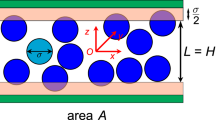
Abstract
We use Gürsey’s statistical mechanics of a one-dimensional fluid to find a formula for the \(P_\textrm{f}/P_\textrm{d}\) ratio in the transport of hard spheres across a membrane through a narrow channel that can accommodate molecular movement only in single file. \(P_\textrm{f}\) is the membrane permeability for osmotic flow and \(P_\textrm{d}\) the permeability for exchange across the membrane in the absence of osmotic flow. The deviation of the ratio from unity indicates the degree of cooperative transport relative to ordinary diffusion of independent molecules. In contrast to an early idea that \(P_\textrm{f}/P_\textrm{d}\) must be equal to the number of molecules in the channel, regardless of the physical nature of the interactions among the molecules, we find a functional dependence on the fractional occupancy of the length of the channel by the hard spheres. We also attempt a random walk calculation for \(P_\textrm{d}\) individually, which gives a result for \(P_\textrm{f}\) as well when combined with the ratio.
Graphical Abstract
The convection/diffusion ratio for hard spheres in single-file transport

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The search for an understanding of how water and ions are transported across cell membranes has always been a central feature of fundamental research in botany, physiology, and medicine [1, 2], acting even very early as the stimulus for van’t Hoff’s quantification of the equilibrium pressure difference across a semi-permeable membrane, and Vegard’s ideas on the mechanism of osmotic water flow through membranes [3, 4]. Urgent contemporaneous needs for efficient energy production and for an increased supply of pure water has augmented biology in creating a vast effort in membrane science and engineering [5,6,7,8].
The internal structure of a membrane determines how water flows through it. Water can flow through a coarse porous membrane, like those used in early research [3, 9], or even capillary walls with pores about 5 nm diameter [10], in more or less its bulk form. Current indications are that the membranes used in the purification of water by reverse osmosis, although densely structured, may be sufficiently porous to accommodate water in bulk form as well [7]. Carbon nanotubes of, say, about 1 nm diameter (two to three water molecules abreast) convey water in interesting structural modifications from bulk [11]. The fast transport inside these nanotubes cannot be modeled by the macroscopic no-slip condition at the walls of the tube [12,13,14,15]. The lipid bilayers comprising animal cell membranes are thought to dissolve small amounts of water, much as oil does, so that transport through them occurs as separate discrete water molecules [2, 4]. Animal cell membranes are punctuated by narrow protein channels [16] through which water molecules can flow, but only in single file [17]. Sufficiently narrow carbon nanotubes can also restrict water flow to single file [18]. The present paper is concerned with certain theoretical questions that arise from specifically single-file transport.
Water transport across membranes can be characterized by two distinct membrane permeabilities, the hydraulic or osmotic (historically, “filtration”) permeability \(P_\textrm{f}\), and the diffusion, or exchange, permeability \(P_\textrm{d}\) [2, 4]. The \(P_\textrm{f}/P_\textrm{d}\) ratio provides useful information about the mechanism of flow. If it equals unity, as for lipid bilayers [2, 19], one may assume that water crosses the membrane as widely separated individual molecules. If it is much larger than unity, then water presumably exists inside the membrane in its usual bulk liquid form, and its flow under applied pressure, or, equivalently, in osmosis, is predominantly convective [9, 20].
Biological membranes are spanned by protein channels so narrow that water molecules traverse them in single file, molecule after molecule [2]. It might have been expected that the osmotic and diffusion permeabilities would be equal for these channels, but as it has turned out, the \(P_\textrm{f}/P_\textrm{d}\) ratio is measurably greater than unity [2, 21,22,23], although not nearly as large as for bulk flow through relatively open synthetic membranes [9, 20]. To explain this experimental observation, an early idea was that the single-file ratio \(P_\textrm{f}/P_\textrm{d}\) should equal N, the number of water molecules in the column, as following from the mutual impenetrability of the water molecules [2]. However, a focus on the permeability ratio was all but lost before resolution of this question. In this paper, we show that the inability of the water molecules in a column to pass each other does not necessarily lead to the answer N for the permeability ratio. Nonetheless, we find that the ratio exceeds unity for a hard sphere model.
