Abstract
This article reviews the different MRI techniques available for the diagnosis, treatment and monitoring of brain metastases with a focus on applying advanced MR techniques to practical clinical problems. Topics include conventional MRI sequences and contrast agents, functional MR imaging, diffusion weighted MR, MR spectroscopy and perfusion MR. The role of radiographic biomarkers is discussed as well as future directions such as molecular imaging and MR guided high frequency ultrasound.
Similar content being viewed by others
Introduction
Brain metastases are the most common central nervous system tumours in adults with a rising incidence due to the increased availability and utilisation of brain imaging and prolonged survival from primary cancers [1–3]. MRI is crucial in making the diagnosis, determining the best course of management, monitoring response to therapy and increasingly in trying to predict prognosis. Rather than reviewing each individual technique and its applications separately, as has been done elsewhere, the different clinical problems encountered in brain metastases are presented and the relevant MRI techniques which can be applied in each scenario addressed to give a practical summary [4–9].
Review
How many brain metastases are present?
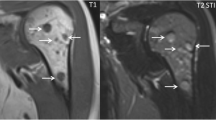
Accurately identifying the number, location and size of brain metastases is important to determine which interventions, if any, are appropriate for a patient. Multiple scoring systems used to predict prognosis take into account the number of lesions, for example the Recursive Partitioning Analysis or RPA classification [10–14]. With respect to detection, localisation and quantification, contrast enhanced MR has been widely demonstrated to be superior to both enhanced CT and non-enhanced MR [15–18] as illustrated in Figure 1. The recommended gadolinium dose in this context is 0.1 mmol/kg and whilst double or triple dose administration has been suggested to increase the detection of small lesions this causes increased false positives and a higher risk of nephrogenic systemic fibrosis therefore it remains an “off label” use [19–21].
A patient known to have melanoma presents with a first seizure and (A) unenhanced CT brain (32-slice scanner, 10 mm slices) taken in the emergency department shows an abnormality in the left frontal lobe with surrounding oedema – they were referred for possible neurosurgical intervention. (B) T1 weighted MRI at 1.5 T with single dose (0.1 mmol/kg) gadolinium contrast detects this lesion but also delineates it further, allowing volume to be assessed accurately and in addition highlights two further areas of abnormality. After staging of the systemic disease and discussion with oncologists and surgeons, the patient was therefore treated with stereotactic radiosurgery to all three areas.
Could novel sequences and agents therefore increase detection without escalating the contrast dose? Different gadolinium based agents, all of which may have slightly differing relaxivity profiles have been compared and these studies are summarised elsewhere. At present gadobutrol appears to identify the greatest number of lesions with the greatest contrast to noise ratio whilst having a lower risk of nephrogenic systemic fibrosis (along with the other “cyclic” structured gadolinium based agents [22]). MR sequences are developing rapidly but are not always explicitly evaluated against existing protocols. Magnetisation saturation transfer (MT) imaging has been directly compared to gradient echo T1 sequences and its addition reduced by half the standard dose of contrast needed for detection leading some to advocate it over ever increasing contrast doses [23, 24]. More recently, 3D T1 weighted “spoiled gradient echo” (SPGR) and T2 weighted post contrast FLAIR sequences have been shown to detect submillimetric (<3 mm) abnormalities and more sensitively assess leptomeningeal disease [25, 26]. The spatial resolution of the acquisition differs depending on the indication for the scan and this has important implications for detection and management which need to be kept in mind. For example if a treatment is decided upon with the multidisciplinary team and the patient but then a surgical or stereotactic radiosurgery (SRS) “planning” scan of higher spatial resolution is obtained, the latter will be more sensitive for detection of metastases than a conventional diagnostic scan and multiple, previously unseen lesions may be identified which render that treatment inappropriate [27].
Is this solitary lesion a metastasis, an abscess or a high grade glioma?
A patient presenting with no known primary cancer and a solitary ring enhancing brain lesion may be suspected of having a brain metastasis, a primary cerebral tumour such as glioblastoma or a cerebral abscess, and despite careful history and exam, misdiagnoses may still occur [28]. Distinguishing abscess from tumour is perhaps the most clinically relevant question and in this regard diffusion-weighted MRI (DWI) has been most useful. Diffusion-weighted images detect free water movement and allow a surrogate of diffusion to be calculated for each voxel to generate apparent diffusion coefficient (ADC) maps. A large number of observational studies have shown that for a solitary cystic or necrotic contrast enhancing lesion, restriction of diffusion on pre-operative MRI is predictive of abscess [29–32]. Modifications to imaging protocols have increased sensitivity, including detection of early capsule formation during abscess development and even prediction of cellularity of abscesses at higher b-values and field strengths [33, 34]. However, there are persistent cautions that some metastatic lesions may show restricted diffusion and mimic the appearances of abscesses, for example two different studies have reported this pattern for non-small cell lung carcinoma metastases, while others have also described it for lymphoma [35–38].
