Abstract
Background
Poecilimon and Isophya are the largest genera of the tribe Barbitistini and among the most systematically complicated and evolutionarily intriguing groups of Palearctic tettigoniids. We examined the genomic organization of 79 taxa with a stable chromosome number using classical (C–banding, silver and fluorochrome staining) and molecular (fluorescence in situ hybridization with 18S rDNA and (TTAGG) n telomeric probes) cytogenetic techniques. These tools were employed to establish genetic organization and differences or similarities between genera or species within the same genus and determine if cytogenetic markers can be used for identifying some taxonomic groups of species.
Results
Differences between the karyotypes of the studied genera include some general changes in the morphology of the X chromosome in Isophya (in contrast to Poecilimon). The number of major rDNA clusters per haploid genome divided Poecilimon into two main almost equal groups (with either one or two clusters), while two rDNA clusters predominated in Isophya. In both genera, rDNA loci were preferentially located in the paracentromeric region of the autosomes and rarely in the sex chromosomes. Our results demonstrate a coincidence between the location of rDNA loci and active NORs and GC-rich heterochromatin regions. The C/DAPI/CMA3 bands observed in most Poecilimon chromosomes suggest the presence of more families of repetitive DNA sequences as compared to the heterochromatin patterns in Isophya.
Conclusions
The results show both differences and similarities in genome organization among species of the same genus and between genera. Previous views on the systematics and phylogenetic grouping of certain lineages are discussed in light of the present cytogenetic results. In some cases, variation of chromosome markers was observed to correspond with variation in other evolutionary traits, which is related to the processes of ongoing speciation and hybridization in zones of secondary contact. It was concluded that the physical mapping of rDNA sequences and heterochromatin may be used as an additional marker for understanding interspecific relationships in these groups and their routes of speciation.
Similar content being viewed by others
Background
Isophya and Poecilimon represent the most evolutionarily successful genera of the tribe Barbitistini (sometimes regarded as a subfamily), accounting for about 82% species of this taxon. Both genera represent herbivorous short-winged bushcrickets with complex acoustic behavior [1]. Their center of diversification and, possibly, origin, is the Pontic region, and especially Anatolia, which is home to a vast number of taxa (a total of 136 Poecilimon species and 86 Isophya species; [2, 3]) with restricted ranges [4, 5]. A great number of taxa are local endemics; nevertheless, they often occur in huge numbers and thus may damage crops [6, 7].
Several attempts to classify these two groups have been made, mainly on a smaller scale (within genera) and using various approaches, such as morphology [6, 8, 9], bioacoustic and morphological traits [5, 10–17], cytogenetic data [18–20], and molecular phylogenies [21, 22]. Yet, many questions and doubts remain unanswered due to the large number of taxa, the vast recent radiation in some lineages and, possibly, the considerable contribution of present and past hybridization events [5, 20].
Ribosomal DNA genes (rDNA) and active nucleolus organizer regions (NORs) are very useful chromosome markers for interspecific comparisons. Over the last few years, fluorescence in situ hybridization (FISH) techniques have been extensively used for understanding the karyotype structure and evolution of various insects, especially coleopterans (e.g. Scarabaeinae [23]), lepidopterans [24], and orthopterans (e.g. Acrididae grasshoppers) [25–30]. In other Orthoptera, e.g. tettigoniids, repeated DNA has been analyzed by silver impregnation (Ag-NORs) and chromosomal mapping with a view to location of the major ribosomal DNA cluster (18S rDNA probe) and telomere repeats (TTAGG) n in the subfamilies Saginae [31, 32], Bradyporinae [33], and Phaneropterinae [20, 34–36].
To date, the chromosomal organization (i.e. the distribution of heterochromatin and the location of 18S loci and active NORs) of Barbitistini was analyzed in species belonging to eight genera of this tribe. In these lineages the latter markers proved to be good for understanding genomic differentiation and distinguishing between species and evolutionary lines [37]. In this work, we present a detailed cytogenetic analysis of 79 taxa belonging to the genera Poecilimon and Isophya. We examined their chromosomal characteristics, the number and distribution of major rDNA clusters using FISH with an 18S rDNA probe, and active NOR locations by silver staining. In FISH experiments, we also used the telomeric (TTAGG) n probe for better identification of chromosome ends. In addition, some Isophya species/subspecies/populations for which rDNA cluster data were previously available [20] were included in our analysis. Furthermore, conventional methods, such as C-banding and fluorochrome CMA3 and DAPI staining, were used to analyze heterochromatin composition in representatives of both genera. The main objectives of the present work were to test (1) how the structure and distribution of rDNA clusters and heterochromatin affect the genomic organization of Poecilimon and Isophya, (2) whether the general tendencies in chromosomal organization correspond to morphoacoustic specializations, and (3) whether these tendencies reflect recent concepts concerning the systematics and phylogeny of both genera.
