Abstract
A chelating N-heterocyclic carbene palladium complex (3) containing N-(3-bromopropyl)-N’-thioether di-functionalized imidazol-2-ylidene was synthesized by the direct metalation of N-(3-bromopropyl)-N’-thioether di-functionalized imidazolium bromide (2) with Pd(OAc)2 in the presence of NaOAc and NaBr at room temperature using CH2Cl2 as solvent. The structure was characterized by NMR, HR-MS and X-ray crystallography. The results showed that complex 3 consists of two independent molecules with configuration of SC, RS in molecule a, and SC, SS in molecule b, respectively. The crystal is triclinic, P1 space group, with a = 8.4815(3), b = 8.6338(3), c = 19.2371(7) Å, V = 1245.66(8) Å3, and Z = 2 (at 293(2)K).
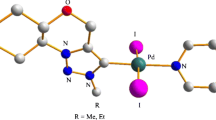
Graphical Abstract
A chelating N-heterocyclic carbene palladium complex (3) consists of two independent molecules with configurations of SC, RS and SC, SS respectively has been synthesized through the direct metalation of the precursor imidazolium bromide (2).



Similar content being viewed by others
Data Availability
CCDC 2312021 contains supplementary crystallographic data for complex 3. These data may be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223–336-033; or e-mail: deposit@ccdc.cam.ac.uk. Atomic coordinates for the optimized structure are available from L. Yang upon request.
References
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) An overview of N-heterocyclic carbenes. Nature 510:485–496. https://doi.org/10.1038/nature13384
Sorbelli D, Belanzoni P, Belpassi L (2021) Tuning the Gold(I)-carbon σ bond in Gold-Alkynyl complexes through structural modifications of the NHC ancillary ligand: effect on spectroscopic observables and reactivity. Eur J Inorg Chem 2021:2401–2416. https://doi.org/10.1002/ejic.202100260
Rojisha VC, Purushothaman I, Parameswaran SDP (2021) Nature of CN bond in N-heterocyclic carbene. Chem Phy Lett 765:138312. https://doi.org/10.1016/j.cplett.2020.138312
Baishya A, Kumar L, Barman MK, Peddarao T, Nembenna S (2016) Air stable N-heterocyclic carbene-carbodiimide (“NHC-CDI”) adducts: zwitterionic type bulky amidinates. ChemistrySelect 1:498–503. https://doi.org/10.1002/slct.201600019
Weetman C, Notman S, Arnold PL (2018) Destruction of chemical warfare agent simulants by air and moisture stable metal NHC complexes. Dalton Trans 47:2568–2574. https://doi.org/10.1039/C7DT04805J
Hashim II, Scattolin T, Tzouras NV, Bourda L, Hecke KV, Ritacco I, Caporaso L, Cavallo L, Nolan SP, Cazin CSJ (2022) Straightforward synthesis of [Cu(NHC)(alkynyl)] and [Cu(NHC)(thiolato)] complexes (NHC= N-heterocyclic carbene). Dalton Trans 51:231–240. https://doi.org/10.1039/D1DT03710B
Wang Q, Dai Z, Di X, Huang Q, Wang Y, Zhu J (2020) Pd (NHC)(cinnamyl) Cl-catalyzed Suzuki cross-coupling reaction of aryl sulfonates with arylboronic acids. Mol Divers 24:903–911. https://doi.org/10.1007/s11030-019-10001-4
Migulin VA (2023) Pd(iv)-induced nucleophile delivery in a cascade double Heck reaction. Org Chem Front 10(4):977–989. https://doi.org/10.1039/D2QO01983C
Lei P, Wang Y, Zhang C, Hu Y, Feng J, Ma Z, Liu X, Szostak R, Szostak M (2022) Sonogashira cross-coupling of aryl ammonium salts by selective C-N activation catalyzed by air-and moisture-stable, highly active [Pd(NHC)(3-CF3-An)Cl2](An= Aniline) precatalysts. Org Lett 2434:6310–6315. https://doi.org/10.1021/acs.orglett.2c02534
Zhou T, Li G, Nolan SP, Szostak M (2019) [Pd(NHC)(acac)Cl]: well-defined, air-stable, and readily available precatalysts for Suzuki and Buchwald-Hartwig cross-coupling (TRansamidation) of amides and esters by N-C/O-C activation. Org Lett 21:3304–3309. https://doi.org/10.1021/acs.orglett.9b01053
Fliedel C, Labande A, Manoury E, Poli R (2019) Chiral N-heterocyclic carbene ligands with additional chelating group (s) applied to homogeneous metal-mediated asymmetric catalysis. Coord Chem Rev 394:65–103. https://doi.org/10.1016/j.ccr.2019.05.003
Yuan D, Huynh HV (2012) Sulfur-functionalized N-heterocyclic carbene complexes of Pd(II): syntheses, structures and catalytic activities. Molecules 17:2491–2517. https://doi.org/10.3390/molecules17032491
Nayan Sharma K, Satrawala N, Kumar Joshi R (2018) Thioether-NHC-ligated Pd(II) complex for crafting a filtration-free magnetically retrievable catalyst for Suzuki-Miyaura coupling in water. Eur J Inorg Chem 2018:1743–1751. https://doi.org/10.1002/ejic.201800209