Our primary tool will be the analysis given by Gürsey in 1950 of a one-dimensional fluid [24], one of the few exact results of statistical mechanics. The molecules of the fluid are freely moving, but subject to short-range interaction forces that prevent the molecules from passing through one another. It is precisely this latter property that Gürsey understood would permit exact evaluation of the partition function by application of the convolution theorem. The short-range interactions allowed are otherwise quite general, although for present considerations we restrict our application to a hard sphere potential (impenetrable balls, all with the same diameter, with no forces between balls when not in contact).
The present paper supersedes a previous one where, due to an unrealistic boundary condition, we erroneously proposed that \(P_\textrm{f}\) and \(P_\textrm{d}\) should be equal [25].
2 Background
The osmotic permeability coefficient \(P_\textrm{f}\) for the molar water flux \(J_\textrm{w}\) across a membrane (number of moles per unit membrane area per second) as induced either by an applied pressure difference \(\Delta P\) or an osmolyte concentration difference \(\Delta C_\textrm{s}\) (osmolyte concentration is the total concentration of impermeable solute) is defined by the equation [2],
which can be called the Fundamental Law of Osmosis [4]. As follows from Debye’s analysis of the van’t Hoff equation [26, 27], an osmolarity difference induces a pressure gradient across the membrane identical [3, 4] to the gradient induced by an applied pressure difference [28].
It is of interest to compare the value of \(P_\textrm{f}\) with the value of a different permeability coefficient \(P_\textrm{d}\) for the same membrane, defined by,
where \(J^{*}_\textrm{w}\) is the molar flux of a tracer water isotope across the membrane as induced by a concentration difference \(\Delta C_\textrm{w}^{*}\) of tracer water with equal pressures on both sides of the membrane and no osmolyte present [2]. From its definition, we see that \(P_\textrm{d}\) measures the rate of water exchange across the membrane in the absence of any driving forces, and due only to the self-diffusion of the water molecules, that is, their thermal random movement.
For membranes allowing bulk, or convective, flow of water,
where the total observed osmotic flow is understood to consist of independent convective and diffusive components, the latter due to the stochastic movements of water molecules that are random in the absence of directed forces but biased in the direction of a force such as a pressure gradient [4]. The osmotic permeability \(P_\textrm{f}\) can be measured experimentally from its definition, Eq. (1), and also the diffusion permeability from its definition, Eq. (2), but the convective component \(P_\textrm{c}\) can only be inferred by difference from Eq. (3). In this way, Mauro [9] and Robbins and Mauro [20] were able to find that the convective component strongly dominates in a series of synthetic collodion membranes, indicating the presence of bulk water inside the membrane. The diffusive component is always present, however, and becomes increasingly significant with increasing density of membrane polymer material [20]. For lipid bilayers, the same experimental procedures (once the difficulty of stirring the solution right up to the membrane is overcome [2]) show the absence of a convective component. Both \(P_\textrm{f}\) and \(P_\textrm{d}\) are independently measured and found to be equal [2, 19], meeting the expectation that water crosses the lipid bilayer as widely separated molecules.
It is useful to rearrange Eq. (3) to the form,
For the synthetic collodion membranes, since measurements show that \(P_\textrm{f}/P_\textrm{d}~>>~1\), it follows that \(P_\textrm{c}>>P_\textrm{d}\), in words, convection dominates diffusion. But if \(P_\textrm{f}/P_\textrm{d}=1\), as in lipid bilayers, \(P_\textrm{c}=0\), and there is no convective water flow.
In the setting of biological cell membranes, a significant part of water exchange and flow is directly across the lipid bilayer, 10–20% [2, 23]. But biological membranes are punctuated by proteins like the aquaporins [16] that provide narrow channels allowing efficient single-file flow of water molecules, and these pores carry most of the transmembrane flow. In fact, it was the measurements of \(P_\textrm{f}/P_\textrm{d}\) ratios, and their observed values substantially greater than unity, that first suggested the existence of these protein channels [21, 22].
The reason that \(P_\textrm{f}/P_\textrm{d}\) greatly exceeds unity in situations involving bulk water is obvious. The diffusion permeability \(P_\textrm{d}\) measures the rate of random thermal movement of the water molecules in macroscopically quiescent bulk water. The osmotic permeability \(P_\textrm{f}\) measures the much greater rate of pressure-driven collective bulk flow. In the following, we show that \(P_\textrm{f}\) can be greater than \(P_\textrm{d}\) in single-file flow for essentially the same reason, but the collective nature of osmotic flow in this case is described differently.
