Abstract
Purpose of Review
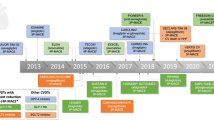
Seven trials of new agents to treat type 2 diabetes (T2DM) have been performed to assess cardiovascular (CV) safety. A significant amount of information regarding the effects of drugs in three classes is available, with new data from multiple other trials expected shortly. This article provides a summary of recently completed trials.
Recent Findings
The dipeptidyl peptidase-4 inhibitors studied thus far do not alter the risk of major adverse CV events (MACE). Glucagon like peptide-1 receptor agonists liraglutide and semaglutide, and the sodium glucose cotransporter-2 inhibitor empagliflozin, significantly reduced the risk of MACE. Empagliflozin also decreased the risk of hospitalization for heart failure. Agents demonstrating a CV outcome benefit also improved parameters of renal function.
Summary
Several newer antihyperglycemic agents have been found to reduce the risk of important CV complications in high-risk patients with T2DM. Future trials are needed to assess the effects of additional drugs and the impact of therapy in lower risk patients and provide additional information regarding non-CV safety outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose and risk of cause specific death. N Engl J Med. 2011;364(9):829–41. doi:10.1056/NEJMoa1008862.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. doi:10.1016/S0140-6736(98)07019-6.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in typediabetes. N Engl J Med. 2008;359(15):1577–89. doi:10.1056/NEJMoa0806470.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi:10.1056/NEJM199309303291401.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9. doi:10.1056/NEJM200002103420603.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi:10.1056/NEJMoa052187.
Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi:10.1056/NEJMoa0802743.
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi:10.1056/NEJMoa0802987.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi:10.1056/NEJMoa0808431.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. doi:10.2337/dc12-0413.
Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC Jr, et al. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012;35(2):409–14. doi:10.2337/dc11-0996.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21071_Avandia_Approv.pdf. Accessed 30 Apr 2017.
https://www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm143413.pdf. Accessed 30 Apr 2017.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi:10.1056/NEJMoa072761.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35. doi:10.1016/S0140-6736(09)60953-3.
•• Guidance for Industry. Diabetes Mellitus — Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). December 2008. https://www.fda.gov/downloads/Drugs/.../Guidances/ucm071627.pdf. Accessed 30 Apr 2017. This document outlines the requirements and specifications for cardiovascular outcomes trials of new medications for diabetes.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus.14 May 2012 CPMP/EWP/1080/00 Rev. 1. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 30 Apr2017.
•• Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi:10.1056/NEJMoa1307684. Primary results for saxagliptin CVOT
•• White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. doi:10.1056/NEJMoa1305889. Primary results for alogliptin CVOT
•• Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. doi:10.1056/NEJMoa1501352. Primary results for sitagliptin CVOT
•• Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi:10.1056/NEJMoa1509225. Primary results for lixisenatide CVOT
•• Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. doi:10.1056/NEJMoa1603827. Primary results for liraglutide CVOT
•• Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi:10.1056/NEJMoa1607141. Primary results for semaglutide CVOT
•• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi:10.1056/NEJMoa1504720. Primary results for empagliflozin CVOT
Coch RW, Green JB. Current cardiovascular outcomes trials in type 2 diabetes: perspectives and insight. Nutr Metab Cardiovasc Dis. 2016;26(9):767–72.
• Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–88. doi:10.1161/CIRCULATIONAHA.114.010389. In-depth analysis of heart failure findings from SAVOR-TIMI 53 CVOT
Zannad Z, Cannon CP, Cushman WC, Bakris GL, Menon V, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76. doi:10.1016/S0140-6736(14)62225-X.
Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32(5):834–8. doi:10.2337/dc08-1755.
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–6. doi:10.1053/j.gastro.2011.02.018.
Raz I, Bhatt DL, Hirshberg B, Mosenzon O, Scirica BM, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor Saxagliptin. Diabetes Care. 2014;37(9):2435–41. doi:10.2337/dc13-2546.
Buse JB, Bethel MA, Green JB, Stevens SR, Lokhnygina Y, et al. Pancreatic safety of sitagliptin in the TECOS study. Diabetes Care. 2017;40(2):164–70. doi:10.2337/dc15-2780.
Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284–6. doi:10.2337/dc15-1707.
Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(4):696–705. doi:10.2337/dc14-1850.
Mosenzon O, Wei C, Davidson J, Scirica BM. Yanuv, et al. incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(11):2142–50. doi:10.2337/dc15-1068.
Josse RG, Majumdar SR, Zheng Y, Adler A, Bethel MA, et al. Sitagliptin and risk of fractures in type 2 diabetes: results from the TECOS trial. Diabetes Obes Metab. 2017;19(1):78–86. doi:10.1111/dom.12786.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi:10.1056/NEJMoa1515920.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rebecca Herbst, Wilburn Bolton, and Afreen Shariff declare that they have no conflict of interest. Jennifer B. Green report grants and personal fees from Merck, grants from AstraZeneca, grants from GlaxoSmithKline, personal fees from Daiichi, and personal fees from Boehringer Ingelheim.
Human and Animal Rights and Informed Consent
This article does not contain any previously unpublished data from human or animal studies.
Additional information
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Herbst, R., Bolton, W., Shariff, A. et al. Cardiovascular Outcome Trial Update in Diabetes: New Evidence, Remaining Questions. Curr Diab Rep 17, 67 (2017). https://doi.org/10.1007/s11892-017-0898-8
Published:
DOI: https://doi.org/10.1007/s11892-017-0898-8