Results
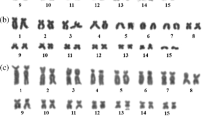
All analyzed Poecilimon and Isophya species showed a 2n = 31 karyotype in the males and a 2n = 32 karyotype in the females with an X0/XX sex determination system. Acrocentric autosomes were divided into two groups: four long (1–4) and eleven medium or short ones (5–15), both of which gradually decreased in size and sometimes minor length differences in chromosome pairs might cause problems with their precise identification. Combining the results of all cytogenetic markers allowed for the identification of homologous chromosome targets, yet sometimes the ordering of particular pairs could be imprecise. In most Poecilimon species, the X chromosome was acrocentric, except for subacrocentric chromosomes in P. jonicus tessellatus, P. martinae, and P. macedonicus (Table 1). Similarly to the results published previously [19], most Isophya species analyzed in this study exhibited a subacrocentric X (Table 2).
Cytogenetic mapping of ribosomal and telomeric DNA and NORs
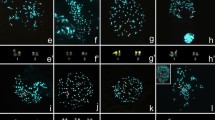
Cytogenetic maps of 18S rDNA were obtained for 39 Poecilimon taxa (Table 1) and 40 Isophya taxa (taken together with those previously published [20]) (Table 2). Representative hybridized metaphasic chromosomes or bivalents are shown in Figures 1a–h, 1a’–h’ and 2a–i, and 2a’–i’. In both genera, the number of rDNA sites per haploid genome ranged from one to five; they were located on the autosomes, and rarely on the sex chromosome – in P. macedonicus (Figure 1e), P. marmaraensis (not shown), P. ukrainicus/fussii (Figure 1f), and also I. pavelii ([20] – see Figure 2a), I. major (not shown), and one population of I. rectipennis (Figure 2e).
Examples of FISH with both 18S rDNA (green) and telomeric DNA (red) probes in spermatogonial metaphase (a, c, d, g, h) or diakinesis (b, e, f), as well as karyotypes arranged from these divisions (a’–h’) and silver staining in diakinesis (i–k) and diplotene (l) of chromosomes for the following Poecilimon species: P. cervus (a, a’), P. bosphoricus (b, b’, i), P. chopardi (c, c’, j), P. klisuriensis (d, d’), P. macedonicus (e, e’, k), P. ukrainicus/fussii (f, f’), P. affinis (g, g’), and P. zwicki (h, h’, l). White arrowheads point to rDNA clusters near the centromeric or distal regions of the chromosomes. Hybridization areas vary in size between some homologous chromosomes, which are marked with an asterisk (*). Black arrowheads indicate the presence of one (i), two (j, k), or four (l) active NORs. 18S rDNA signals in mitotic metaphase/diakinesis coincide with NORs. Bar = 10 μm.
Examples of FISH with both 18S rDNA (green) and telomeric DNA (red) probes in spermatogonial metaphases (a, c, d, e, f, g) or diakinesis (b, h, i) as well as karyotypes arranged from these divisions (a’–h’) and silver staining in diakinesis (l) and diplotene (j, k, m) of chromosomes for the following Isophya species: I. thracica (a, a’, j), I. nervosa (b, b’), I. cf. armena (c, c’, k), I. brunneri (d, d’), I. rectipennis – Bulgarian population (e, e’) and Turkish population (f, f’), I. schneideri (g, g’, l), I. rizeensis (h, h’), and I. yaraligozi (i, i’, m). In the karyotypes, white arrowheads indicate the chromosomal location of rDNA clusters and an asterisk (*) marks differences in size between some homologous chromosomes (a’, c’, e’, f’, i’). Black arrowheads indicate the presence of one (j), two (k), three (l), or four (l) active NORs which coincide with 18S rDNA signals. Bar = 10 μm.
Most Poecilimon taxa carried one (50%) or two (45%) rDNA loci, and only two species exhibited four loci (5%). When a single rDNA cluster was detected, it was always located on a small pair near the paracentromeric region (one of the pairs 9–12; Figure 1a,b); only in P. jonicus tessellatus it appeared in the interstitial region (not shown). Two rDNA clusters were evident on long/medium and short chromosome pairs near the paracentromeric region (Figure 1c,d), except for P. macedonicus (distal location on the X chromosome; Figure 1e) and P. aff. glandifer (interstitially near its distal region, probably on the 3rd or 4th pair; not shown). FISH revealed rDNA on four bivalents only in P. affinis (in the paracentromeric region; Figure 1g) and P. zwicki (paracentromeric or distally located; Figure 1h). While the number of 18S rDNA loci varied from one to five in Isophya, most species showed two locations (62.7%); rarely one (11.8%), three (11.8%), four (7.8%), or five (5.9%) clusters (including polymorphism between populations in some species) (Figure 2a–i). Most 18S rDNA loci were situated in different-sized autosomes in the paracentromeric region, however distally to the centromere on a long pair (3) in I. straubei ssp. (not shown), as well as on the subacrocentric X chromosome and a medium-sized bivalent (pair 5) in I. rectipennis from both Bulgarian populations (Figure 2e and see Figure 2c,d in [20]).
Individuals from different populations demonstrated the same rDNA-FISH signal location in Poecilimon brunneri, P. roseoviridis, P. macedonicus, P. schmidtii, Isophya miksici, I. longicaudata longicaudata, I. modestior (from Bulgarian populations), I. rhodopensis petkovi, and I. tosevski. On the other hand, interpopulation variation in the rDNA signal pattern observed in P. armeniacus and I. rectipennis (Figure 2e,f), was also previously reported in I. bureschi, I. rhodopensis rhodopensis, and I. rh. rhodopensis/I. rh. leonorae intermediate forms [20] (Table 1 and 2). Sometimes the rDNA cluster varied in size between homologous chromosomes (Table 1 and 2, see the chromosome number marked with an asterisk; Figures 1’ and 2a’ ,c’ ,e’ ,f’ ,i’).