Distinguishing metastases from high grade glioma has proven more difficult using DWI although in theory the region of vasogenic oedema around metastases should show greater free diffusion than the more cellular, infiltrated region around a high grade glioma. Studies have varied in their cutoff for distinguishing the two pathologies as well as the methodology of how to take the reading (does one use the lowest ADC value, the mean of multiple measurements and which areas should one sample in the peri-tumoural region?) and there is no agreement about reliability [39–43]. Diffusion tensor imaging (DTI) is an enhancement of DWI with more diffusion gradients and directions used during acquisition. This allows more advanced metrics than just the average diffusion coefficient to be calculated and disruption of white matter tracts to be visualised. Again, the theory that high grade glioma is an invasive tumour which infiltrates white matter tracts whereas metastases deform them is supported but not consistently repeated by studies examining DTI metrics such as fractional anisotropy which may be superior to ADC alone [44–47].
Further advanced techniques have therefore been combined with diffusion imaging in this context to try and increase sensitivity, most commonly MR spectroscopy [48]. Single and multivoxel magnetic resonance spectroscopy (MRS) provides information about the metabolic profile of specific regions in and around a lesion [49, 50] as shown in Figure 2. Proton MRS has previously shown discriminatory power between high grade glioma and metastases by measurement of the choline/NAA ratio [51, 52]. Novel spectroscopic markers continue to be investigated, including lipid and macromolecule concentrations by proton MRS [53] and phosphate metabolites by 31P MRS[54].
An elderly patient was referred with hemiparesis and suspected to have a stroke. MRI demonstrated a lesion in the left hemisphere which on (A) T1 weighted axial image post gadolinium at 1.5 T is shown to have a solid and ring enhancing portion. (B) The associated ADC map shows considerably reduced diffusion at the site of the solid portion of the lesion with increased diffusion due to vasogenic oedema in the white matter surrounding the mass. (C) Single voxel proton MRS of the lesion yields an abnormal spectrum with a large lipid and lactate peak, reduced NAA, reduced Cr and slightly elevated Cho. This pointed to a metastasis, glioma or lymphoma as opposed to an abscess. There was time to optimise the patient for surgery and begin steroid treatment before the lesion was resected and confirmed to be a renal cell carcinoma.
Further information on physiological activity can be gathered by combining with MR perfusion studies. MRI perfusion permits the generation of maps of relative cerebral blood flow (rCBF) and volume (rCBV) which are measures of vascularity. High grade glioma pathologically shows neovascularisation and infiltration of surrounding brain, hence the peritumoral rCBV is higher than for a metastasis [55, 56]. Using the same dataset and different post processing techniques, diffusion susceptibility contrast or DSC MRI allows prediction of tissue permeability, a measure of blood-brain barrier disruption and this accordingly increases around a metastasis, where there is increased capillary permeability and therefore vasogenic oedema [57, 58].
In summary, multimodal MR used singly or in combination has improved our ability to distinguish metastases from primary cerebral tumours or abscesses. Despite the application of these advanced MR techniques of diffusion, perfusion and spectroscopy to solve this clinical problem [59] it remains a fact that for accessible, larger lesions surgery is often undertaken and tissue diagnosis obtained regardless in order to move forward treatment. The role and evidence for MRI in surgical planning is therefore the next area to consider.
What information does the neurosurgeon need and how can MRI provide it?
