intensive glucose lowering treatment (oral or insulin) versus a standard treatment (standard care), less intensive glycaemic lowering treatment, or placebo (intensive glycaemic treatment could be defined either by a specified HbA1c target or by treatment intensification); trials using clinically relevant outcomes; and participants aged 18 or older with type 2 diabetes were included [14]. We analyzed the articles and supplemental documents (web appendices) of RCTs included in our meta-analysis that evaluated the efficacy of intensive blood-glucose control [14]. We especially looked for the intake of ACEIs, AIIRAs, fibrates, statins, and aspirin which have a proven efficacy on diabetic complications [9–13]. When the p-value for concomitant treatments was not specified in the publications, it was directly calculated and a statistical significance of 0.05 was determined. Authors were contacted for additional data when necessary.
Results
A total of eight open-label RCTs were found with the systematic review [1, 3, 15–20]. Only two publications specified concomitant treatments received by patients during the study. However, they did not publish data about all five therapeutic classes of interest (see the ACCORD and ADVANCE studies in Table 1) [18, 19]. We contacted the authors of all trials, and received additional data from one study [16] (see Table 2). In total, only three studies (37,5%) reported data about concomitant treatments. In the ADVANCE study, data is only available on 86% of included patients [18]. There is a statistically significant difference (p = 0.02) in taking aspirin, which was more prescribed in the intensively treated group (Table 3). In the ACCORD study, data is available for 96% of included patients [19]. The intake of ACEIs is significantly more frequent in the conventional treatment group (p = 0.02) (Table 3). In the Kumamoto study, there was no statistically significant difference between groups (Table 3) [21].
Discussion
Our study highlights the lack of publications on concomitant treatments in trials assessing intensive blood-glucose control treatments in T2D. Only three out of eight RCTs (37.5, the ACCORD, ADVANCE, and Kumamoto (after direct contact with authors) studies reported the intake of these treatments without specifying the drugs, even though their efficacy on outcome measures had been proven. In two studies (ACCORD and AVANCE), statistically significant differences at 5% were seen in both groups treated with specific medications without controlling to what extent they influence study results. This lack of data is harmful, because the interpretation of study results may be distorted and lead to incorrect recommendations for clinical practice. Pooling proportions of cointerventions and looking for an interaction between differences in cointerventions and the effect of glycemic control on outcomes is feasible using meta-analysis and meta-regression techniques. This would help us reach our second objective, "if concomitant treatments are reported in clinical trials, to assess their possible confounding effect on outcome." However, this would require a minimum of 5 trials for each covariate. We were only able to retrieve data on concomitant treatments for three trials (two published and one obtained from the authors). Therefore, we felt that meta-regression would not be appropriate and would give unrobust results [22].
The example of UKPDS 33 [1] is a prime example of this. When it was published in The Lancet in 1998, it showed that intensive blood-glucose control was effective against the onset of microvascular complications and long-term macrovascular complications. Yet, the only outcome with a statistically significant change was the “retinal photocoagulation” outcome: RR = 0.71; CI 95% [0.53-0.96]. This outcome was added during the study and let the authors conclude that the treatment was effective against all diabetic complications: “any diabetes-related endpoints” (RR = 0.88 ; CI 95% [0.79-0.99]). There was also a difference in blood pressure (BP) between some groups: at six-year follow-up, the chlorpropamide-treated group showed a mean BP that was much higher than other groups (143/82 mmHg vs 138/80 mmHg, p < 0.001). UKPDS authors emphasized that the proportion of patients treated with an antihypertensive drug was different (p = 0.022) depending on the group: 43% for the chlorpropamide-treated group compared to 34%, 36% and 38% in other groups (respectively due to lifestyle and diet guidelines, glibenclamide, and insulin). Yet, UKPDS 38 [8] showed that treating BP could help reduce the risk of developing diabetic retinopathy. The double-blind, placebo-controlled DIRECT-2 RCT also showed that candesartan increases the rate of retinopathy regression in T2D by 34% (RR = 1.34; CI 95% (1.08-1.68)) [23]. In insulin-dependent (ID) diabetes, enalapril and losartan also proved to be effective on diabetic retinopathy regardless of BP (OR = 0.35 : CI 95% [0.14-0.85], OR = 0.30 ; [0.12-0.73] respectively) [24]. The FIELD [9] and ACCORD-Lipid [25] studies (two double-blind placebo-controlled RCTs) showed that fenofibrate was effective on retinopathy in T2D, regardless of the decrease in serum lipids. The efficacy of fenofibrate on this outcome measure seemed even higher than for blood-glucose control. Since UKPDS 34 was published, metformin has been considered to be the most effective treatment for overweight patients with T2D [3]. For overall mortality, the risk ratio of metformin compared to lifestyle and diet guidelines was 0.64, CI 95% [0.45-0.91]. However, a recent meta-analysis showed that metformin was not necessarily more effective than other treatments [26]. So, the positive result observed in UKPDS 34 may just be artificial, especially since in the same study, only the combination of metformin and sulfonamides was deleterious compared to sulfonamides alone (for overall mortality: RR = 1.6, CI 95% [1.02-2.52]). It would have been essential to know which concomitant treatments were present in this study. In a letter to the authors of UKPDS after the 10-year follow-up publication, the question of concomitant treatments came up: “Information on accompanying treatment during the study is necessary in order to interpret the mortality data.” [27] Surprisingly, UKPDS authors did not respond to this [28].