FISH with the (TTAGG) n probe (tDNA-FISH) was used for spermatogonial or oogonial mitosis and spermatocyte nuclei at different stages of meiosis. In all species of both genera, tDNA-FISH signals were detected at the distal ends of most chromosomes, but showed variation in size and intensity on the autosomes and X chromosomes in some species (Figure 1a–h; 2a–i).
In all the species examined in the present study, rDNA-FISH signals were co-localized with the active NORs visualized by AgNO3 staining (Figures 1i–l, 2j–m). A lack of a full congruency between rDNA location and NOR activity has been previously reported for some autosomes and the X chromosome only in four Isophya species [20].
Heterochromatin patterns revealed by banding techniques
Table 3 shows variation among the analyzed species and genera using C–banding (only double/thick C–bands) and DAPI/CMA3 patterns. Heterochromatin blocks can be characterized as DAPI-/CMA3+ (GC-rich), DAPI+/ CMA3- (AT-rich) or DAPI+/CMA3+ (containing both AT- and GC-rich regions). Generally, thick C-bands on most chromosomes showed bright DAPI+ and CMA3+ signals in pericentromeric or, rarely, interstitial or distal regions. In such cases, DAPI and CMA3 blocks were located very close to each other, but only bright CMA3 signals coincided with 18S rDNA and active NORs (Figure 3a,b). In some species AT-bands were not detected (designated as DAPI- and marked as “0” in Table 2), while bright CMA3+ bands were co-localized with rDNA-FISH/NOR signals (Tables 1, 2, 3; Figure 3c–d). Unfortunately, it was not possible to separate DAPI/CMA3 regions to compare them with rDNA locations in Poecilimon bischoffi, P. orbelicus, P. aff. ledereri, and P. marmaraensis.
C-, DAPI (blue), and CMA 3 (green) stained heterochromatin and selected chromosomes (with C-bands, DAPI, CMA 3 , 18S rDNA, NOR) of spermatogonial metaphase in I. major (a) and I. modestior from the Bulgarian population (b) as well as of diplotene in P. martinae (c) and Isophya sp. (d). In the selected chromosomes, C/DAPI/CMA3 blocks were located very close to each other, but bright CMA3 signals coincided with 18S rDNA and active NORs (a,b); thick C-bands were DAPI-, whereas bright CMA3+ signals were co-localized with rDNA-FISH/NOR signals (c,d); heteromorphism in the paracentromeric region of 1/2 in Isophya sp. (d) seems to be corroborated by all staining methods. The arrows (left panels) indicate selected chromosomes (right panels). Bar = 10 μm.
Some species in both studied genera exhibited heteromorphism in terms of rDNA-FISH signal size/strength and C/DAPI/CMA3 bands between homologue chromosomes or different-sized autosomes (as indicated with an asterisk in Tables 1, 2, 3); for example, in P. zwicki (Figure 1h’), I. thracica (Figure 2a’), I. cf. armena (Figure 2c’), I. rectipennis (Turkish and Bulgarian populations; Figure 2e’ ,f’), I. yaraligozi (Figure 2i’), and Isophya sp. (Figure 3d).
Discussion
By mapping rDNA (and also potential NORs) and two heterochromatin classes we set out to determine whether these methods may be used to identify chromosome markers useful for studying the genomic organization and diversity of Poecilimon and Isophya and for distinguishing evolutionary lineages. The differences and similarities revealed between the two genera are listed below.
Differences in genome organization revealed by cytogenetic markers
Given the stable karyotype in both genera (2n = 31), variation in the number of acrocentric chromosomes involved only the sex chromosomes. This could be due to the occurrence of a pericentric inversion that converted the original/ancestral acrocentric X chromosome to a subacro-/submetacentric one. These karyotype characteristics were found in 35 out of 42 Isophya taxa (present study; [19]), but in only three out of 39 Poecilimon species/subspecies (present study; [38]). Thus, changes in the morphology of the X chromosomes are common in Isophya, in contrast to Poecilimon and other Barbitistini [39].
Based on the number of rDNA clusters, the genus Poecilimon was divided into two main, almost equal, groups carrying the FISH signal on one or two chromosomes. In turn, the prevalent number of rDNA locations in Isophya was two, while either one or 3–5 locations occurred in some cases. Three rDNA clusters were usually connected with a large amount of heterochromatin in the chromosome set (also [19] for Isophya). A high number of NORs/rDNA loci with variable positions is characteristic of some other Barbitistini (Barbitistes, Polysarcus, Phonochorion; [37]), involving groups of species of recent origin. In both analyzed genera, most species had rDNA on one or two chromosomes, which makes it difficult, especially in Poecilimon, to assess their ancestral status. The presence of paracentromeric rDNA loci only on a single bivalent was previously observed in other Phaneropterinae species: the European Odontura[36] and Phaneroptera falcata[40], as well as in the African Lunidia viridis[34] and four species of the genus Eurycorypha[35]. In other Tettigoniidae, single rDNA-positive FISH signals have been observed in European representatives of Saginae and Bradyporinae [32, 35]. In addition, one active NOR seems to be a typical feature of karyotypes with the ancestral chromosome number in Tettigoniinae [41].