3 The \(P_\textrm{f}/P_\textrm{d}\) ratio in single-file flow
We start with the equation,
where \(D^{*}\) is the self-diffusion coefficient of water molecules inside a membrane of thickness L. If the water molecules cross the membrane through a protein channel, we assume for simplicity that the length of the channel also equals L. With reference to the definition of \(P_\textrm{d}\) in Eq. (2), the driving force for tracer diffusion across the membrane on the molecular level is not the concentration difference between tracer concentrations in the reservoirs bathing the membrane but the tracer concentration gradient inside the membrane. Since the factor 1/L does not appear explicitly in the definition, it must be included as a factor in any expression for \(P_\textrm{d}\). The constant factor K accounts for the dependence of the permeability on the amount of water in the membrane relative to the outside reservoirs. Equivalently, it accounts for the probability that a molecule enters and exits the channel, and for interactions with the walls of the channel once the molecule is inside. An explicit expression for K, given subsequently, is not needed in this section.
Next, we consider osmotic water flow across the membrane. As discussed above in the Background section, it is caused by a pressure gradient. Thus for a membrane containing liquid water, the flow is predominately bulk, or convective, flow. A lipid bilayer contains a slight amount of water dissolved in it as discrete independent molecules, and in osmosis these molecules are transported across the bilayer by a pressure gradient [4]. The molecules drift in a stochastic diffusive manner in the direction of lower pressure. In single file also, the movement of water molecules in osmosis is best described in terms of the diffusive movement of the discrete molecules in the column, since the water does not exist there in bulk. We therefore postulate the same starting point for \(P_\textrm{f}\) as for \(P_\textrm{d}\),
but for \(P_\textrm{f}\) the diffusion coefficient D may not be equal to the self-diffusion coefficient \(D^{*}\). The \(P_\textrm{f}/P_\textrm{d}\) ratio is therefore,
Notice that the partition coefficient K cancels from the ratio \(P_\textrm{f}/P_\textrm{d}\), being the same for both permeabilities.
The concentration dependence of diffusion coefficients is crucial for present purposes. In self-diffusion, the environment of a thermally agitated molecule is on average symmetric, and the influence of surrounding molecules is not biased in any particular direction. But in diffusive flow caused by a concentration gradient, the more numerous molecules on one side of any given diffusing molecule exert a stronger concentration effect (from intermolecular forces) than the less numerous ones on the other side. Because of this asymmetry, we can write [29,30,31,32,33,34],
where in this equation \(\mu ^{\textrm{ex}}\), in dimensionless units of \(k_\textrm{B}T\), is the “excess” part of the chemical potential of the diffusing species, which accounts for non-ideal concentration effects, and therefore tends to zero when the concentration is low and intermolecular interactions become negligible. For the concentration derivative in the single-file case of diffusing hard spheres, we have defined \(\phi \) as a measure of the linear density of the spheres,
In words, then, \(\phi \) is the average fraction of the length of the column occupied by the spheres, each sphere possessing diameter a.
The interaction between hard spheres is entirely repulsive (the potential energy of interaction jumps from zero to infinity when two spheres make contact). It follows that the more numerous hard spheres at the high concentration side of a given sphere will push that sphere toward the low concentration side, augmenting self-diffusion. The derivative in Eq. (8) is thus expected to be positive. We confirm the expectation by making an exact calculation. From the statistical mechanical analysis of a linear assembly of molecules as presented in 1950 by Gürsey [24], we find for the special case of hard spheres in single file [34],
Both terms in this expression increase when \(\phi \) increases. When \(\phi \) tends to zero, so does \(\mu ^{\textrm{ex}}\).
Graphical representation of Eq. 11 for the \(P_\textrm{f}/P_\textrm{d}\) ratio as a function of fractional occupancy of single-file channel with hard spheres
The result for the \(P_\textrm{f}/P_\textrm{d}\) ratio follows,
Notice that in this section we did not attempt to calculate \(P_\textrm{f}\) and \(P_\textrm{d}\) individually but only their ratio, which is plotted in Fig. 1. At low densities, a regime where the molecules do not interact at all, so that the effect of asymmetric interactions in osmotic flow vanishes, \(\phi \rightarrow 0\), and we see that \(P_\textrm{f}\) becomes equal to \(P_\textrm{d}\). But as \(\phi \) increases, the expression for the \(P_\textrm{f}/P_\textrm{d}\) ratio becomes significantly greater than unity, until finally diverging at \(\phi =1\), when the spheres are in contact (closest packed) at infinitely high interaction energy, and the slightest asymmetry of a hard sphere environment has a dominantly strong effect.