Ruhling A, Schaepe K, Rakers L, Vonhoren B, Tegeder P, Ravoo BJ, Glorius F (2016) Modular bidentate hybrid NHC-thioether ligands for the stabilization of palladium nanoparticles in various solvents. Angew Chem Int Ed 55:5856–5860. https://doi.org/10.1002/anie.201508933
Liu Y, Kean ZS, d’Aquino AI, Manraj YD, Mendez-Arroyo J, Mirkin CA (2017) Palladium(II) weak-link approach complexes bearing hemilabile N-heterocyclic carbene-thioether ligands. Inorg Chem 56:5902–5910. https://doi.org/10.1021/acs.inorgchem.7b00543
Mechrouk V, Maisse-François A, Bellemin-Laponnaz S, Achard T (2023) β-Alkylation through dehydrogenative coupling of primary alcohols and secondary alcohols catalyzed by thioether-functionalized N-heterocyclic carbene ruthenium complexes. Eur J Inorg Chem 26:e202300188. https://doi.org/10.1002/ejic.202300188
Seo H, Park HJ, Kim BY, Lee JH, Son SU, Chung YK (2003) Synthesis of P- and S-functionalized chiral imidazolium salts and their Rh and Ir complexes. Organometallics 22:618–620. https://doi.org/10.1021/om020878u
Abel R, David M, Manuel A, Eleuterio Á, José ML, Rosario F (2006) Synthesis, structure, and applications of N-dialkylamino-N‘-alkylimidazol-2-ylidenes as a new type of NHC ligands. Organometallics 25(26):6039–6046. https://doi.org/10.1021/om060788f
Stephen JR, Abel R, David M, Manuel A, Eleuterio Á, José ML, Rosario F (2007) Imidazo [1,5-a] pyridin-3-ylidene/thioether mixed C/S ligands and complexes thereof. Organometallics 26(10):2570–2578. https://doi.org/10.1021/om070063r
Krishna D, Wu M, Chiang M, Li Y, Leung PH, Pullarkat SA (2013) N-heterocyclic carbene C, S palladium (II) π-Allyl complexes: synthesis, characterization, and catalytic application in allylic amination reactions. Organometallics 32(8):2389–2397. https://doi.org/10.1021/om400110t
Krishnan D, Pullarkat SA, Wu M, Li Y, Leung PH (2013) Synthesis, structural characterisation and stereochemical investigation of chiral sulfur-functionalised N-heterocyclic carbene complexes of palladium and platinum. Chem Eur J 19(17):5468–5475. https://doi.org/10.1002/chem.201204320
Yang L, Yuan J, Mao P, Guo Q (2015) Chelating palladium complexes containing pyridine/pyrimidine hydroxyalkyl di-functionalized N-heterocyclic carbenes: synthesis, structure, and catalytic activity towards C-H activation. RSC Adv 5:107601–107607. https://doi.org/10.1039/C5RA21183B
Guo M, MA. Sc Thesis, Synthesis and catalytic properties of NCS pincer N-heterocyclic carbene palladium complexes, Henan University of Techonology, Henan, P. R. China (2023).
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341. https://doi.org/10.1107/S0021889808042726
Palatinus L, Chapuis G (2007) SUPERFLIP-a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Cryst 40:786–790. https://doi.org/10.1107/S0021889807029238
Palatinus L, van der Lee A (2008) Symmetry determination following structure solution in P1. J Appl Cryst 41:975–984. https://doi.org/10.1107/S0021889808028185
Palatinus L, Prathapa SJ, van Smaalen S (2012) EDMA: a computer program for topological analysis of discrete electron densities. J Appl Cryst 45:575–580. https://doi.org/10.1107/S0021889812016068
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8. https://doi.org/10.1107/S2053229614024218
Meng X, Yang L, Liu Q, Dong Z, Yuan J, Xiao Y, Mao P (2015) Amide functionalized pyridine/pyrimidine chelating N-heterocyclic carbene palladium complexes: synthesis, structure, and catalysis for C-5 arylation of imidazoles. Chin J Org Chem 42:3747–3756. https://doi.org/10.6023/cjoc202206005
Acknowledgements
This work was supported by the Natural Science Foundation of Henan Province (No. 242300420186) and the Innovative Funds Plan of Henan University of Technology (No. 2020ZKCJ29).
Author information
Authors and Affiliations
Contributions
Y. Xiao, Y. Liu, and Y. Wang carried out synthesis and characterization. L. Yang, J. Yuan, and L. Qu carried out data analysis, partly wrote the main manuscript and acquire fund. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Liu, Y., Yang, L. et al. Synthesis and Crystal Structure of a Chelating N-Heterocyclic Carbene Palladium Complex Containing N-(3-Bromopropyl)-N’-thioether di-functionalized Imidazol-2-ylidene. J Chem Crystallogr (2024). https://doi.org/10.1007/s10870-024-01012-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10870-024-01012-7