Thick C/DAPI/CMA3 bands were observed on most chromosomes in Poecilimon suggesting the presence of more families of repetitive DNA sequences in this genus as compared to the heterochromatin patterns in Isophya. Thus, these bands appear to be a feature differentiating the karyotypes of the two genera (Table 3). A high amount of heterochromatin was earlier reported for some other genera of Barbitistini [37].
Similarities in genome organization revealed by cytogenetic markers
All the studied species had an ancestral (for Tettigoniidae) diploid chromosome number, and thus the variability in the number and location of rDNA loci probably resulted from transposition involving mobile elements or ectopic recombination. Similar mechanisms have been suggested for Acrididae [25], Lepidoptera [24], and Scarabaeinae [23]. Generally, rDNA loci are coincident with active NORs and GC-rich heterochromatin, which indicates the presence of multiple repetitive DNA sequences. Some species in both genera showed different intensities of rDNA hybridization signals on homologous pairs of autosomes, and also heteromorphism in the pattern of heterochromatin distribution (indicated by an asterisk in Tables 1, 2, 3). These differences were detected consistently by all the banding techniques used and observed in those chromosomes in which heterochromatin occurred in large quantities (i.e. in the form of large paracentromeric heterochromatic blocks). Such intraspecific polymorphism may be the result of different mechanisms, i.e. tandem duplication of ribosomal genes, unequal meiotic crossing-over, translocation rearrangements, or homologous recombination [23, 42, 43].
Our results point to some general patterns in the structure of heterochromatin and NOR/18S rDNA locations: (1) DAPI and CMA3 staining cause bright fluorescence co-occurring with thick paracentromeric C-blocks, suggesting the presence of a high number of AT- and CG-base pairs; (2) sometimes CMA3 does not detect active NORs but a special type of GC-rich heterochromatin associated with this region [44]; (3) NOR/rDNA-FISH regions show a bright CMA3 signal; (4) the pattern of distribution of GC-rich blocks indicates some level of dynamism in the genome content of heterochromatin areas and may be related to specific changes characterizing groups of taxa. Thus, different heterochromatin types suggest the occurrence of specific rearrangements of repetitive DNA families that have evolved during the diversification of Isophya and Poecilimon and are characteristic of certain phylogroups.
A comparison of cytogenetic and taxonomic traits
The evolution of repeated DNA families is dominated by genomic events such as duplication and spreading which may impede tracking the evolutionary history of the sequence, and thus prevent using these as genetic markers. However, in some cases we find concordance of cytogenetic data with phylogenetic traits such as morphology, bioacoustics, and molecular data. Previously published phylogenies [22] and speculations on species groupings using morphology and behavior are discussed below and summarized in Tables 1, 2, 3 (the first column). The European representatives of the Poecilimon ampliatus group (Table 1, group 1) sensu stricto[45] with monophyletic origin [22] show a more or less uniform location of two paracentromeric rDNA clusters on long and short autosome pairs, while the Anatolian species along with P. orbelicus (regarded as a monophyletic lineage except P. armeniacus[3]) exhibit only one FISH signal. This may provide additional data for refining species relationships by exploring correlations with morphological and behavioral traits [10, 45]. Interesting examples deserving particular attention include the little known P. glandifer and P. ataturki, which were placed within the P. ampliatus group [46], possibly on account of having an abdominal tergal gland (a structure that is occasionally found within several groups of Barbitistini). And though their affinities have not been phylogenetically studied yet, there is some evidence (Chobanov et al., unpublished data) supporting their cytogenetic distinctness from group 1. An example of concordance of the present data with morphological, behavioral, and molecular traits is group 2, including taxa of the P. syriacus group [14]. Two groups of sibling species are divided between one and two rDNA-FISH signal positions – the P. bosphoricus group (group 3) [17] and the P. brunneri group (group 5) [10, 22]. No proposed relationships between these were reflected in the chromosome markers, except for the phylogenetic affinities of representatives of the P. bosphoricus group with one chromosome rDNA location (P. miramae + P. cervus + P. heinrichi + P. bosphoricus), which are polyphyletic with respect to those with two locations (P. anatolicus and P. turcicus), according to mtDNA data [22]. The four rDNA clusters in P. zwicki (the last being a basal member of the P. brunneri group according to molecular data [22]) and P. affinis probably represent autapomorphies.