Despite the divergence at closest packing, Eq. (11) yields realistic values for the \(P_\textrm{f}/P_\textrm{d}\) ratio when the density parameter \(\phi \) is given in a realistic range. For gramicidin A pores [2], the measured value of \(P_\textrm{f}/P_\textrm{d}\) is 5.3, which matches Eq. (11) for \(\phi =0.57\). The value of \(P_\textrm{f}/P_\textrm{d}\) measured [23] for aquaporin is 12.7, matched by Eq. (11) when \(\phi =0.72\). It is worth observing that for values of \(\phi \) greater than 0.5, the average single-file spacing of hard spheres cannot accommodate another sphere. In other words, in a model such as we have been exploring, it is not necessary for meaningful interpretation of “full occupancy” that \(\phi \) be assigned the value unity.
As a further example, we may recall Robbins and Mauro’s measurements of \(P_\textrm{f}/P_\textrm{d}\) for synthetic collodion membranes [20]. For the membrane most dense in polymer material, the measured value of \(P_\textrm{f}/P_\textrm{d}\) was 36. The passage of water across membranes of higher density could not be detected. We therefore take the number 36 as a speculative measure of a maximum value that evolutionary selection could have reached for \(P_\textrm{f}/P_\textrm{d}\) by means of a narrow protein pore in exploiting the advantages of concerted flow over diffusion of isolated molecules into and out of a cell. The corresponding value of \(\phi \) from Eq. (11) is 0.83.
4 The individual permeabilities
We regard the result for the \(P_\textrm{f}/P_\textrm{d}\) ratio as our central one. However, at the cost of further modeling, we will also try to find individual expressions for the osmotic and diffusion permeabilities. Actually, we need only an expression for the latter, since \(P_\textrm{f}\) will follow immediately from their ratio.
For \(P_\textrm{d}\) in Eq. (5), we need expressions for both K and \(D^{*}\). The water partition coefficient \(K=C_\textrm{w,m}/C_\textrm{w}^{0}=v_\textrm{w}^{0}C_\textrm{w,m}\), that is, the ratio of water concentrations (number of molecules per unit volume) inside the membrane and in the ideally dilute reservoirs, where \(v_\textrm{w}^{0}\) is the volume per molecule of pure water. For the concentration \(C_\textrm{w,m}\) of water in the membrane, the volume of the membrane is AL, where A is the area of a cross section, and if the water crosses through n single-file channels, each containing N hard-sphere “water” molecules, then \(C_\textrm{w,m}=nN/AL=n\phi /aA\), where we have used the fractional occupancy \(\phi \) of the channel, Eq. (9). We therefore have \(K=(nv_\textrm{w}^{0}/aA)\phi \).
As in traditional diffusion theory, we describe the thermal movement of the molecules in the channel by a random walk, in this case one-dimensional. The mean free path \(l=(1-\phi )l_{0}\), where \(l_{0}\) is the mean free path when \(\phi \rightarrow 0\). In words, \(l_{0}\) is the mean free path for a molecule in a channel so sparsely occupied that other molecules offer negligible interference with its thermal movement. The only obstacles to movement of the water molecule when \(\phi \rightarrow 0\) comes from its interactions with the fixed molecular groupings comprising the walls of the channel. In a one-dimensional random walk, \(D^{*}=(1/2)\nu l^{2}=(1/2)\nu l_{0}^{2}(1-\phi )^{2}\), where \(\nu \) is the collision frequency, which in this model we assume independent of \(\phi \).
With these expressions for K and \(D^{*}\), we arrive at a formula for the diffusion permeability,
where for the prefactor,
The dependence of \(P_\textrm{d}\) on \(\phi \) is shown in Fig. 2. When \(\phi \rightarrow 0\), the permeability tends to zero. This behavior is reasonable, since water molecules cannot cross the membrane if the membrane holds no water. The permeability rises to a maximum value at \(\phi =1/3\), then falls back to zero at \(\phi =1\), where the hard spheres are touching and diffusional movement in the channel is suppressed. This condition is never achieved for temperatures above zero, and the mean field calculation given here should be understood as meaningful only if \(\phi \) is not too close to unity.
