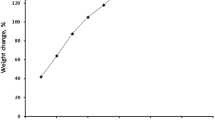
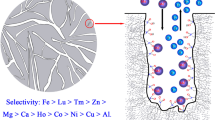
Abstract
Heavy metal ions such as hafnium, zirconium, vanadium, nickel, iron, and uranium were separated from petroleum ash leach liquor using ion-exchange resins Dowex 1×8, A2S3B8, and Amberlite (IRA-420). The separation was studied using Fourier transform IR spectroscopy (FT-IR) to confirm the adsorption of the metal ions onto the organic resins. Possible reactions between the metal ions and organic resins were suggested on the basis of the FT-IR spectra. The results show that the Dowex resin takes up U, Hf, and Zr most efficiently. This fact can be attributed to the ability of the Dowex resin to reduce both vanadium(V) and iron(III) metal ions to the lower valence state. This reduction facilitates the absorption of U, Hf, and Zr ions. The results obtained by ICP-OES and FT-IR are compared.




Similar content being viewed by others
REFERENCES
Xu, L., Xiao, Y., Van Sandwijk, A., Xu, Q., and Yan, Y., J. Nucl. Mater., 2015, vol. 466, p. 21.
Abdelkader, A.M. and El-Kashif, E., ISIJ Int., 2007, vol. 47, p. 25.
Abdelkader, A.M., Daher, A., Abdelkareem, R.A., and El-Kashif, E., Metall. Mater. Trans., 2007, vol. B38, p. 35. https://doi.org/10.1134/S0031918X1413016
Shahab, S., Yadollah, Y., and Mohammadreza, K.Z., J. Hazard. Mater., 2008, vol. 156, p. 583.
Abdelkader, A.M. and Fray, D.J., Electrochim. Acta, 2012, vol. 64, p. 10.
Susanta, L., Kakoli, D.N., Ramaswami, A., and Das, N.R., Appl. Radiat. Isot., 2000, vol. 52, p. 1399.
Taghizadeh, M., Ghanadi, M., and Zolfonoun, E., J. Nucl. Mater., 2011, vol. 412, p. 334.
Raju, B., Hwa, Y.L., and Man, S.L., J. Radioanal. Nucl. Chem., 2013, vol. 295, p. 1537.
Smolik, M., Jakobik-Kolon, A., and Poranski, M., Hydrometallurgy, 2009, vol. 95, p. 350.
Ling, Y.W. and Man, S.L., Hydrometallurgy, 2016, vol. 160, p. 12.
Agata, J., Marek, S., and Hanna, J., Hydrometallurgy, 2013, vol. 140, p. 77.
Pravin, U.S., Ram, S.L., and Rupa, S.M., Open J. Phys. Chem., 2011, vol. 1, p. 45.
Abd El-Hamid, A.M., Zahran, M.A., Abou Elthana, S.M., Khalid, F.M., and Kamal, H.M., RSC Adv., 2015, vol. 5, p. 80245. https://doi.org/10.1039/C5RA11563A
Abd El-Hamid, A.M., Zahran, M.A., Khalid, F.M., and Mahmoud, A.H., RSC Adv., 2014, vol. 4, p. 12506. https://doi.org/10.1039/C3RA44523B
Jha, M.C., Meyer, G.A., and Wicker, G.R., J. Met., 1981, vol. 33, p. 48.
Karbanee, N., van Hille, R.P., and Lewis, A.E., Ind. Eng. Chem. Res., 2008, vol. 47, p. 1596. https://doi.org/10.1021/ie0711224
Mani, P. and Suresh, S., Rasayan J. Chem., 2009, vol. 2, p. 307.
Theo, K.J., Leisel, H., and Ray, L.F., J. Raman Spectrosc., 2004, vol. 35, p. 969.
Trevor, J.F., Zirconium-89 complexes for cell tracking with positron emission tomography: PhD Thesis, School of Physical Sciences, Univ. of Kent at Canterbury, 2015.
Farmer, V.C., Handbook of the Infrared Spectra of Minerals, London: Mineralogical society of Great Britain, 1974.
Frost, R.L., Kloprogge, T., Martens, W.N., and Williams, P., Neues Jahrb. Mineral., Monatsh., 2002, vol. 11, p. 481.
Periasamy, A., Muruganand, S., and Palaniswamy, M., Rasayan J. Chem., 2009, vol. 2, no. 4, p. 981.
Hans, H.A., Am. Mineral., 1965, vol. 150, p. 1553.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ACKNOWLEDGMENTS
The authors are grateful to the memory of Prof. Mohamed Mahdy for continuous encouragement and sincere help during this research. In addition, the authors are grateful to the members of the Pilot Plant Experimental Department t as well as to the colleagues from the Chemical Analysis Department, especially from the Inductively Coupled Plasma Laboratory and Yellow Cake Purification Department, for their kind assistance in analytical control of the study and for valuable advices and sincere help.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
El-Hamid, A.M.A., Zahran, M.A., Ahmed, Y.M.Z. et al. Separation of Heavy Metal Ions from Petroleum Ash Liquor Using Organic Resins and FT-IR Study of the Process. Radiochemistry 62, 243–250 (2020). https://doi.org/10.1134/S1066362220020125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362220020125