The genus Isophya shows a more complicated pattern of rDNA/heterochromatin organization than Poecilimon. In most taxa, two 18S rDNA sequences were located on two chromosome pairs. This seems to be a plesiomorphic state for the group, characteristic of the most primitive lineages [5], for example, I. hospodar, I. straubei (Table 2, group 1), and I. rectipennis (group 2). Yet, all of these exhibit quite a peculiar location of the studied markers. In some taxa, a significant variation of chromosome markers (Tables 2, 3) corresponds to distinct intraspecific and intrapopulation genetic and/or morphoacoustic variation [5, 21, 47]. Three to five FISH signal-positive chromosomes have been found in taxa of recent origin and/or populations where hybridization by secondary contact of haplotypes is suspected. The latter case concerns the I. modesta group of species [5, 19], and especially the I. rhodopensis complex, representing a few subspecies inhabiting a large area of secondary contact between possibly formerly isolated populations, where intrapopulation variation in the number of rDNA clusters is observed. This is usually connected with ongoing speciation and hybridization in zones of secondary contact [5, 21, 47].
According to our results analyzed in conjunction with known systematic and phylogenetic data transposition and recombination, or, alternatively, occasional loss of rDNA fragments clusters, may have often occurred multiple times in different lineages of Isophya and, rarely, in Poecilimon. Similar events resulting in a variable heterochromatin structure have been observed in other groups of Barbitistini, mostly taking place within or between taxa of recent origin [37].
Conclusions
The cytogenetic study presented herein constitutes the next step towards a better understanding of chromosomal organization and evolution within Phaneropterinae. We have outlined some general tendencies of chromosomal organization within Isophya and Poecilimon. These may result in unique species-specific characters or involve homoplastic changes in distinct lineages. And although chromosomal distribution of repeated DNA sequences could represent intrinsic aspects of the evolutionary dynamics of the repeated DNA families, our results have sometimes reflected inferences based on morphological, behavioral, and/or gene-sequence data. Thus, mapping of rDNA sequences and heterochromatin may in some cases be used as an additional marker for understanding relationships and routes of speciation within Barbitistini.
Material and methods
A total of 95 specimens of 39 Poecilimon species/subspecies and 112 specimens of 40 Isophya taxa (including 21 previously described species/subspecies [20]) were studied. Male adults and nymphs and female nymphs were collected from 2006 to 2012 in Eastern Europe and Turkey. Details of taxon names and their possible grouping and collection sites are given in Tables 1 and 2. Chromosome preparations were obtained from the gonads of last instar nymphs or adults. Testes and ovarioles were incubated in hypotonic solution (0.9% sodium citrate), fixed in modified Carnoy’s solution – ethanol: acetic acid (3: 1), and stored at 2°C until use. Chromosome preparations for the examination of nucleolus organizer regions (NORs) and fluorescence in situ hybridization (FISH) experiments were made by tissue squashing using 45% acetic acid, subsequent removal of cover slips by the dry ice technique, and air-drying. The silver staining method (AgNO3) for NOR location was performed according to the protocol by Warchałowska-Śliwa and Maryańska-Nadachowska [48]. Constitutive heterochromatin was revealed by the C-banding technique as described by Sumner [49]. In order to identify GC- and AT-rich regions, the preparations were stained with CMA3 and DAPI, respectively [50].
FISH with ribosomal 18S DNA (rDNA) genes and the telomeric sequence (TTAGG) n was performed exactly as described in Warchałowska-Śliwa et al.[32]. Preparations were counterstained with DAPI (4,6-diamidino-2-phenylindole) and mounted in an anti-fade medium with DABCO. Images for FISH were captured using an AXIOSCOP 2 (Zeiss) microscope equipped with a CCD camera, filter set, and an ISIS5 image processing package (Metasystems GmbH) at the Microscopic Centre of the Institute of Cytology and Genetics, SB RAS, Novosibirsk, Russia. Slides with silver staining impregnation were examined under a Nikon Eclipse 400 light microscope fitted with a CCD DS-U1 camera and an NIS-Elements BR 3.0 image analyzing system (Nikon). At least 10 meiotic divisions (from diplotene to metaphase I) per male and at least three spermatogonial and/or oogonial metaphases (for some specimens) were analyzed using three techniques: FISH, AgNO3 staining, and classical cytogenetic methods (C-banding and DAPI/CMA3 staining). In each species, both the rDNA-FISH pattern and the location of active NORs were always recorded on meiotic bivalents in prophase I in the same individuals.
Abbreviations
- FISH:
-
Fluorescence in situ hybridization
- CMA3:
-
Chromomycin A3
- DAPI:
-
4’6 diamidino-2- phenylindole
- NOR:
-
Nucleolar organizer region
- tDNA-FISH:
-
Telomeric probe
- rDNA:
-
Ribosomal DNA
- BG:
-
Bulgaria
- GR:
-
Germany
- MK:
-
Macedonia
- PL:
-
Poland
- RU:
-
Russia
- SR:
-
Serbia
- TR:
-
Turkey
- UA:
-
Ukraine
- SL:
-
Slovenia
- a:
-
acrocentric
- sa:
-
subacrocentric
- sm:
-
submetacentric
- p:
-
paracentromeric
- d:
-
distal
- i:
-
interstitial
- i–d:
-
interstitial near the distal end
- C:
-
C–bands
- 0:
-
DAPI + signal not visualized.
References
Heller K-G: Evolution of song pattern in east Mediterranean Phaneropterinae: Constraints by the communication system. The Tettigoniidae: biology, systematics and evolution. Edited by: Bailey WJ, Rentz DCF. 1990, Bathurst (Crawford House Press) & Berlin et al: Springer, 130-151.