With \(P_\textrm{d}\) in hand and Eq. (11) for the \(P_\textrm{f}/P_\textrm{d}\) ratio, the result for \(P_\textrm{f}\) follows,
Thus \(P_\textrm{f}\) rises linearly with \(\phi \) from zero at zero occupancy (for the same physical reason that \(P_\textrm{d}\) vanishes there) to its maximum value B at \(\phi =1\).
Since \(P_\textrm{d}\) vanishes at \(\phi =1\) in this model, an interesting interpretation of the course of the \(P_\textrm{f}/P_\textrm{d}\) ratio as a function of \(\phi \) (see Fig. 1) is that it describes a transition from all-diffusive flow at low densities, \(P_\textrm{f}/P_\textrm{d}\rightarrow 1\), to all-convective flow at \(\phi =1\). The critical point for the transition is at \(\phi =1\), where the diffusive component disappears. Of course, we know that one-dimensional systems do not exhibit phase transitions, which again shows the limitations of our mean-field interpretation of \(\phi \).
We can arrive at a numerical result if we convert \(P_\textrm{f}\) to the osmotic permeability per pore \(p_\textrm{f}=(A/n)P_\textrm{f}\) [2]. For the gramicidin A pore, \(p_\textrm{f}\) has been measured in two laboratories by distinct procedures as \(1 \times 10^{-14}\; \hbox {cm}^{3}\)/s and \(6 \times 10^{-14}\;\hbox {cm}^{3}\)/s [2]. In our model, the combination \((1/2)\nu l_{0}^{2}\) is the self-diffusion constant for water molecules in the pore under conditions of low fractional occupancy. If we use for it the value of the self-diffusion constant of bulk water, along with \(\phi =0.57\) (see discussion involving gramicidin A in Sect. 3), the diameter a of a water molecule, and \(L= 2.5\) nm for the length of the gramicidin A pore, we get \(5.6 \times 10^{-14}\;\hbox {cm}^{3}\)/s for \(p_\textrm{f}\) from Eq. (14).
5 Discussion
For water transport across lipid bilayers, the \(P_\textrm{f}/P_\textrm{d}\) ratio is found to be equal to unity, as expected for diffusive movement of sparsely distributed water molecules dissolved in an oily environment [2, 4, 19]. For synthetic membranes more receptive to liquid water, the ratio is orders of magnitude greater than unity, indicating the dominance of bulk liquid flow [9, 20]. There was an early focus, currently all but lost, on the interesting question of why the \(P_\textrm{f}/P_\textrm{d}\) ratio is greater than unity for water molecules moving in single file across narrow protein channels spanning biological membranes [2]. The answer was that \(P_\textrm{f}/P_\textrm{d}=N\), the number of water molecules in the channel, as a seemingly plausible condition if the passage of one molecule requires previous passage of all molecules ahead of it [2]. In this view, the permeability ratio equals N regardless of how large N might be (how long the pore), or of the fractional occupancy \(\phi \) of the pore, or of the nature of the liquid (it need not be water, the degree of hydrogen bonding does not matter), or of the interaction potential of any theoretical particles used as a model (hard spheres, Lennard–Jones 6–12, etc.).
In arriving at the stark answer N for the permeability ratio in single-file flow, no use was made of the development in the theory of liquids of one-dimensional models that could be solved exactly [24, 35, 36]. In fact, the no-pass condition is inherent in these liquid models, as well as in the calculations of this paper. For example, Gürsey’s evaluation of the partition function integral for a one-dimensional fluid explicitly uses the condition that the coordinate \(x_{i}\) of the ith molecule is integrated only between the coordinates \(x_{i-1}\) and \(x_{i+1}\) of the two neighboring molecules. But even though in developing our analysis we have made use of no-pass models, we did not arrive at the answer N for the single-file \(P_\textrm{f}/P_\textrm{d}\) ratio.
There is also a more recent simulation that finds \(P_\textrm{f}/P_\textrm{d}=N\) (or \(N+1\)) [37]. However, the authors do not simulate the laboratory measurements that define the two permeabilities according to Eq. (1) and Eq. (2). Instead, they essentially assume the answer N in advance by characterizing \(P_\textrm{f}\) as the rate for a molecule to take a single step in the channel, while \(P_\textrm{d}\) is taken as the rate, expected to be N times smaller, for a molecule to take all steps corresponding to the entire length of the channel.