Image guided surgery allows the surgeon to plan the safest route to the tumour preoperatively and to maximise safe resection intraoperatively. Conventional post contrast T1 weighted images with thin slice protocols are generally acquired for image guidance software registration and the increased spatial resolution of such sequences compared to the usual diagnostic scans (and thus potential to detect more lesions than first seen) has been highlighted in the discussion of detection. Diffusion imaging allows images of white matter tracts and their relationship to tumour to be delineated. DTI acquisitions, in addition to generating quantitative parameters, can permit tractography by estimating the directionality of fibre tracts, permitting the tracking of fibre bundles in 3D space. The methodology for this post processing varies and a number of non-proprietary software solutions in addition to those supplied by manufacturers are available. Further refinements to the methodology, including use of higher order algorithms to resolve ambiguous directionality on voxels may further increase reliability [60]. Functional MR detects changes in blood oxygen level or BOLD signal in metabolically more active areas during application of a stimulus or performance of a task. This is particularly useful to localise language areas or motor cortex. Few series have examined the role of fMRI in metastasis resection alone but in those that have significant benefits in terms of motor recovery, preservation and therefore quality of life have been demonstrated [61]. Commercially available software can integrate functional and tractography sequences into a single merged dataset for use in theatre as shown in Figure 3, with improved outcomes for series looking exclusively at metastases [62, 63].
A patient known to have breast cancer with a manually dexterous job presented with intermittent left hand and arm weakness and was found (A) to have a solitary ring enhancing lesion in the premotor area on T1W MRI with gadolinium. (B) functional MRI performing a hand tapping and gripping task determined the location of hand function and this was used as the “seed” region of interest on a DTI scan to produce a representation of the motor tracts. (C) these were used to generate a 3D object and fused with an anatomical planning scan (1 mm slices) using commercially available software (StealthViz™ with StealthDTI™ by Medtronic, running on an S7 workstation) to produce images that were used intraoperatively for image guided resection, avoiding the tracts (shown in red, with tumour rendered in green).
Where is the original, primary cancer?
In cases of multiple metastases or solitary lesions with no known primary, MRI may give useful diagnostic clues as to the original tumour. Some primaries may have particular signal characteristics even on conventional MR. For example melanoma metastases may show high signal on non-enhanced T1 sequences due to the effect of melanin and mucinous metastases may show low T2 signal compared with the expected hyper-intensity on this sequence. The metabolic profile has been investigated for metastases of differing primary tumours as well as the surrounding brain with no abnormal spectra reported outside of the lesion itself. Metabolic features on MRS have shown limited value in predicting primary type. However, raised mobile lipid content has been proposed as a weak sign of a colonic origin for metastasis [64].Others have tried to use the diffusion characteristics of particular metastases to distinguish the primary and whilst it has been shown that ADC values are higher in well differentiated adenocarcinoma metastases than poorly differentiated types this may simply reflect cellularity and could not predict the primary, only suggest a narrower differential [36]. Likewise in a series with a variety of primary cancers including lung and breast, restricted diffusion could not be correlated with any primary nor could ADC predict primary pathology [35]. Spectroscopy was combined with perfusion MR to show that differences in choline-creatine ratios between metastases of lung and breast cancer correlated with differences in relative cerebral blood volume but tissue was not available for comparison to look for a unifying pathological basis [65].
In summary, MRS may be used to distinguish primary tumour origin. However more than one advanced MR metric may need to be combined in order to robustly generate models that differentiate the primary lesion and though these studies would likely be retrospective, ideally some image guided correlation of regions of interest on the advanced MR with the final tissue samples is needed, as has been performed for glioma [45].
Are these brain metastases responding to chemo/radio-therapy?
Beyond diagnosis, MRI may be used to monitor response to treatment as part of clinical and radiological follow up. This may be immediate, as in post-operative MRI to determine if there has been a complete resection or delayed, i.e. has the metastasis responded chemo- or radio-therapy. In general, conventional sequences are utilized but one area of particular clinical interest in following cases up is ongoing or increasing enhancement following surgery or stereotactic radiosurgery to a metastasis and whether this represents radio-necrosis or recurrence [66]. Standard patterns of changes in brain metastases are seen on conventional MRI with perilesional oedema, central hypointensity on T2 weighted imaging at 2-6 months followed by blurring of the enhancement margin, reduction in enhancing volume over time and formation of a glial scar [67–69]. These changes and difficulty in interpreting responses are illustrated in Figure 4. Although attempts have been made to build discriminatory measures from conventional MRI [70] performance has proven disappointing [71]. Blood flow would be expected to differ significantly between areas of necrosis and recurrence. MR perfusion has shown higher rCBV in recurrent metastases after radiosurgery compared to areas of radionecrosis and reduction in CBF over time for treated lesions [72, 73]. Furthermore, the first reading of CBF following treatment was highly predictive of the final response, even though pre-operative readings could not predict this. Diffusion imaging may reflect cellularity and ADC readings from metastases treated with radiosurgery, taken immediately post treatment may be tracked to determine if the lesion is responding to therapy, manifest as increasing ADC as compared to a recurring or necrotic lesion where the changes in ADC differ. The initial ADC value may also predict final response to the treatment [74]. In conjunction with perfusion MRI, this suggests the novel application of advanced MR readings, not just as diagnostic tools but as predictors of future treatment response or biological markers.