Eades DC, Otte D, Cigliano MM, Braun H: Orthoptera Species File Online. 2013, Version 2.0/4.1. Available from: http://Orthoptera.SpeciesFile.org/ (18.10.2013)
Boztepe Z, Kaya S, Çıplak B: Integrated systematics of the Poecilimon luschani species group (Orthoptera, Tettigoniidae): radiation as a chain of populations in a small heterogeneous area. Zool J Linnean Soc. 2013, 169: 43-69. 10.1111/zoj.12058.
Çıplak B: Biogeography of Anatolia: the marker group Orthoptera. Mem Soc Entomol Ital. 2004, 82 (2): 357-372.
Chobanov DP, Grzywacz B, Iorgu I, Ciplak B, Ilieva M, Warchałowska-Śliwa E: Review of the Balkan Isophya (Orthoptera: Phaneropteridae) with particular emphasis on the Isophya modesta group and remarks on the systematics of the genus based on morphological and acoustic data. Zootaxa. 2013, 3658: 1-81. 10.11646/zootaxa.3658.1.1.
Bey-Bienko GY: Orthoptera. Sect. 2. Leaf bush-crickets (Phaneropterinae). 1954, Moscow - Leningrad: New Series 59. Fauna of the USSR. Zoological Institute of the Academy of Sciences of the USSR, 385-II
Can E: Zur Kenntnis von Isophya amplipennis Br. v. W., I. pavelii Br. v. W. und I. tenuicerca Rme. (Orth. Tettigoniidae), als Schädlinge von Eichenniederwäldern in Südosteuropa. I Zeitschrift für Angewandte Entomologie. 1959, 43: 387-411.
Ramme W: Beiträge zur Kenntnis der palaearktischen Orthopterenfauna (Tettig. et Acrid.). Mitt Zool Mus Berlin. 1933, 18: 416-434.
Ramme W: Zur Systematik, Faunistik und Biologie der Orthopteren von Südost-Europa und Vorderasien. Mitt Zool Mus Berlin. 1951, 27: 1-432.
Heller K-G: Zur Bioakustik und Phylogenie der Gattung Poecilimon (Orthoptera, Tettigoniidae, Phaneropterinae). Zool Jahrb Abt Anat Onot Tier. 1984, 111: 69-117.
Heller K-G: Bioakustik der europäischen Laubheuschrecken. 1988, Weikersheim: Josef Margraf, 358-
Sevgili H, Çiplak B, Heller K-G, Demirsoy A: Morphology, bioacoustics and phylogeography of the Isophya major group (Orthoptera: Tettigoniidae: Phaneropterinae): a species complex occurring in Anatolia and Cyprus. Eur J Entomol. 2006, 103: 657-671. 10.14411/eje.2006.086.
Heller K-G, Korsunovskaya OS, Sevgili H, Zhantiev RD: Bioacoustics and systematics of the Poecilimon heroicus-group (Orthoptera: Phaneropteridae: Barbitistinae). Eur J Entomol. 2006, 103: 853-865. 10.14411/eje.2006.116.
Heller K-G, Sevgili H, Reinhold K: A re-assessment of the Poecilimon syriacus group (Orthoptera 8. Tettigonioidea, Phaneropteridae) based on bioacoustics, morphology and molecular data. Insect Syst Evol. 2008, 39: 361-379. 10.1163/187631208788784309.
Heller K-G, Willemse L, Odé B, Volleth M, Feist R, Reinhold K: Bioacoustics and systematics of the Poecilimon hamatus group (Tettigonioidea: Phaneropteridae: Poecilimon: Hamatopoecilimon n. subg.). J Orthopt Res. 2011, 20: 81-95. 10.1665/034.020.0108.
Chobanov DP, Heller K-G: Revision of the Poecilimon ornatus group (Orthoptera: Phaneropteridae) with focus on Bulgaria and Macedonia. Eur J Entomol. 2010, 107: 647-672. 10.14411/eje.2010.073.
Kaya S, Ciplak B, Chobanov D, Heller K-G: Poecilimon bosphoricus group (Orthoptera, Phaneropterinae): iteration of morpho-taxonomy by song characteristics. Zootaxa. 2012, 3225: 1-71.
Warchałowska-Śliwa E, Heller K-G, Maryańska-Nadachowska A, Lehmann A: Chromosome evolution in the genus Poecilimon (Orthoptera, Tettigonioidea, Phaneropteridae). Folia biol (Kraków). 2000, 48: 127-136.
Warchałowska-Śliwa E, Chobanov DP, Grzywacz B, Maryańska-Nadachowska A: Taxonomy of the genus Isophya (Orthoptera, Phaneropteridae, Barbitistinae): Comparison of karyological and morphological data. Folia biol (Kraków). 2008, 56: 227-241. 10.3409/fb.56_3-4.227-241.
Grzywacz B, Maryańska-Nadachowska A, Chobanov DP, Karamysheva T, Warchałowska-Śliwa E: Comparative analysis of the location of rDNA in the Palaearctic bushcricket genus Isophya (Orthoptera: Tettigoniidae: Phaneropterinae). Eur J Entomol. 2011, 108: 509-517. 10.14411/eje.2011.066.