The hard sphere model adopted in the present paper is certainly not an accurate portrayal of water molecules. Can the values of \(P_\textrm{f}/P_\textrm{d}\) exceeding unity for single-file flow of water be explained, as here for hard spheres, by invoking only ordinary thermal and pressure driven movements of independent molecules, or are specific properties of water molecules necessarily involved, such as their propensity (in an inhospitable carbon nanotube environment, for example [38]) to link by direct hydrogen bonding into a connected chain, like monomers in a polymer? In this case, it would be difficult to justify the asymmetric distribution underlying our use of Eq. (8), or the picture of freely moving small molecules used here to calculate P d individually.
The tools in the exploding field of flow in confined spaces have not yet been brought to bear on the \(P_\textrm{f}/P_\textrm{d}\) ratio for single-file flow. For a review emphasizing theoretical aspects, see [39].
Data Availability
No data associated in the manuscript.
References
B. Alberts, R. Heald, A. Johnson, D. Morgan, M. Raff, K. Roberts, P. Walter, J. Wilson, T. Hunt, Molecular Biology of the Cell, 7th edn. (Garland Press, New York, 2022)
A. Finkelstein, Water Movement through Lipid Bilayers, Pores, and Plasma Membranes (Wiley, New York, 1987)
L. Vegard, On the free pressure in osmosis. Proc. Camb. Philos. Soc. 15, 13–23 (1908)
G.S. Manning, A. Kay, The physical basis of osmosis. J. Gen. Physiol. 155, 10, e202313332 (2023)
B. Park, J. Kamcev, L.M. Robeson, M. Elimelech, B.D. Freeman, Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017)
D. Kitto, J. Kamcev, Manning condensation in ion exchange membranes: a review on ion partitioning and diffusion models. J. Polym. Sci. 60, 2929–2973 (2022)
M. Heiranian, H. Fan, L. Wang, X. Lu, M. Elimelech, Mechanisms and models for water transport in reverse osmosis membranes: history, critical assessment, and recent developments. Chem. Soc. Rev. 52, 8455–8480 (2023)
X. Li, G. Jiang, M. Jian, C. Zhao, J. Hou, A.W. Thornton, X. Zhang, J.Z. Liu, B.D. Freeman, H. Wang, L. Jiang, H. Zhang, Construction of angstrom-scale ion channels with versatile pore configurations and sizes by metal-organic frameworks. Nature Commun. 14, 286 (2023)
A. Mauro, Nature of solvent transfer in osmosis. Science 126, 252–253 (1957)
C.C. Michel, F.E. Curry, Microvascular permeability. Physiol. Rev. 79, 703–761 (1999)
J.R. Bordin, J.S. Andrade, A. Diehl, M.C. Barbosa, Enhanced flow of core-softened fluids through narrow nanotubes. J. Chem. Phys. 140, 194504 (2014)
M. Heiranian, N.R. Aluru, Nanofluidic transport theory with enhancement factors approaching one. ACS Nano 14, 272–281 (2020)
J.R. Bordin, A. Diehl, M.C. Barbosa, Relation between flow enhancement factor and structure for core-softened fluids inside nanotubes. J. Phys. Chem. B 117(23), 7047–7056 (2013)
K.P. Lee, H. Leesea, D. Mattia, Water flow enhancement in hydrophilic nanochannels. Nanoscale 4, 2621–2627 (2012)
X. Qin, Q. Yuan, Y. Zhao, S. Xie, Z. Liu, Measurement of the rate of water translocation through carbon nanotubes. Nano Lett. 11(5), 2173–2177 (2011)
S.H. White, G. von Heijne, D.M. Engelman, Cell Boundaries: How Membranes and Their Proteins Work (Garland Science, New York, 2022)
A. Horner, P. Pohl, Single-file transport of water through membrane channels. Faraday Discuss. 209, 9–33 (2018)
Z. Wan, Y. Gao, X. Chen, X.C. Zeng, J.S. Francisco, C. Zhu, Anomalous water transport in narrow-diameter carbon nanotubes. PNAS 119(39), e2211348119 (2022)
R. Fettiplace, D.A. Haydon, Water permeability of lipid membranes. Physiol. Rev. 60, 510–550 (1980)
E. Robbins, A. Mauro, Experimental study of the independence of diffusion and hydrodynamic permeability coefficients in collodion membranes. J. Gen. Physiol. 43, 523–532 (1960)
G. Hevesy, E. Hofer, A. Krogh, The permeability of the skin of frogs to water as determined by D2O and H2O. Skand. Arch. Physiol. 72, 199–214 (1935)