A lack of blinding may overestimate the effect studied from 17% to 34% [29–31]. However, not blinding can also lead to a lack of difference because the control group does not “stay constant.” For instance, the MRFIT study observed the effect of the multifactorial care management of cardiovascular risk on 12,000 patients compared to usual care. After seven years of follow-up, no difference between patient groups was observed for overall mortality or coronary events. One of the authors’ hypotheses was that the control group (usual care) had changed its health habits. Smoking had dropped from 59% to 46%, diastolic BP from 91 to 84 mmHg, and antihypertensive drug intake had increased from 19% to 47%. Because the cardiovascular risk of this control group decreased, the study had insufficient statistical power and could not demonstrate a statistically significant difference [32]. Yudkin [33] and Gale [34] call this phenomenon the “Hawthorne effect”: the study itself may change patients’ and doctors’ behavior. This is more of a problem in open-label studies where patients and doctors know what the study drug is. So it is appropriate that CONSORT 2010 recommends in Section 11b (on the blinding of RCTs) that co-intervention similarities [35] must be described and verified, which was not required in 2001 [36]. Concerning T2D treatment, the demonstration of blood-glucose control efficacy seems to be affected by the lack of publications on concomitant treatments whose effect on diabetic complications is already proven. However, it remains to be determined to what extent the results are affected by this bias.
Conclusions
Few concomitant treatments were published in this sample. There is a potential risk of observer bias in studies assessing the efficacy of blood-glucose control in T2D.
Authors’ information
RB: general practitioner and lecturer
IS: general practitioner and lecturer
SE: general practitioner and lecturer
MC: consultant
TBA: cardiologist, pharmacologist, and lecturer
BK: pediatric pharmacologist and professor
CC: endocrinologist, pharmacologist, and lecturer
FG: cardiologist and professor
References
UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998, 352: 837-853.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association: Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009, 32: 193-203. 10.2337/dc08-9025.
UK Prospective Diabetes Study (UKPDS) Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998, 352: 854-865.
Nathan DM: Some answers, more controversy, from UKPDS. Lancet. 1998, 352: 832-833.
Ewart RM: The case against agressive treatment of type 2 diabetes: critique of the UK prospective diabetes study. BMJ. 2001, 323: 854-858. 10.1136/bmj.323.7317.854.
McCormack J, Greenlahg T: Seeing what you want to see in randomised controlled trials : versions and perversions of UKPDS data. BMJ. 2000, 320: 1720-1723. 10.1136/bmj.320.7251.1720.
Schulz KF, Grimes DA: Blinding in randomised trials: hiding who got what. Lancet. 2002, 359: 696-700. 10.1016/S0140-6736(02)07816-9.
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators: Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005, 366: 1849-1861.
Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, D’Emden MC, Crimet DC, O’Connell RL, Colman PG, FIELD study investigators: Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007, 370: 1687-1697. 10.1016/S0140-6736(07)61607-9.
UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998, 317: 703-713. 10.1136/bmj.317.7160.703.
