Abstract
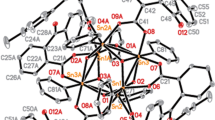
Two new complexes, [Cu(Phen)(o-Hbza)(H2O)2][Cu(Phen)(o-Hbza)Cl]2(o-Hbza) (I) and [Cu(Phen)(H2O)(p-Hbza)Cl] H2O (II) (o-Hbza = o-hydroxybenzoic acid, p-Hbza = p-hydroxybenzoic acid, Phen = 1,10-phenanthroline), have been synthesized and characterized by single-crystal X-ray diffraction methods (CIF files CCDC nos. 975524 (I) and 975525 (II)), elemental analyses, IR spectroscopy, thermal analyses as well as magnetic measurements. Compound I is extended into 1D chains through hydrogen-bonding and further assembled into 2D supramolecular layers through π⋯π interactions. Compound II is pairwise aggregated to form H-bonded dimuclear motifs, which are held together by π⋯π interactions into 1D chains and further assembled into 2D supramolecular networks by O-H⋯Cl hydrogen bonds. The magnetic behaviors of I and II obey the Curie-Weiss law with χm = C/(T − θ) with the Curie constant C = 1.283(2) cm3 mol−1 K and the Weiss constant θ = 0.284(4) K for I, as well as C = 0.424(1) cm3 mol−1 K and θ = 0.040(4) K for II, indicating weak ferromagnetic interactions between the Cu2+ ions.
Similar content being viewed by others
References
Mamula, O. and Zelewsky, A.V., Coord. Chem. Rev., 2003, vol. 242, p. 87.
Coordination Polymers: Design, Analysis and Applications, Batten, S.R., Neville, S.M., and Turner, D.R., Eds., Cambridge: RSC, 2009.
Sangeetha, N.M. and Maitra, U., Chem. Soc. Rev., 2005, vol. 34, p. 821.
Corma, A., Rey, F., Rius, J., et al., Nature, 2004, vol. 431, p. 287.
Schmitt, W., Baissa, E., Mandel, A., et al., Angew. Chem. Int. Ed., 2001, vol. 40, p. 3577.
Sozzani, P., Bracco, S., Comotti, A., et al., Angew. Chem. Int. Ed., 2005, vol. 44, p. 1816.
Campidelli, S., Brandmuller, T., Hirsch, A., et al., Chem. Commun., 2006, p. 4282.
Petitjean, A., Puntoriero, F., Campagna, S., et al., Eur. J. Inorg. Chem., 2006, p. 3878.
Desiraju, G.R., Acc. Chem. Res., 1996, vol. 29, p. 441.
Desiraju, G.R., Acc. Chem. Res., 2002, vol. 35, p. 565.
Desiraju, G.R., Angew. Chem. Int. Ed., 2007, vol. 46, p. 8342.
Fan, E., Vicent, C., Geib, S.J., and Hamilton, A.D., Chem. Mater., 1994, vol. 6, p. 1113.
Garcia-Garibay, M.A., Angew. Chem. Int. Ed., 2007, vol. 46, p. 8945.
Stang, P.J. and Olenyuk, B., Acc. Chem. Res., 1997, vol. 30, p. 502.
Lemoine, P., Viossat, B., Morgant, G., et al., J. Inorg. Biochem., 2002, vol. 89, p. 18.
Korah, R., Kalita, L., and Murugavel. R., Indian J. Chem., Sect. A: Inorg., Bio-Inorg., Phys., Theor. Anal. Chem., 2011, vol. 50, p. 763.
Bain, G.A. and Berry, J.F., J. Chem. Educ., 2008, vol. 85, p. 532.
Sheldrick, G.M., SHELXS-97, Program for Crystal Structure Solution, Göttingen (Germany): Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXL-97, Program for Crystal Structure Refinement, Göttingen (Germany): Univ. of Göttingen, 1997.
Addison, A.W. and Rao, N., Dalton Trans., 1984, p. 1349.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Interscience-Wiley, 1986.
Campbell, G.C. and Haw, J.F., Inorg. Chem., 1998, vol. 27, p. 3706.
Ginsberg, A.P. and Lines, M.E., Inorg. Chem., 1972, vol. 11, p. 2289.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Lin, C.J., Zheng, Y.Q., Zhang, D.Y. et al. Syntheses, crystal structures, and characterization of copper(II) carboxylate complexes incorporating o-hydroxybenzoic acid and p-hydroxybenzoic acid. Russ J Coord Chem 40, 932–942 (2014). https://doi.org/10.1134/S1070328414120094
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328414120094