Monitoring of treatment response after stereotactic radiosurgery. A patient with known metastatic melanoma underwent treatment to a left frontal lesion (A) and at 3 months (B) the characteristic changes of a blurred margin and necrosis followed by formation of a glial scar at 6 months (C) are seen. However in a similar appearing patient (D) with a right parietal lesion from metastatic renal cell carcinoma the lesion shows more florid changes at 3 months (E) with increased contrast enhancement at the margins and the possibility of progression as opposed to radionecrosis is raised. Multi-modal techniques described in the text may show promise in deciding how to proceed at this stage. The patient was well and on monoclonal antibody chemotherapy, with observation the lesion eventually regressed at 6 months (F) and is currently stable.
Could radiographic biomarkers predict survival?
As described in many studies here, various metrics or measurements may be taken from the different advanced MR images described using the device workstation or non-proprietary software. A biological marker or “biomarker” is strictly any such reading which is used for diagnostic or prognostic purposes. Even conventional MR may provide such markers and it has been shown that for solitary metastases in one series, the degree of oedema on the preoperative T2 weighted scan was related to the degree of angiogenesis, brain invasiveness and overall survival with reduced oedema surprisingly being a worse prognostic factor [75]. The role of ADC and CBF in predicting response of metastases to SRS has been discussed in the preceding section. In a recent study preoperative DWI and ADC readings were assessed in single metastases which were subsequently resected. A reduced overall survival was found irrespective of adjuvant treatments and there was a denser reticulin (stromal) matrix in patients with low tumour ADC compared to the group average [76]. These measures could potentially be incorporated into the prognostic models mentioned such as the recursive partitioning score (RPA) or graded prognosis assessment (GPA) score; widely validated predictors of survival in brain metastases patients which combine clinical information such as age, status of the primary cancer and extracranial disease amongst others [10, 77]. Further standardisation of the post processing and measurement workflow is needed, however, before MRI metrics could be confidently used in clinical practice.
What are the emerging directions of MRI in brain metastases management?
As cancer diagnostics and therapeutics become increasingly related three areas of emerging MR technology with practical applications to brain metastases stand out. Integration of cancer staging via PET-MR offers one potential means of incorporating further functional data in real time without losing all the knowledge already acquired about the behaviour and characteristics of metastases on MRI [78]. Novel contrast agents including those that can identify molecular targets are in development in animals and an agent based on iron oxide particles which binds to a vascular cell adhesion molecule common to human metastases has been used to visualise micro-metastases at MRI, suggesting the possibility of diagnosing and possibly treating brain metastases long before they were previously even detected [79]. Finally the application of MR as an intraoperative tool for guiding minimally invasive therapies such as laser coagulation or high intensity focused ultrasound is no longer conceptual, with several small series of treated tumours including metastases [80, 81]. MR technology will continue to enhance diagnosis but is now being used to predict prognosis and being incorporated into the treatment of metastases too [82].
Conclusions
This article has summarised the current evidence for the practical application of advanced MRI techniques including diffusion weighted imaging, tractography, perfusion studies, functional MRI and MR spectroscopy in common clinical scenarios relating to brain metastases. Previously the literature has predominantly focused on applying these modalities to intrinsic brain tumours such as glioma but there appears to be an increasing recognition of the burden of metastatic brain disease and the need for novel applications of MR technology to solve the sort of practical clinical problems described and provide more prognostic information. It may be that this occurs by development of further conventional sequences and better contrast media, including at a molecular level, or by combination of the existing possibilities to generate multimodal datasets. Evidence of practical, clinical utility specific to metastases needs to be gathered at each stage to justify the development of - and determine the best use of - these expensive resources.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- ADC:
-
Apparent diffusion coefficient
- ROI:
-
Region of interest
- MRS:
-
Magnetic resonance spectroscopy
- DWI:
-
Diffusion weighted imaging
- FLAIR:
-
Fluid attenuated inversion recovery
- rCBV:
-
Relative cerebral blood volume
- CBF:
-
Cerebral blood flow
- fMRI:
-
Functional magnetic resonance imaging
- RPA:
-
Recursive partitioning analysis
- GPA:
-
Graded prognostic assessment
- SRS:
-
Stereotactic radiosurgery
- DTI:
-
Diffusion tensor imaging
- DSC:
-
Dynamic susceptibility contrast.
