Abstract
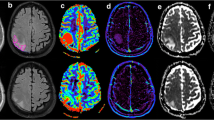
This study compared the effectiveness of relative cerebral blood volume, apparent diffusion coefficient, and spectroscopic imaging in differentiating between primary high-grade gliomas and solitary metastases. A 3.0-T MR unit was used to perform proton MR spectroscopy, diffusion imaging, and conventional MR imaging on 26 patients who had solitary brain tumors (14 high-grade gliomas and 12 metastases). All diagnoses were confirmed by biopsy. Twelve perfusion MR studies (8 high-grade gliomas and 4 metastases) were also performed. The results showed that the choline to creatine ratio and relative cerebral blood volume in the peritumoral regions of high-grade gliomas were significantly higher than they were in the metastases. The apparent diffusion coefficient values in tumoral and peritumoral regions of metastases were significantly higher than they were in the primary gliomas. Although conventional MR imaging characteristics of solitary metastases and primary high-grade gliomas may sometimes be similar, the peritumoral perfusion-weighted and spectroscopic MR imaging enable distinction between the two. Diffusion-weighted imaging techniques were complementary techniques to make a differential diagnosis between the two malignant tumors.



Similar content being viewed by others
References
Kaminogo M, Ishimaru H, Morikawa M et al. (2001) Diagostic potential of short echo time MR spectroscopy of gliomas with single-voxel and point-resolved spatially localized proton spectroscopy of brain. Neuroradiology 43:353–363
Luyten PR, Marien AJ, Heindel W et al. (1990) Metabolic imaging of patients with intracranial tumors: H-1 MR spectroscopic imaging and PET. Radiology 176:791–799
Alger JR, Frank JA, Bizzi A et al. (1990) Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxyglucose PET. Radiology 177:633–641
Nelson SJ, Huhn S, Vigneron DB et al. (1997) Volume MRI and MRSI techniques for the quantitation of treatment response in brain tumors. Magn Reson Imaging 7:1146–1152
Vigneron DB, Bollen A, McDermott M et al. (2001) Three-dimensional magnetic resonance spectroscopic imaging of histologically confirmed brain tumors. Magn Reson Imaging 19:89–101
Krabbe K, Gideon P, Wagn P, Hansen U, Thosem C, Madsen F (1997) MR diffusion imagng of human intracranial tumors. Diagn Neuroradiol 39:483–489
Roland G, Daniel B, Vigneron N et al. (2001) Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 21:357–366
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial neoplasms. J Neurosurg 66:865–874
Henry RG, Vigneron DB, Fischbein NJ et al. (2000) Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 21:357–366
Castillo M, Kwock L (1998) Proton MR spectroscopy of common brain tumors. Neuroimaging Clin N Am8:733–752
McBride DQ, Miller BL, Nikas DL et al. (1995) Analysis of brain tumors using 1H magnetic resonance spectroscopy. Surg Neurol 44:137–144
Shimuzu H, Kumabe T, Tominaga T et al. (1996) Non-invasive evaluation of malignat brain tumors wityh proton MR spectroscopy. AJNR Am J Neuroradiol 17:737–747
Knopp EA, Cha S, Johnson G et al. (1999) Glial neoplasms: dynamic contrast enhanced T2-weighted MR imaging. Radiology 211:791–798
Tien RD, Felsberg GJ, Friedman H, Brown M, McFall J (1994) MR imaging of high-grade cerebral gliomas: value of diffusion-weighted echoplanar pulse sequences. AJR Am J Roentgenol 162:671–677
Brunberg JA, Chenevert TL, MckeeverPE et al. (1995) In vivo MR determination of water diffusion coefficients and diffusion anisotropy: correlation with structural alteration in gliomas of cerebral hemispheres. AJNR Am J Neuroradiol 16:361–371
Lu S, Ahn D, Johnson G, Cha S (2003) Peritumoral diffusion tensor imaging of high- grade gliomas and metastatic brain tumos. AJNR Am J Neuroradiol 24:937–941
Steen RG (1992) Edema and tumor perfusion: characterization by quantitative proton MR imaging. AJR Am J Roentgenol 158:259–264
Zhang M, Olsson Y (1997) Hematogenous metastases of the human brain: characteristics of peritumoral brain changes—a review. J Neurooncol 35:81–89
Hossman KA, Bloink M (1981) Blood flow and regulation of blood flow in experimental peritumoral edema. Stroke 12:211–217
Burger P (1990) Classification, grading, and patterns of spread of malignant gliomas. In: Apuzzo ML (eds) Neurosurgical topics: malignant cerebral glioma. American Association of Neurological Surgeons, Park Ridge, 3–17
Aronen HJ, Gazit IE, Louis DN et al. (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Poptani H, Gupta RK, Roy R, Pandey R, Jain VK, Chhabra DK (1995) Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. Am J Neuroradiol 16:1593–1603
Bruhn H, Frahm J, Gyngell ML et al. (1989) Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology 172:541–548
Preul MC, Caramanos Z, Collins DL et al. (1996) Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat Med 2:323–325
Sugahara T, Korogi Y, Kochi M et al. (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Li X, Lu y, Pirzkall A, McKnight T, Nelson S (2002) Analysis of the spatial characteristics of metabolic abnormalities in newly diagnosed glioma patients. J Magn Reson Imaging 16:229–237
Ikehira H, Miyamoto T, Yasukawa T et al. (1995) Differences in metabolic and morphological reactions after radiation therapy: proton NMR spectroscopy and imaging of patients with intracranial tumors. Radiat Med 13:199–204
Usenius T, Usenius J, Tenhunen M et al. (1995) Radiation-induced changes in human brain metabolites as studied by 1H nuclear magnetic resonance spectroscopy in vivo. Int J Radiat Oncol Biol Phys 33:719–724
Walecki J, Sokol M, Pieniazek P et al. (1999) Role of short TE 1H-MR spectroscopy in monitoring of postoperation irradiated patients. Eur J Radiol 30:154–161
Taylor JS, Langston JW, Reddick WE et al. (1996) Clinical value of proton magnetic resonance spectroscopy for differentiating recurrent or residual brain tumor from delayed cerebral necrosis. Int J Radiat Oncol Biol Phys 36:1251–1261
Ricci PE, Pitt A, Keller PJ, Coons SW, Heiserman JE (2000) Effect of voxel position on single-voxel MR spectroscopy findings. AJR Am J Neuroradiol 21:367–337
Meng L, Soonmee C, Knopp EA, Johnson G, Arnett BS, Litt AW (2002) High grade-gliomas and solitary metasases: differentiaition by using perfusion and proton spectroscopic MR imaging. Radiology 222:715–721
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiang, I., Kuo, YT., Lu, CY. et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46, 619–627 (2004). https://doi.org/10.1007/s00234-004-1246-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1246-7