Grzywacz-Gibała B, Chobanov D, Warchałowska-Śliwa E: Preliminary phylogenetic analysis of the genus Isophya (Orthoptera: Phaneropteridae) based on molecular data. Zootaxa. 2010, 2621: 27-44.
Ullrich B, Reinhold K, Niehuis O, Misof B: Secondary structure and phylogenetic analysis of the internal transcribed spacers 1 and 2 of bush crickets (Orthoptera: Tettigoniidae: Barbitistini). J Zool Sys Evol Res. 2010, 48 (3): 219-228.
Cabral-de-Mello DC, Oliveira SB, Moura RC, Martins C: Chromosomal organization of the 18S and 5S rRNAs and histone H3 genes in Scarabaeinae coleopterans: insights into the evolutionary dynamics of multigene families and heterochromatin. BMC Genetics. 2011, 12: 88-
Nguyen P, Sahara K, Yoshido A, Marec F: Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica. 2010, 138: 343-354. 10.1007/s10709-009-9424-5.
Cabrero J, Camacho JPM: Location and expression of ribosomal RNA genes in grasshoppers: Abundance of silent and cryptic loci. Chromosome Res. 2008, 16: 595-607. 10.1007/s10577-008-1214-x.
Loreto V, Cabrero J, López-León MD, Camacho JPM, Souza MJ: Comparative analysis of rDNA location in five Neotropical gomphocerinae grasshopper species. Genetica. 2008, 132: 95-101.
Cabrero J, López-León MD, Teruel M, Camacho JPM: Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshoppers. Chromosome Res. 2009, 17: 397-404. 10.1007/s10577-009-9030-5.
Oliveira NL, Cabral-de-Mello DC, Rocha MF, Loreto V, Martins C, Moura RC: Chromosomal mapping of rDNAs and H3 histone sequences in grasshopper Rhammatocerus brasiliensis (Acrididae, Gomphocerinae): extensive chromosomal dispersion and co-localization of 5SrDNA/H3 histone clusters in the A complement and B chromosome. Mol Cytogenet. 2011, 4: 24-10.1186/1755-8166-4-24.
Rocha MF, Melo NK, Souza MJ: Comparative cytogenetics analysis of two grasshopper species of the tribe Abracrini (Ommatolampinae, Acrididae). Genet Mol Biol. 2011, 34: 214-219. 10.1590/S1415-47572011000200008.
Jetybayev IE, Bugrov AG, Karamysheva TV, Camacho JPM: Chromosomal localization of ribosomal and telomeric DNA provides new insights on the evolution of Gomphocerinae grasshoppers. Cytogenet Genome Res. 2012, 138: 36-45. 10.1159/000341571.
Warchałowska-Śliwa E, Maryańska-Nadachowska A, Heller K-G: Cytogenetic variability of the genus Saga Charp. (Orthoptera, Tettigoniidae, Saginae): heterochromatin differentiation. Caryologia. 2007, 60: 29-36. 10.1080/00087114.2007.10589544.
Warchałowska-Śliwa E, Grzywacz B, Maryańska-Nadachowska A, Karamysheva TV, Rubtsov NB, Chobanov DP: Chromosomal differentiation among bisexual European species of Saga (Orthoptera: Tettigoniidae: Saginae) detected by both classical and molecular methods. Eur J Entomol. 2009, 106: 1-9. 10.14411/eje.2009.001.
Warchałowska-Śliwa E, Grzywacz B, Maryańska-Nadachowska A, Karamysheva TV, Chobanov DP, Heller K-G: Cytogenetic variability among Bradyporinae species (Orthoptera: Tettigoniidae). Eur J Entomol. 2013, 110 (1): 1-12. 10.14411/eje.2013.001.
Hemp C, Heller K-G, Warchałowska-Śliwa E, Hemp A: A new genus and species of African Phaneropterinae (Orthoptera: Tettigoniidae), with data on its ecology, bioacoustics and chromosomes. Org Divers Evol. 2010, 10: 215-226. 10.1007/s13127-010-0013-3.
Hemp C, Heller K-G, Warchałowska-Śliwa E, Grzywacz B, Hemp A: Biogeography, ecology, acoustics and chromosomes of East African Eurycorypha Stål species (Orthoptera, Phaneropterinae) with the description of new species. Org Divers Evol. 2013, DOI: 10.1007/s13127-012-0123-1
Warchałowska-Śliwa E, Maryańska-Nadachowska A, Grzywacz B, Karamysheva T, Lehmann AW, Lehmann GUC, Heller K-G: Changes in the numbers of chromosomes and sex determination system in bushcrickets of the genus Odontura (Orthoptera: Tettigoniidae: Phaneropterinae). Eur J Entomol. 2011, 108: 183-195. 10.14411/eje.2011.025.
Warchałowska-Śliwa E, Grzywacz B, Maryańska-Nadachowska A, Karamysheva TV, Heller K-G, Lehmann AW, Lehmann GUC, Chobanov DP: Comparative physical mapping of the rDNA and heterochromatin in eight bushcricket genera of the tribe Barbitistini (Orthoptera: Tettigoniidae: Phaneropterinae). Genome. 2013, 56 (11): 667-676. 10.1139/gen-2013-0119.