C.V. Paganelli, A.K. Solomon, The rate of exchange of tritiated water across the human red cell membrane. J. Gen. Physiol. 41, 259–277 (1957)
J.C. Mathai, S. Mori, B.L. Smith, G.M. Preston, N. Mohandas, M. Collinsi, P.C.M. van Zijl, M.L. Zeidel, P. Agre, Functional analysis of aquaporin-1 deficient red cells. J. Biol. Chem. 271, 1309–1313 (1996)
F. Gürsey, Classical statistical mechanics of a rectilinear assembly. Proc. Cambr. Phil. Soc. 46, 182–194 (1950)
G.S. Manning, The relation between osmotic flow and tracer solvent diffusion for single-file transport. Biophys. Chem. 3, 147–152 (1975)
P. Debye, Kinetische theorie der gesetze des osmotischen drucks bei starken elektrolyten. Phys. Z. 24, 334–338 (1923)
G.S. Manning, Binary diffusion and bulk flow through a potential energy profile: a kinetic basis for the thermodynamic equations of flow through membranes. J. Chem. Phys. 49, 2668–2675 (1968)
L. Wang, J. He, M. Heiranian, H. Fan, L. Song, Y. Li, M. Elimelech, Water transport in reverse osmosis membranes is governed by pore flow, not a solution-diffusion mechanism. Sci. Adv. 9, eadf8488 (2023)
S.R. de Groot, P. Mazur, Non-equilibrium Thermodynamics (Dover, New York, 1984)
J.G. Kirkwood, Transport of ions through biological membranes from the standpoint of irreversible thermodynamics, in Ion Transport Across Membranes. ed. by T. Clarke (New York, Academic Press, 1954), pp.119–127
C. Tanford, Physical Chemistry of Macromolecules (Wiley, New York, 1961)
R.A. Robinson, R.H. Stokes, Electrolyte Solutions (Dover, Mineola, 2002)
J. Kamcev, D.R. Paul, G.S. Manning, B.D. Freeman, Accounting for frame of reference and thermodynamic non-idealities when calculating salt diffusion coefficients in ion exchange membranes. J. Membr. Sci. 537, 396–406 (2017)
G.S. Manning, Deviation from the Einstein relation of the single file diffusion coefficient. Biophys. Chem. 5, 389–394 (1976)
A. Santos, A Concise Course on the Theory of Classical Liquids (Springer, Switzerland, 2016)
J. Frenkel, Kinetic Theory of Liquids (Dover, New York, 1955)
G. Portella, B.L. de Groot, Determinants of water permeability through nanoscopic hydrophilic channels. Biophys. J . 96, 925–938 (2009)
G. Hummer, J.C. Rasaiah, J.P. Noworyta, Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001)
N. Kavokine, R.R. Netz, L. Bocquet, Fluids at the nanoscale: from continuum to subcontinuum transport. Annu. Rev. Fluid Mech. 53, 377–410 (2021)
Acknowledgements
The author is grateful to Alan Kay for many interesting discussions.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Consider the ordinary diffusion of a solute in water (or any other solvent), or the pressure equalization in a real gas (which can also be regarded as a diffusion process, given the one-to-one relation between gas pressure and number density, or concentration). The flux of particles \({\textbf{j}}\) is related to the negative gradient of chemical potential \(\mu \) by the friction coefficient at uniform concentration f,
where c is solute or gas concentration. With units chosen such that \(k_{\textrm{B}}T=1\), the self-diffusion coefficient \(D^{*}\) is 1/f (Einstein relation), and the relevant part of \(\mu \) can be written as \(\ln c + \mu ^\textrm{ex}\), where the ”excess” term accounts for interactions among the solute or gas molecules,
Since \(\mu ^\textrm{ex}\) depends on c, and for the diffusion process, c depends on location \({\textbf{r}}\),
Finally, then,
from which the diffusion constant D is identified as,
or Eq. 8 of the text. It is also Equation (127), Chapter XI, in De Groot and Mazur [29], and Equation (21-8) in Tanford [31].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manning, G.S. A hard sphere model for single-file water transport across biological membranes. Eur. Phys. J. E 47, 27 (2024). https://doi.org/10.1140/epje/s10189-024-00419-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-024-00419-6