References
Soffietti R, Rudā R, Mutani R: Management of brain metastases. J Neurol 2002, 249: 1357–1369. 10.1007/s00415-002-0870-6
Gavrilovic IT, Posner JB: Brain metastases: epidemiology and pathophysiology. J Neuro-Oncol 2005, 75: 5–14. 10.1007/s11060-004-8093-6
Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G: Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 2011, 22(1):1–6. v 10.1016/j.nec.2010.08.007
Fink KR, Fink JR: Imaging of brain metastases. Surg Neurol Int 2013, 4(Suppl 4):S209-S219.
Mills SJ, Thompson G, Jackson A: Advanced magnetic resonance imaging biomarkers of cerebral metastases. Cancer Imaging 2012, 12: 245–252. 10.1102/1470-7330.2012.0012
Young RJ, Sills AK, Brem S, Knopp EA: Neuroimaging of metastatic brain disease. Neurosurgery 2005, 57(5 SUPPL):S4–10-S4–23.
Cha S: Neuroimaging in neuro-oncology. Neurotherapeutics 2009, 6(3):465–477. 10.1016/j.nurt.2009.05.002
Kaal EC, Taphoorn MJ, Vecht CJ: Symptomatic management and imaging of brain metastases. J Neurooncol 2005, 75(1):15–20. 10.1007/s11060-004-8094-5
Brandão LA, Shiroishi MS, Law M: Brain tumors. A multimodality approach with diffusion-weighted imaging, diffusion tensor imaging, magnetic resonance spectroscopy, dynamic susceptibility contrast and dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging Clin N Am 2013, 21(2):199–239. 10.1016/j.mric.2013.02.003
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997, 37: 745–751. 10.1016/S0360-3016(96)00619-0
Jenkinson MD, Haylock B, Shenoy A, Husband D, Javadpour M: Management of cerebral metastasis: evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer 2011, 47(5):649–655. 10.1016/j.ejca.2010.11.033
Nieder C, Marienhagen K, Astner ST, Molls M: Prognostic scores in brain metastases from breast cancer. BMC Cancer 2009, 9: 105. 10.1186/1471-2407-9-105
Siu TL, Jeffree RL, Fuller JW: Current strategies in the surgical management of cerebral metastases: an evidence-based review. J Clin Neurosci 2011, 18(11):1429–1434. 10.1016/j.jocn.2011.04.002
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M: Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012, 30(4):419–425. 10.1200/JCO.2011.38.0527
Davis PC, Hudgins PA, Peterman SB, Hoffman JC Jr: Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. Am J Neuroradiol 1991, 12(2):293–300.
Yokoi K, Kamiya N, Matsuguma H, Machida S, Hirose T, Mori K, Tominaga K: Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest 1999, 115(3):714–719. 10.1378/chest.115.3.714
Schellinger PD, Meinck HM, Thron A: Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol 1999, 44(3):275–281. 10.1023/A:1006308808769
Kruger S, Mottaghy FM, Buck AK, Maschke S, Kley H, Frechen D, Wibmer T, Reske SN, Pauls S: Brain metastasis in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for diagnosis in the initial staging. Nuklearmedizin 2011, 50(3):101–106.
Schneider G, Kirchin MA, Pirovano G, Colosimo C, Ruscalleda J, Korves M, Salerio I, La Noce A, Spinazzi A: Gadobenate dimeglumine-enhanced magnetic resonance imaging of intracranial metastases: Effect of dose on lesion detection and delineation. J Magn Reson Imaging 2001, 14: 525–539. 10.1002/jmri.1216
Sze G, Johnson C, Kawamura Y, Goldberg SN, Lange R, Friedland RJ, Wolf RJ: Comparison of single- and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol 1998, 19(5):821–828.
Yuh WTC, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ: The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. Am J Neuroradiol 1995, 16(2):373–380.
Anzalone N, Essig M, Lee SK, Dörfler A, Ganslandt O, Combs SE, Picozzi P: Optimizing contrast-enhanced magnetic resonance imaging characterization of brain metastases: relevance to stereotactic radiosurgery. Neurosurgery 2013, 72(5):691–701. 10.1227/NEU.0b013e3182889ddf
Ginsberg LE, Lang FF: Neuroradiologic screening for brain metastases–can quadruple dose gadolinium be far behind? AJNR Am J Neuroradiol 1998, 19(5):829–830.