Messina A, Ippolito S, Lombardo F: Cariologia di alcune specie Europee di Phaneropterinae (Insecta, Orthoptera). Animalia. 1975, 2: 215-224.
Warchałowska-Śliwa E, Heller K-G: C-banding patterns of some species of Phaneropterinae (Orthoptera, Tettigoniidae) of Europe. Folia biol (Kraków). 1998, 46: 177-181.
Warchałowska-Śliwa E: Karyotype characteristics of katydid orthopterans (Ensifera, Tettigoniidae) and remarks on their evolution at different taxonomic levels. Folia biol (Krakòw). 1998, 46: 143-176.
Warchałowska-Śliwa E, Heller K-G, Maryańska-Nadachowska A: Cytogenetic variability of European Tettigoniidae (Orthoptera, Tettigoniidae): Karyotypes, C- and Ag-NOR-banding. Folia biol (Krakòw). 2005, 53: 161-171. 10.3409/173491605775142800.
Cabral-de-Mello DC, Martins C, Souza MJ, Moura RC: Cytogenetic mapping of 5S and 18S rRNAs and H3 histone genes in four ancient Proscopiidae grasshopper species: contribution to understanding the evolutionary dynamics of multigene families. Cytogenet. Genome. Res. 2011, 132: 89-93. 10.1159/000317476.
Bressa MJ, Franco MJ, Toscani MA, Papeschi AG: Heterochromatin polymorphism in Holhymenia rubiginosa (Heteroptera: Coreidae). Eur J Entomol. 2008, 105: 65-72. 10.14411/eje.2008.009.
Schneider MC, Rosa SP, Almeida MC, Costa C, Cella DM: Chromosomal similarities and differences among four Neotropical Elateridae (Conoderini and Pyrophorini) and other related species, with comments on the NOR pattern in Coleoptera. J Zool Sys Evol Res. 2007, 45: 308-316. 10.1111/j.1439-0469.2006.00398.x.
Heller K-G, Lehmann A: Taxonomic revision of the European species of the Poecilimon ampliatus-group (Orthoptera Phaneropteridae). Mem Soc Entomol Ital. 2004, 82: 403-422.
Ünal M: Phaneropterinae (Orthoptera: Tettigoniidae) from Turkey and the Middle East II. T Am Entomol Soc. 2010, 136: 125-183. 10.3157/061.136.0203.
Grzywacz B, Warchałowska-Śliwa E: Polymorphism of the genus Isophya (Orthoptera, Phaneropteridae, Barbitistinae) revealed by RAPD. Folia Biol (Krakòw). 2008, 56: 153-157. 10.3409/fb.56_3-4.153-157.
Warchałowska-Śliwa E, Maryańska-Nadachowska A: Karyotypes, C-bands, NORs location in spermatogenesis of Isophya brevipennis Brunner (Orthoptera: Phaneropteridae). Caryologia. 1992, 45: 83-89. 10.1080/00087114.1992.10797213.
Sumner AT: A simple technique for demonstrating centromere heterochromatin. Exp Cell Res. 1972, 75: 304-306. 10.1016/0014-4827(72)90558-7.
Schweizer D: Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 1976, 58: 307-324. 10.1007/BF00292840.
Acknowledgements
This research was supported by the Ministry of Science and Higher Education of Poland, grant N N303 611738 to E. Warchałowska-Śliwa, and by the research agreement between the Polish Academy of Sciences and Bulgarian Academy of Sciences (project coordinators E. Warchałowska-Śliwa and P. Michailova). Comparative studies of type specimens in the collections of the Naturkunde Museum (Berlin), Naturhistorisches Museum (Vienna), the Natural History Museum (London), and Hungarian Natural History Museum (Budapest) by D.P. Chobanov were financed by the SYNTHESYS (European Commission’s Research Infrastructures Network funded under FP7) grants DE-TAF-3950, AT-TAF-546, GB-TAF-1320, HU-TAF-2202, respectively. T.V. Karamysheva was partially supported by the Mianowski Fund – Foundation for the Promotion of Science, Poland (2009) and RFBR, research project No. 14-04-00086 a. We owe special thanks to Battal Ciplak and Sarp Kaya (Akdeniz University, Antalya, Turkey) for their cooperation in the field studies in Turkey, to Sergey Ivanov (Taurida National University “V.I. Vernadsky”, Simferopol, Ukraine) and Yuri Budashkin (Karadagh Nature Reserve, Ukraine) for their invaluable help in collecting samples in Crimea, and to Gerlind U.C. Lehmann and Arne W. Lehmann for collecting individuals in Slovenia. Thanks are due to two anonymous reviewers for their useful comments significantly improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BG, DPCH, EW-Ś made major contributions to conception and design of analyses, interpretation of data and writing the manuscript. AM-N, TVK, K-GH took part in data analysis. All authors interpreted the results, have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
About this article
Cite this article
Grzywacz, B., Chobanov, D.P., Maryańska-Nadachowska, A. et al. A comparative study of genome organization and inferences for the systematics of two large bushcricket genera of the tribe Barbitistini (Orthoptera: Tettigoniidae: Phaneropterinae). BMC Evol Biol 14, 48 (2014). https://doi.org/10.1186/1471-2148-14-48
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2148-14-48