Abstract
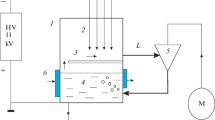
Results are presented from experimental studies of decomposition of toluene (C6H5CH3) in a polluted air flow by means of a steady-state atmospheric pressure glow discharge at different water vapor contents in the working gas. The experimental results on the degree of C6H5CH3 removal are compared with the results of computer simulations conducted in the framework of the developed kinetic model of plasma chemical decomposition of toluene in the N2: O2: H2O gas mixture. A substantial influence of the gas flow humidity on toluene decomposition in the atmospheric pressure glow discharge is demonstrated. The main mechanisms of the influence of humidity on C6H5CH3 decomposition are determined. The existence of two stages in the process of toluene removal, which differ in their duration and the intensity of plasma chemical decomposition of C6H5CH3 is established. Based on the results of computer simulations, the composition of the products of plasma chemical reactions at the output of the reactor is analyzed as a function of the specific energy deposition and gas flow humidity. The existence of a catalytic cycle in which hydroxyl radical OH acts a catalyst and which substantially accelerates the recombination of oxygen atoms and suppression of ozone generation when the plasma-forming gas contains water vapor is established.
Similar content being viewed by others
References
A. N. Trushkin and I. V. Kochetov, Plasma Phys. Rep. 38, 407 (2012).
A. M. Vandenbroucke, R. Morrent, N. De Geyter, and C. Leys, J. Hazard. Mater. 195, 30 (2011).
R. Rudolph, K. P. Francke, and H. Miessner, Plasma Chem. Plasma Process. 22, 401 (2002).
Yu. S. Akishev, V. B. Karalnik, I. V. Kochetov, et al., in Proceedings of the 16th International Symposium on Plasma Chemistry, Taormina, 2003, p. 226.
R. Vertriest, R. Morrent, J. Dewulf, et al., Plasma Sources Sci. Technol. 12, 4121 (2003).
H. M. Lee and M. B. Chang, Plasma Chem. Plasma Process. 23, 541 (2003).
Guo Yu-fang, Ye Dai-qi, Chen Ke-fu, and Tian Ya-feng, Chem. Plasma Proc. 26, 237 (2006).
M. Kogoma, Y. Miki, K. Tanaka, and K. Takahashi, Plasma Process. Polym. 3, 727 (2006).
Chang Ming Du, Jian Hua Yan, and B. Cheron, Plasma Sources Sci. Technol. 16, 791 (2007).
J. Van Durme, J. Dewulf, W. Sysmans, et al., Chemosphere 68, 1821 (2007).
N. Blin-Simiand, F. Jorand, Z. Belhadj-Miled, et al., Int. J. Plasma Environ. Sci. Technol. 1, 64 (2007).
R. A. Korzekwa, M. G. Grothaus, R. K. Hutcherson, et al., Rev. Sci. Instrum. 69, 1886 (1998).
S. Futamura, T. Terasawa, and M. Sugasawa, in Proceedings of the 18th International Symposium on Plasma Chemistry, Kyoto, 2007.
D. N. Chin, C. W. Park, and C. W. Hahn, Bull. Korean Chem. Soc. 21, 228 (2000).
Z. Falkenstein and J. J. Coogan, J. Phys. D 30, 817 (1997).
Y. Akishev, O. Goossens, T. Callebaut, et al., J. Phys. D 34, 2875 (2001).
Y. Akishev, A. Deryugin, I. Kochetov, et al., J. Phys. D 26, 1632 (1993).
Yu. S. Akishev, A. A. Deryugin, V. B. Karal’nik, et al., Plasma Phys. Rep. 20, 511 (1994).
K. H. Becker, E. H. Fink, W. Groth, et al., Faraday Discuss. Chem. Soc., No. 53, 35 (1972).
J. Balamuta and M. F. Golde, J. Chem. Phys. 76, 2430 (1982).
Y. Itikawa and N. Mason, J. Phys. Chem. Ref. Data 34, 1 (2005).
J. A. Manion, R. E. Huie, R. D. Levin, et al., NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Ver. 7.0, Release 1.6.4, Data version 2008.12 (National Inst. of Standards, Technology, Gaithersburg, MD, 2008), http://kinetics.nist.gov/
L. W. Sieck, T. J. Buckley, J. T. Herron, and D. S. Green, Plasma Chem. Plasma Process. 21, 441 (2001).
N. Blin-Simiand, F. Jorand, L. Magne, et al., Plasma Chem. Plasma Process. 28, 429 (2008).
Master Chemical Mechanism (University of Leeds, Leeds, UK, 2010), http://mcm.leeds.ac.uk/MCM/
J. A. Miller and C. F. Melius, Combust. Flame 91, 21 (1992).
R. Knystautas, J. H. S. Lee, J. E. Shepherd, and A. Teodorczyk, Combust. Flame 115, 424 (1998).
H.-Y. Zhang and J. T. McKinnon, Combust. Sci. Technol. 107, 261 (1995).
W. J. Pitz and C. K. Westbrook, Combust. Flame 63, 113 (1986).
M. Deminsky, V. Chorkov, G. Belov, et al., Comput. Mater. Sci. 2, 169 (2003).
Yu. S. Akishev, M. E. Grushin, I. V. Kochetov, et al., Plasma Phys. Rep. 26, 157 (2000).
H. H. Kim, A. Ogata, and S. Futamura, J. Phys. D 38, 1292 (2005).
A. Ogata, H. H. Kim, S. M. Oh, et al., in Proceedings of the International Conference on Electrostatic Precipitation, Cairns, 2006, p. 1.
V. T. Samoilovich, V. I. Gibalov, and K. V. Kozlov, Physical Chemistry of Barrier Discharge (Izd. Mosk. Gos. Univ., Moscow, 1989) [in Russian].
R. Ono and R. Oda, Int. J. Plasma Environ. Sci. Technol. 1, 123 (2007).
V. A. Bityurin, E. A. Filimonova, and G. V. Naidis, IEEE Trans. Plasma Sci. 37, 911 (2009).
M. B. Zheleznyak and E. A. Filimonova, Teplofiz. Vys. Temp. 36, 557 (1998).
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.N. Trushkin, M.E. Grushin, I.V. Kochetov, N.I. Trushkin, Yu.S. Akishev, 2013, published in Fizika Plazmy, 2013, Vol. 39, No. 2, pp. 193–209.
Rights and permissions
About this article
Cite this article
Trushkin, A.N., Grushin, M.E., Kochetov, I.V. et al. Decomposition of toluene in a steady-state atmospheric-pressure glow discharge. Plasma Phys. Rep. 39, 167–182 (2013). https://doi.org/10.1134/S1063780X13020025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063780X13020025