Heart Outcomes Prevention Evaluation Study Investigators: Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000, 355: 253-259.
Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group: Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004, 363: 757-767.
Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS, American Diabetes Association: Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American diabetes association, a scientific statement of the American heart association, and an expert consensus document of the American college of cardiology foundation. Diabetes Care. 2010, 33: 1395-1402. 10.2337/dc10-0555.
Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C: Effect of intensive glucose-lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes mellitus: a meta-analysis of randomised controlled trials. BMJ. 2011, 343: d4169-10.1136/bmj.d4169.
UGDP: Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VIII. Evaluation of insulin therapy: final report. Diabetes. 1982, 31: 1-81. 10.2337/diabetes.31.1.1.
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin dependent diabetes mellitus: a randomized prospective 6 year study. Diab Res Clin Pract. 1995, 28: 103-117. 10.1016/0168-8227(95)01064-K.
Abraira C, Colwell J, Nuttall F, Sawin CT, Henderson W, Comstock JP, Emanuele NV, Levin SR, Pacold I, Lee HS: Cardiovascular events and correlates in veterans affairs diabetes feasibility trial. Veterans affairs cooperative study on glycemic control and complications in type II diabetes. Arch Intern Med. 1997, 157: 181-188. 10.1001/archinte.1997.00440230053007.
The ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008, 358: 2560-2572.
The ACCORD study group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008, 358: 2545-2559.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators: Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009, 360: 129-139. 10.1056/NEJMoa0808431.
Shichiri M, Kishikawa H, Ohkubo Y, Wake N: Long-term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000, 23: B21-B29.
Thompson SG, Higgins JP: How should Meta-regression analyses be undertaken and interpreted?. Stat Med. 2002, 21: 1559-1573. 10.1002/sim.1187.
Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N, DIRECT Programme Study Group: Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-protect 2): a randomised placebo-controlled trial. Lancet. 2008, 372: 1385-1393. 10.1016/S0140-6736(08)61411-7.
Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009, 361: 40-51. 10.1056/NEJMoa0808400.
Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ, ACCORD Study Group: Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010, 363: 233-244. Erratum in: N Engl J Med 2011, 364:190
Lamanna C, Monami M, Marchionni N, Mannucci E: Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2011, 13: 221-228. 10.1111/j.1463-1326.2010.01349.x.
Mühlhauser I: Follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2009, 360: 417-
Holman RR, Matthews DR, Neil HA: Follow-up of intensive glucose control in type 2 diabetes. The authors reply. N Engl J Med. 2009, 360: 418-
Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995, 273: 408-412. 10.1001/jama.1995.03520290060030.
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP: Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet. 1998, 352: 609-613. 10.1016/S0140-6736(98)01085-X.
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA: Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008, 336: 601-605. 10.1136/bmj.39465.451748.AD. Epub 2008 Mar 3
Multiple Risk Factor Trial Research Group: Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982, 248: 1465-1477.
Yudkin J: Hyperglycaemia as a cardiovascular risk factor in diabetes. BMJ. 2008, 372: 1036-1037.
Gale EAM: The Hawthorne studies – a fable for our time?. QJM. 2004, 97: 439-449. 10.1093/qjmed/hch070.
Schulz KF, Altman DG, Moher D, CONSORT Group: CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010, 340: c332-10.1136/bmj.c332.
Moher D, Schulz KF, Altman DG: The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001, 357: 1191-1194. 10.1016/S0140-6736(00)04337-3.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2288/13/107/prepub
Acknowledgements
The authors would like to thank Cléa Foret for translating the manuscript and Kent Neal (supported by the French Cochrane Center) for proofreading the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors do not have any competing interests to declare.
Authors’ contributions
RB, CC, and FG conceived the study. RB, IS, and SE extracted the data and reviewed the selected papers. RB and CC performed the statistical analysis. RB, IS, SE, CC, FG, and TBA drafted the manuscript. MC, BK, CC, TBA, and FG helped interpret the results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Boussageon, R., Supper, I., Erpeldinger, S. et al. Are concomitant treatments confounding factors in randomized controlled trials on intensive blood-glucose control in type 2 diabetes? a systematic review. BMC Med Res Methodol 13, 107 (2013). https://doi.org/10.1186/1471-2288-13-107
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2288-13-107