Haba D, Pasco Papon A, Tanguy JY, Burtin P, Aube C, Caron-Poitreau C: Use of half-dose gadolinium-enhanced MRI and magnetization transfer saturation in brain tumors. Eur Radiol 2001, 11(1):117–122. 10.1007/s003300000493
Chen W, Wang L, Zhu W, Xia L, Qi J, Feng D, Luo X: Multicontrast single-slab 3D MRI to detect cerebral metastasis. AJR Am J Roentgenol 2012, 198(1):27–32. 10.2214/AJR.11.7030
Kakeda S, Korogi Y, Hiai Y, Ohnari N, Moriya J, Kamada K, Hanamiya M, Sato T, Kitajima M: Detection of brain metastasis at 3T: comparison among SE, IR-FSE and 3D-GRE sequences. Eur Radiol 2007, 17(9):2345–2351. 10.1007/s00330-007-0599-9
Nagai A, Nagai A, Shibamoto Y, Mori Y, Hashizume C, Hagiwara M, Kobayashi T: Increases in the number of brain metastases detected at frame-fixed, thin-slice MRI for gamma knife surgery planning. Neuro Oncol 2010, 12(11):1187–1192. 10.1093/neuonc/noq084
Brodbelt A: Delaying emergency treatment for patients with cerebral abscesses and brain tumours: a consequence of the neuroscience MDT? Br J Neurosurg 2012, 26(3):325. 10.3109/02688697.2012.680628
Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M: Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol 1999, 20(7):1252–1257.
Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, Ueda S, Sato H: Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging 1996, 14(9):1113–1116. 10.1016/S0730-725X(96)00237-8
Kim YJ, Chang KH, Song IC, Kim HD, Seong SO, Kim YH, Han MH: Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol 1998, 171(6):1487–1490. 10.2214/ajr.171.6.9843275
Krabbe K, Gideon P, Wagn P, Hansen U, Thomsen C, Madsen F: MR diffusion imaging of human intracranial tumours. Neuroradiology 1997, 39: 483–489. 10.1007/s002340050450
Fertikh D, Krejza J, Cunqueiro A, Danish S, Alokaili R, Melhem ER: Discrimination of capsular stage brain abscesses from necrotic or cystic neoplasms using diffusion-weighted magnetic resonance imaging. J Neurosurg 2007, 106(1):76–81. 10.3171/jns.2007.106.1.76
Tomar V, Yadav A, Rathore RK, Verma S, Awasthi R, Bharadwaj V, Ojha BK, Prasad KN, Gupta RK: Apparent diffusion coefficient with higher b-value correlates better with viable cell count quantified from the cavity of brain abscess. AJNR Am J Neuroradiol 2011, 32(11):2120–2125. 10.3174/ajnr.A2674
Duygulu G, Ovali GY, Calli C, Kitis O, Yünten N, Akalin T, Islekel S: Intracerebral metastasis showing restricted diffusion: correlation with histopathologic findings. Eur J Radiol 2010, 74(1):117–120. 10.1016/j.ejrad.2009.03.004
Hayashida Y, Hirai T, Morishita S, Kitajima M, Murakami R, Korogi Y, Makino K, Nakamura H, Ikushima I, Yamura M, Kochi M, Kuratsu JI, Yamashita Y: Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol 2006, 27(7):1419–1425.
Holtas S, Geijer B, Strömblad LG, Maly-Sundgren P, Burtscher IM: A ring-enhancing metastasis with central high signal on diffusion-weighted imaging and low apparent diffusion coefficients. Neuroradiology 2000, 42(11):824–827. 10.1007/s002340000431
Stadnik TW, Chaskis C, Michotte A, Shabana WM, van Rompaey K, Luypaert R, Budinsky L, Jellus V, Osteaux M: Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 2001, 22(5):969–976.
Bulakbasi N, Kocaoglu M, Farzaliyev A, Tayfun C, Ucoz T, Somuncu I: Assessment of diagnostic accuracy of perfusion MR imaging in primary and metastatic solitary malignant brain tumors. AJNR Am J Neuroradiol 2005, 26: 2187–2199.
Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T: Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 2006, 58(3):394–403. 10.1016/j.ejrad.2005.12.032
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO, Liu GC: Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 2004, 46(8):619–627.
Lee EJ, terBrugge K, Mikulis D, Choi DS, Bae JM, Lee SK, Moon SY: Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol 2011, 196(1):71–76. 10.2214/AJR.10.4752
Wang S, Kim S, Chawla S, Wolf RL, Knipp DE, Vossough A, O'Rourke DM, Judy KD, Poptani H, Melhem ER: Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage 2009, 44(3):653–660. 10.1016/j.neuroimage.2008.09.027
Byrnes TJ, Barrick TR, Bell BA, Clark CA: Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed 2011, 24(1):54–60. 10.1002/nbm.1555
Price SJ, Gillard JH: Imaging biomarkers of brain tumour margin and tumour invasion. Br J Radiol 2011, 84 Spec No 2: S159–67.
Tsuchiya K, Fujikawa A, Nakajima M, Honya K: Differentiation between solitary brain metastasis and high-grade glioma by diffusion tensor imaging. Br J Radiol 2005, 78(930):533–537. 10.1259/bjr/68749637
Toh CH, Wei KC, Ng SH, Wan YL, Lin CP, Castillo M: Differentiation of brain abscesses from necrotic glioblastomas and cystic metastatic brain tumors with diffusion tensor imaging. AJNR Am J Neuroradiol 2011, 32(9):1646–1651. 10.3174/ajnr.A2581
Bulakbasi N, Kocaoglu M, Ors F, Tayfun C, Uçöz T: Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol 2003, 24(2):225–233.
Pinker K, Stadlbauer A, Bogner W, Gruber S, Helbich TH: Molecular imaging of cancer: MR spectroscopy and beyond. Eur J Radiol 2012, 81(3):566–577. 10.1016/j.ejrad.2010.04.028
van der Graaf M: In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J 2010, 39(4):527–540. 10.1007/s00249-009-0517-y
Server A, Josefsen R, Kulle B, Maehlen J, Schellhorn T, Gadmar Ø, Kumar T, Haakonsen M, Langberg CW, Nakstad PH: Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol 2010, 51(3):316–325. 10.3109/02841850903482901
Wong CS, Chu TYC, Ma JKF: Peri-tumoural magnetic resonance spectroscopy to differentiate solitary primary intra-axial high-grade glioma and brain metastasis: a pilot study. J Hong Kong College Radiologists 2010, 13(4):195–198.
Crisi G, Orsingher L, Filice S: Lipid and macromolecules quantitation in differentiating glioblastoma from solitary metastasis: A short-echo time single-voxel magnetic resonance spectroscopy study at 3 T. J Comput Assist Tomogr 2013, 37(2):265–271. 10.1097/RCT.0b013e318282d2ba
Ha DH, Choi S, Oh JY, Yoon SK, Kang MJ, Kim KU: Application of 31P MR spectroscopy to the brain tumors. Korean J Radiol 2013, 14(3):477–486. 10.3348/kjr.2013.14.3.477
Blasel S, Jurcoane A, Franz K, Morawe G, Pellikan S, Hattingen E: Elevated peritumoural rCBV values as a mean to differentiate metastases from high-grade gliomas. Acta Neurochir 2010, 152(11):1893–1899. 10.1007/s00701-010-0774-7
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW: High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002, 222: 715–721. 10.1148/radiol.2223010558
Lacerda S, Law M: Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am 2009, 19(4):527–557. 10.1016/j.nic.2009.08.007
Thompson G, Mills SJ, Stivaros SM, Jackson A: Imaging of brain tumors: perfusion/permeability. Neuroimaging Clin N Am 2010, 20(3):337–353. 10.1016/j.nic.2010.04.008
Server A, Graff BA, Døli Orheim TE, Schellhorn T, Josefsen R, Gadmar OB, Nakstad PH: Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology 2011, 53(5):319–330. 10.1007/s00234-010-0740-3
Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A: White matter fiber tractography: why we need to move beyond DTI. J Neurosurg 2013, 118(6):1367–1377. 10.3171/2013.2.JNS121294
Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C: Operative treatment of subcortical metastatic tumours in the central region. J Neurooncol 2011, 103(3):567–573. 10.1007/s11060-010-0420-5
Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW: Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 2005, 56(5):1021–1033.
Tan TC, Black PM: Image-guided craniotomy for cerebral metastases: techniques and outcomes. Neurosurgery 2007, 61(1 Suppl):349–356. discussion 356–7
Chernov MF, Ono Y, Kubo O, Hori T: Comparison of 1H-MRS-detected metabolic characteristics in single metastatic brain tumors of different origin. Brain Tumor Pathol 2006, 23(1):35–40. 10.1007/s10014-006-0198-5
Huang BY, Kwock L, Castillo M, Smith JK: Association of choline levels and tumor perfusion in brain metastases assessed with proton MR spectroscopy and dynamic susceptibility contrast-enhanced perfusion weighted MRI. Technol Cancer Res T 2010, 9: 327–337.
Kang TW, Kim ST, Byun HS, Jeon P, Kim K, Kim H, Lee JI: Morphological and functional MRI, MRS, perfusion and diffusion changes after radiosurgery of brain metastasis. Eur J Radiol 2009, 72(3):370–380. 10.1016/j.ejrad.2008.08.009
Friedman DP, Morales RE, Goldman HW: MR imaging findings after stereotactic radiosurgery using the gamma knife. AJR Am J Roentgenol 2001, 176(6):1589–1595. 10.2214/ajr.176.6.1761589
Lunsford LD, Kondziolka D, Maitz A, Flickinger JC: Black holes, white dwarfs and supernovas: imaging after radiosurgery. Stereotact Funct Neurosurg 1998, 70(Suppl 1):2–10.
Peterson AM, Meltzer CC, Evanson EJ, Flickinger JC, Kondziolka D: MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology 1999, 211(3):807–814. 10.1148/radiology.211.3.r99jn48807
Dequesada IM, Dequesada IM, Quisling RG, Yachnis A, Friedman WA: Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 2008, 63(5):898–903. discussion 904 10.1227/01.NEU.0000333263.31870.31
Stockham AL, Tievsky AL, Koyfman SA, Reddy CA, Suh JH, Vogelbaum MA, Barnett GH, Chao ST: Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol 2012, 109(1):149–158. 10.1007/s11060-012-0881-9
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S: Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2009, 30(2):367–372.
Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ, Vandertop WP: Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 2009, 256(6):878–887. 10.1007/s00415-009-5034-5
Goldman M, Boxerman JL, Rogg JM, Norén G: Utility of apparent diffusion coefficient in predicting the outcome of Gamma Knife-treated brain metastases prior to changes in tumor volume: a preliminary study. J Neurosurg 2006, 105(Suppl):175–182.
Spanberger T, Berghoff AS, Dinhof C, Ilhan-Mutlu A, Magerle M, Hutterer M, Pichler J, Wöhrer A, Hackl M, Widhalm G, Hainfellner JA, Dieckmann K, Marosi C, Birner P, Prayer D, Preusser M: Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis 2013, 30(4):357–368. 10.1007/s10585-012-9542-9
Berghoff AS, Spanberger T, Ilhan-Mutlu A, Magerle M, Hutterer M, Woehrer A, Hackl M, Widhalm G, Dieckmann K, Marosi C, Birner P, Prayer D, Preusser M: Preoperative diffusion-weighted imaging of single brain metastases correlates with patient survival times. PLoS One 2013, 8(2):e55464. 10.1371/journal.pone.0055464
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W: A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008, 70(2):510–514. 10.1016/j.ijrobp.2007.06.074
Torigian DA, Zaidi H, Kwee TC, Saboury B, Udupa JK, Cho ZH, Alavi A: PET/MR imaging: technical aspects and potential clinical applications. Radiology 2013, 267(1):26–44. 10.1148/radiol.13121038
Serres S, Soto MS, Hamilton A, McAteer MA, Carbonell WS, Robson MD, Ansorge O, Khrapitchev A, Bristow C, Balathasan L, Weissensteiner T, Anthony DC, Choudhury RP, Muschel RJ, Sibson NR: Molecular MRI enables early and sensitive detection of brain metastases. Proc Natl Acad Sci U S A 2012, 109(17):6674–6679. 10.1073/pnas.1117412109
Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF: Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery 2012, 71(1 Suppl Operative):133–144. 144–5
Ram Z, Cohen ZR, Harnof S, Tal S, Faibel M, Nass D, Maier SE, Hadani M, Mardor Y: Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006, 59(5):949–955. discussion 955–6
Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, Kassell NF, Elias WJ: Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery. J Neurosurg 2013, 118(2):215–221. 10.3171/2012.10.JNS12449
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RZ drafted the article with assistance from KD, MB and MR. MR and MB assisted with finding figures. CW, MDJ, MR significantly edited the article. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zakaria, R., Das, K., Bhojak, M. et al. The role of magnetic resonance imaging in the management of brain metastases: diagnosis to prognosis. cancer imaging 14, 8 (2014). https://doi.org/10.1186/1470-7330-14-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1470-7330-14-8